Abstract
In polygynous species, mate choice is an integrated part of sexual selection. However, whether mate choice occurs independently of the genetic relatedness among mating pairs has received little attention, although inbreeding may have fitness consequences. We studied whether genetic relatedness influenced females' choice of partner in a highly polygynous ungulate—the reindeer (Rangifer tarandus)—in an experimental herd during two consecutive rutting seasons; the herd consisting of 75 females in 1999 and 74 females in 2000 was exposed to three 4.5-year-old adults and three 1.5-year-old young males, respectively. The females' distribution during peak rut was not influenced by their genetic relatedness with the dominant males of the mating groups. Further, genetic relatedness did not influence the actual choice of mating partner. We conclude that inbreeding avoidance through mating group choice as well as choice of mating partner, two interconnected processes of female mate choice operating at two different scales in space and time, in such a highly female-biased reindeer populations with low level of inbreeding may not occur.
Keywords: relatedness, mate choice, inbreeding avoidance, Rangifer tarandus
1. Introduction
Mating systems express the ways in which individuals of both sexes interact in order to reproduce (Clutton-Brock 1989). In polygynous species, reproductive success varies greatly among males, whereas females' success is comparatively less variable (Trivers 1972). This implies that males increase their reproductive success by increasing their number of mating partners, whereas females increase their reproductive success by improving the quality of their mates (Trivers 1972). Hence, males should compete for access to females, whereas females should be more selective than males in their partner choice (Andersson 1994).
Inbreeding may have deleterious effects on offspring fitness (Frankham et al. 2002). Inbreeding avoidance through female mate choice based on relatedness recognition has therefore been suggested in several vertebrate mating systems (Pusey & Wolf 1996); selection pressure being particularly important in species where passive inbreeding avoidance through dispersal is poorly developed (Pusey & Wolf 1996). The ability to disperse may be restricted by habitat fragmentation (Frankham et al. 2002), and behavioural responses to combat such restrictions have been reported (Stow & Sunnucks 2004; Banks et al. 2005).
Today, the remnant populations of wild European tundra reindeer (Rangifer tarandus tarandus) are mainly distributed in fragmented mountainous areas in southern Norway (Andersen & Hustad 2004). Reproductive success varies greatly among reindeer males and is related to males' phenotypic quality (Røed et al. 2002). Hence, the species' mating system will probably amplify the risk of inbreeding in these small and geographically restricted populations. The female-biased sex ratio among adults, reported in many managed ungulate populations (Ginsberg & Milner-Gulland 1994) including European wild tundra reindeer (Andersen & Hustad 2004), will also contribute to the reduction of the effective population size and increase the populations' inbreeding vulnerability.
Focussing on females' ability to choose mates under two extreme female-biased sex ratio models with different male age structures, as a potential inbreeding mechanism in reindeer, we investigated whether (i) the females' mating group affinity depended on their genetic relatedness to the dominant male of the group and (ii) genetic relatedness influenced their choice of mating partner.
2. Material and methods
(a) Study area and experimental animals
The study was conducted at Kutuharju Field Reindeer Research Station, in Kaamanen, Finland (69° N, 27° E), as a part of a larger reproductive study in reindeer (Røed et al. 2002). The herd consisting of 75 females in 1999 and 74 females in 2000 was exposed to three adult (4.5-year-old, weight: 120–140 kg) males and three young (1.5-year-old, weight: 61–68 kg) males, respectively, all born within the herd. Females and males were individually marked, weighed and blood sampled before the rut. During both rutting seasons, the herd was confined to a 15 km2 fenced area. The males were radio collared. During the rut, from late September to early November, the males were located daily and individual females associated with them recorded. We extracted the rank among males based on agonistic interactions observed during the rut.
Date of peak rut was individually defined for each female by backdating from the birth date of her calf using mean gestation length of observed matings during first oestrus cycle with matching paternities. By adding and removing 3 days to the date of peak rut, we defined the peak week of rut for each female individually. Number of days observed with each male during the peak week of rut was calculated for each female individually. If two or three males were present in the same mating group on a given day, then the score was only given to the highest ranked male.
