Abstract
Apoptosis accompanying negative selection is a central but poorly understood event in T cell development. The Nur77 nuclear steroid receptor and Bim, a proapoptotic BH3-only member of the Bcl-2 family, are two molecules implicated in this process. However, how they relate to each other and how Nur77 induces apoptosis remain unclear. In thymocytes, Nur77 has been shown to induce cell death through a transcriptional-dependent pathway, but in cancer cell lines, Nur77 was reported to induce apoptosis through conversion of Bcl-2 into a killer protein at the mitochondria. Whether this Nur77 transcriptional-independent pathway actually occurs in vivo remains controversial. Using an optimized fractionation protocol for thymocytes, here we report that stimulation of CD4+CD8+ thymocytes results in translocation of Nur77 and its family member Nor-1 to the mitochondria, leading to their association with Bcl-2 and exposure of the Bcl-2 proapoptotic BH3 domain. In two T cell receptor transgenic models of negative selection, F5 and HY, a conformational change of the Bcl-2 molecule in the negatively selected T cell population was similarly observed. Thus, the Nur77 family and Bim pathways converge at mitochondria to mediate negative selection.
Apoptosis is an essential process in eliminating superfluous or potentially dangerous cells in multicellular organisms (1). Dysregulation of this evolutionarily conserved mechanism could lead to cancer, autoimmunity, or degenerative disorders. Apoptosis plays an especially important role in regulating and establishing the T cell repertoire during T cell development. Developing immature T cells or thymocytes are subject to selection based on the binding avidity between their TCR and self-peptide–MHC complexes. Thymocytes that strongly recognize self-peptide–MHC undergo apoptosis through a process termed negative selection, whereas a weak recognition of self-peptide–MHC results in survival and further development. Negative selection ensures that thymocytes leaving the thymus are tolerant to the host's own proteins and thus contributes to prevention of autoimmunity. Mice with a mutation in AIRE, a gene essential for expression of “tissue-specific” antigens in thymic antigen-presenting cells, exhibit severe autoimmunity due to the defective process of antigen presentation for negative selection (2). The signal transduction pathways leading to negative selection in thymocytes are not fully understood. Previous work has implicated only a few molecules, including MINK, JNK, and p38 kinases as well as the downstream effector molecules Bim, a Bcl-2 proapoptotic member, and the Nur77 family of transcription factors (3, 4). Null mutation in Bim or expression of a dominant-negative Nur77 protein resulted in a partial rescue of cells destined to die through negative selection (5–7). The molecular relationship between these pathways, however, is not clear, and how Nur77 causes thymocyte cell death remains to be elucidated.
Nur77 is an orphan nuclear steroid receptor that belongs to the steroid/thyroid hormone receptor superfamily (4). The Nur77 family consists of Nur77, Nor-1, and Nurr1. In thymocytes and T cells, expression of only Nur77 and Nor-1 is induced in response to strong engagement of the TCR and correlates with apoptosis (8). Although Nur77−/− mice have no phenotype, redundancy with Nor-1 is the likely explanation (8, 9). Overexpression of a dominant-negative Nur77 protein, which blocks the activity of all family members, can inhibit apoptosis associated with negative selection (6, 7). Conversely, constitutive expression of either Nur77 or Nor-1 but not Nurr1 in thymocytes leads to massive apoptosis (9). Establishing Nur77's mode of action during negative selection has proven to be elusive so far. As a transcription factor, Nur77 regulates several downstream genes, including TRAIL, Fas ligand, and two genes with poorly characterized function, NDG1 and NDG2. NDG1 can initiate apoptosis through caspase-8, and thus Nur77 transcription might lead to caspase cleavage through the death receptor pathways or caspase-8 (10). In addition, a mutant Nur77 with higher transcriptional activity causes more apoptosis in thymocytes than a mutant Nur77 that exhibits little transcriptional capability (11). Thus, Nur77 transcriptional activity seems to correlate with its apoptotic function. However, a dominant-negative protein of FADD, which can simultaneously inhibit signaling from multiple death receptors, showed no effect on negative selection (12). Thus, it is possible that Nur77 initiates apoptosis through other mechanisms. Interestingly, Nur77 has been reported to be capable of translocating from the nucleus to mitochondria in cancer cell lines (13–17). At the mitochondria of cancer cell lines, Nur77's association with Bcl-2 at a linker region between the BH3 and BH4 domains of Bcl-2 was reported to expose the Bcl-2 BH3 domain that converts Bcl-2 into a proapoptotic molecule (14). Whether this is physiologically relevant or not is not clear. Most of the original experiments were done through overexpression of a Nur77 mutant lacking the DNA binding domain. In thymocytes, Nur77 was reported to localize mainly in the nucleus in one report (18). However, thymocytes are known to be fragile, and cell fractionation studies of thymocytes are notoriously difficult because they contain very little cytoplasm. Using an optimized fractionation protocol, we report here that Nur77 and its family member Nor-1 are present in thymic mitochondria in an export-dependent fashion. We also present evidence that Nur77 and Nor-1 associate with Bcl-2 in stimulated thymocytes. Exposure of the Bcl-2 BH3 domain was detected in in vitro anti-CD3/CD28 or PMA/ionomycin-stimulated thymocytes and in two TCR transgenic models of negative selection. Based on these data, we present a simplified model of apoptosis during negative selection through the mitochondria via Nur77/Nor-1/Bcl-2 and Bim.