During calving, all females were confined to a smaller enclosure (approx. 50 ha) where data on birth date and mother–calf assignments were obtained. Blood samples were obtained from all individuals and analysed for 17 DNA microsatellite loci (Røed et al. 2002) to assess paternities and relatedness. Relatedness (R) was estimated for all pairwise combinations of all females producing a calf and all the available males for each year separately by using the program Relatedness 5.0 (Queller & Goodnight 1989). Jackknifing over all the loci generated standard errors for average relatedness values. Based on paternity of all sires from 1997 and onwards and maternal pedigree dating back to the late 1960s, we were able to trace close relatives within the herd.
(b) Statistical analyses
We used the generalized linear model (Genmod procedure, SAS 2003) with a log link to assess the effect of genetic relatedness on the female's dominant mating group male preference (measured as the number of days spent together with each male during peak rut). To test whether the genetic relatedness influenced choice of mating partner, we compared the relatedness of all potential non-mating pairs with all actual mating pairs by performing a randomization test using the permutation testing in SAS (2003) based on 10 000 random samplings.
3. Results
Forty and 48 paternities were established in 1999 and 2000, respectively, and the genetic relatedness between these mothers and available males is the basis for the further analyses. The heterozygosity value (±s.e.) in the herd was 0.735 (±0.030) across the 17 loci analysed and the mean (±s.e.) relatedness between the males was 0.074 (±0.067) in 1999 and −0.059 (±0.109) in 2000.
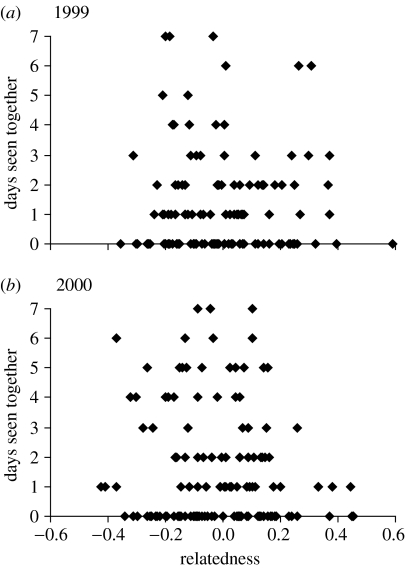
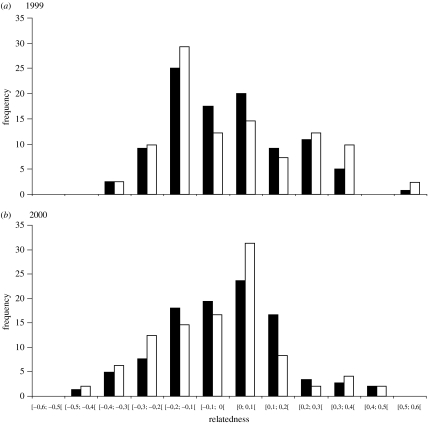
The number of days each female was observed with the dominant males of the mating groups during peak week of rut was not influenced by her genetic relatedness to the males (1999: Χ2=0.01, d.f.=118, p=0.92; 2000: Χ2=1.47, d.f.=142, p=0.23; figure 1). Moreover, the frequency distribution of all potential as well as all actual mating pairs did not diverge from a normal distribution around zero as indicated by the mean (±s.e.) relatedness values of all potential pairs of −0.005 (±0.017) in 1999, −0.014 (±0.015) in 2000, and all actual pairs of 0.011 (±0.034) in 1999 and 0.032 (±0.027) in 2000, respectively (figure 2). There was no difference in relatedness between actual mating pairs and all potential non-mating pairs both in 1999 (permutation test: p=0.50) and in 2000 (permutation test: p=0.40). In 1999, three females were daughters of the three available males and two of them were actually mated by their father; out of two half-sisters, one was mated by their half-brother and out of four aunts, two were mated by their sisters' son. Obviously in 2000, the young males had no daughters in the herd and unfortunately we know only one of the males' fathers. Two of the mothers of the males were available, but none of them was mated by their son. Further, no half-sisters of the males were among the females in 2000. There was no relationship between male rank and number of offspring sired (in 1999, highest ranked male: 19, medium: 3 and lowest: 18; in 2000, this figure was 19, 13 and 17, respectively).
Figure 1.
Number of days each female was observed during her peak week of rut together with the mating group dominant males in relation to her relatedness with the actual males in (a) 1999 and (b) 2000.
Figure 2.
Frequency relatedness distribution by year (a) 1999 and (b) 2000) for all potential mating pairs (solid bars) and all actual mating pairs resulting in sires (open bars).
4. Discussion
Inbreeding depression has been reported to influence traits related to fitness in various species (Keller & Waller 2002). Accordingly, the degree of relatedness between mates may have indirect fitness consequences and induce a selection pressure for active avoidance of close kin as mates, although the evidence in vertebrates is conflicting (Pusey & Wolf 1996). The discrepancies may be contributing to species differences in inbreeding level and life-history strategies (Pusey & Wolf 1996) as well as inbreeding risk (Stow & Sunnucks 2004). The high levels of heterozygosity within the herd, which falls well within the range of wild reindeer populations in Norway (0.630–0.742; K. H. Røed et al. 2006, unpublished data), suggest no ongoing inbreeding in the population.
Female reindeer may express their mating group preference since they live in fission–fusion societies during the rut (Skogland 1989). However, their ability to move relatively freely was not manifested through choice of mating group dominant males with lower genetic relatedness than expected by chance regardless of male age structure (figure 1). Given the low male resource availability, the cost of assessing males may counteract any fitness gain of choosing high-quality mates (Wong & Candolin 2005). It could therefore pay for the females to accept the first suitor regardless of the genetic relatedness to secure successful conception during first oestrus. This tactic will ensure an optimal timing of birth—a critical factor for offspring survival in northern ungulates (Langvatn et al. 2004). However, even with only 4% of males available, the females moved between mating groups during the rut; in 1999, 26 females visited all males, 13 females visited two males, whereas only one was seen together with only one male during their pre-peak and peak weeks of rut. The figures in 2000 were 30, 17 and 1, respectively.
Skogland (1989) argued that change in sex ratio influences the reindeer mating system. Indeed, a male tending tactic of receptive females is common under natural condition where the tundra reindeer aggregate into large mating groups (Skogland 1989). The frequency of aggregation of males during the two most intensive weeks of rut was low. In 1999, all three males were observed together only once, whereas in 2000 all three males were observed together once and two of them were seen together six times. This implies that given a highly female-biased sex ratio, the dominant male will mount most of the females coming into oestrus within his mating group and suggests that female choice of mating group close to conception may be regarded as a precursor for choice of mating partner.
The mean genetic relatedness between actual mated and all potential non-mating pairs did not differ, irrespective of male age structure, indicating random mating in relation to genetic relatedness within the studied herd as also shown in wood bison (Bison bison athabascae; Wilson et al. 2002). In 1999, there was no avoidance of close relatives. Indeed, around 17% of all potential mating pairs had a relatedness greater than 0.2, whereas around 25% of the actual mated pairs had a relatedness greater than 0.2 (figure 2). In 2000, the picture was more unclear. Still, about 8% of all potential mating pairs in 2000 had a relatedness greater than 0.2 and the percentage of actual mated pairs with a relatedness greater than 0.2 was almost identical (figure 2). However, we acknowledge the big variance in relatedness estimators (Lynch & Ritland 1999) and hence the difficulties to distinguish any other relationships than first- and second-orders relatives.
Reindeer/caribou is a classic Ice Age mammal adapted to rapid expansion and retreat in concert with glacial ice-sheet fluctuations (Geist 1999). Its highly migratory lifestyle (Geist 1999) indicates high degree of gene flux between populations. Indeed, man-induced bottlenecks through landscape fragmentation and manipulation of the population structure may affect the long-term viability of the remnant small and fragmented populations of wild European tundra reindeer (Andersen & Hustad 2004). Signs of inbreeding in these small populations have not been detected (K. H. Røed 2006, unpublished data). However, the fragmentation rate has increased the past few decades (Andersen & Hustad 2004) and incomplete behavioural inbreeding avoidance coupled with restricted dispersal may render these populations vulnerable to inbreeding (Frankham et al. 2002).
References
- Andersen, R. & Hustad H. (eds) 2004 Villrein og Samfunn. En veiledning til bevaring og bruk av Europas siste villreinfjell (in Norwegian). Trondheim, Norway: NINA Temahefte 27.
- Andersson M.B. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Banks S.C, Ward S.J, Lindenmayer D.B, Finayson G.R, Lawson S.J, Taylor A.C. The effect of habitat fragmentation on the social kin structure and mating system of the agile antechinus (Antechinus agilis) Mol. Ecol. 2005;14:1789–1801. doi: 10.1111/j.1365-294X.2005.02535.x. doi:10.1111/j.1365-294X.2005.02535.x [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Mammalian mating systems. Proc. R. Soc. B. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Frankham R, Ballou J.D, Briscoe D.A. Cambridge University Press; Cambridge, UK: 2002. Introduction to conservation genetics. [Google Scholar]
- Geist V. San-Hill Press; London, UK: 1999. Deer of the world. Their evolution, behaviour and ecology. [Google Scholar]
- Ginsberg J.R, Milner-Gulland E.J. Sex biased harvesting and population dynamics of ungulates. Cons. Biol. 1994;8:157–166. doi:10.1046/j.1523-1739.1994.08010157.x [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. doi:10.1016/0169-5347(01)02442-9 [Google Scholar]
- Langvatn R, Mysterud A, Stenseth N.C, Yoccoz N.G. Timing and synchrony of ovulation in red deer constrained by short northern summers. Am. Nat. 2004;163:763–772. doi: 10.1086/383594. doi:10.1086/383594 [DOI] [PubMed] [Google Scholar]
- Lynch M, Ritland K. Estimation of pairwise relatedness with molecular markers. Genetics. 1999;152:1753–1766. doi: 10.1093/genetics/152.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey A, Wolf M. Inbreeding avoidance in animals. Trends Ecol. Evol. 1996;11:201–206. doi: 10.1016/0169-5347(96)10028-8. doi:10.1016/0169-5347(96)10028-8 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Røed K.H, Holand Ø, Smith M.E, Gjøstein H, Kumpula J, Nieminen M. Reproductive success in reindeer males in a herd with varying sex ratio. Mol. Ecol. 2002;11:1239–1243. doi: 10.1046/j.1365-294x.2002.01509.x. doi:10.1046/j.1365-294X.2002.01509.x [DOI] [PubMed] [Google Scholar]
- SAS 2003 SAS/STAT guide for personal computers Version 8. Cary, NC: SAS Inst. Inc.
- Skogland T. Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Adv. Ethol. 1989;29:1–71. [Google Scholar]
- Stow A.J, Sunnucks P. Inbreeding avoidance in Cunningham's skinks (Egernia cunninghami) in natural and fragmented habitat. Mol. Ecol. 2004;13:443–447. doi: 10.1046/j.1365-294x.2003.02060.x. doi:10.1046/j.1365-294X.2003.02060.x [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aline; Chicago, IL: 1972. pp. 136–176. [Google Scholar]
- Wilson G.A, Olson W, Strobeck C. Reproductive success in wood bison (Bison bison athabascae) established using molecular techniques. Can. J. Zool. 2002;80:1537–1548. doi:10.1139/Z02-147 [Google Scholar]
- Wong B.B.M, Candolin U. How is female mate choice affected by male competition? Biol. Rev. 2005;80:559–571. doi: 10.1017/S1464793105006809. doi:10.1017/S1464793105006809 [DOI] [PubMed] [Google Scholar]