RESULTS AND DISCUSSION
Nur77 family members are localized to the mitochondria and associate with Bcl-2 in stimulated thymocytes
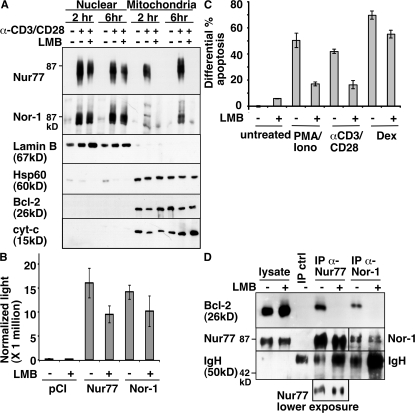
In cancer cell lines, the conversion of Bcl-2 to a proapoptotic form by Nur77 was reported to be mediated by translocation of Nur77 from the nucleus to the mitochondria (14). In thymocytes, Nur77 could be detected easily in the nucleus. However, the mitochondria fraction was very difficult to obtain. We first performed cell fractionation with thymocytes using published protocols (18–20). To monitor fractionation, we used Western blot analysis with antibodies for either a nuclear-specific protein lamin B or mitochondria-specific proteins HSP-60 and cytochrome c. However, we found that pestle homogenization, a step described in all published fractionation protocols, led to rupture of mitochondria in these fragile thymocytes, resulting in impure cell fractionation and very little intact mitochondria. Using a modified protocol involving no pestle homogenization, we have successfully isolated fractions from thymocytes in a consistent manner. Thymocytes were either not stimulated or stimulated with anti-CD3/CD28 for either 2 or 6 h to induce apoptosis. Fractions were then probed with antibodies specific for either Nur77 or Nor-1. In addition to the nucleus, both Nur77 and Nor-1 could be found at the mitochondria in a stimulus-dependent manner (Fig. 1 A). Nur77 was found in the mitochondria fractions from thymocytes stimulated for either 2 or 6 h, whereas a strong Nor-1 signal was found in the 6-h time point. As expected, very little Nur77 or Nor-1 was found in the nucleus or mitochondria fraction of unstimulated thymocytes. Similar results were obtained with PMA/ionomycin-stimulated thymocytes, which also induced cell death, although the kinetics of Nur77/Nor-1 appearance in mitochondria varied depending on the concentrations of PMA and ionomycin used (unpublished data).
Figure 1.
Nur77 and Nor-1 localize to the mitochondria and associate with Bcl-2 in anti-CD3/CD28 or PMA/ionomycin-treated thymocytes. (A) Nuclear and mitochondrial fractions of wild-type thymocytes cultured with 10 μg/ml anti-CD3 and 2 μg/ml anti-CD28 (plate-bound) were isolated and blotted with anti-Nur77 and anti–Nor-1 antibodies. For fraction purity, the blot was also probed with antibodies for lamin B, HSP60, Bcl-2, and cytochrome c. (B) Transcriptional activity of Nur77 and Nor-1 was determined using a luciferase assay (as described in Materials and methods) on 293T cells transiently transfected with the indicated expression plasmids along with a Nur77/Nor-1–dependent luciferase reporter construct and renilla internal control construct. (C) The extent of apoptosis in wild-type thymocytes cultured with 10 μg/ml anti-CD3/2 μg/ml CD28 (plate-bound), 1.25 ng/ml PMA/0.5 μM ionomycin, or 0.5 μM dexamethasone (Dex) was assessed by annexin V staining. Apoptosis was normalized to the untreated controls. (D) Cell lysates from lck-Bcl-2 thymocytes cultured with 2.5 ng/ml PMA/0.5 μM ionomycin were immunoprecipitated (IP) with anti–rabbit IgG (IP crtl), anti-Nur77, and anti–Nor-1 antibodies, followed by blotting with an anti–Bcl-2 antibody. Results shown in A and D represent three to six independent experiments. Data in B and C represent the mean ± SD of three independent experiments. Thymocytes were pretreated for 2 h with 20 nM leptomycin B (LMB) where indicated.
To see if the mitochondria-specific Nur77/Nor-1 signals are export dependent, we added leptomycin B, a nuclear export inhibitor (21). Leptomycin B completely blocked the translocation of Nur77 and Nor-1 to mitochondria, whereas it had a minimal effect on the Nor-1 levels in the nucleus. Although leptomycin B did decrease the levels of nuclear Nur77 to some extent, its effect on Nur77 translocation to mitochondria is more dramatic and complete. Consistent with this notion, leptomycin B had a minimal effect on Nur77 and Nor-1 transactivation activities in a luciferase reporter assay, affirming intact nuclear function of Nur77/Nor-1 (Fig. 1 B). Thus, Nur77 and Nor-1 are transported to the thymic mitochondria from the nucleus after TCR-mediated stimulation. Interestingly, leptomycin B also significantly inhibited the apoptotic activities of anti-CD3/CD28 or PMA/ionomycin in thymocytes but not that of dexamethasone (Fig. 1 C), suggesting that a significant portion of TCR-mediated apoptotic activities occurs outside the nucleus.
To determine if Nur77 family proteins were capable of binding to Bcl-2, coimmunoprecipitation experiments were performed using either Nur77 or Nor-1–specific antibodies, followed by Western blot analysis with the Bcl-2–specific antibodies. Because thymocytes contain very few mitochondria and also express very little Bcl-2, especially for nonstimulated thymocytes, we used lck-Bcl-2 transgenic mice (22). Freshly isolated thymocytes were stimulated for 4 h with PMA/ionomycin. PMA/ionomycin treatment was used for this experiment because it stimulates higher levels of Nur77 and Nor-1 compared with anti-CD3/CD28 treatment. The lysate obtained from PMA/ionomycin-treated thymocytes was immunoprecipitated with rabbit IgG control, anti-Nur77, or anti–Nor-1 antibodies. As shown in Fig. 1 D, Bcl-2 was specifically coimmunoprecipitated with the Nur77 or Nor-1 antibodies, and not with the control rabbit IgG, indicating the interaction of Nur77 and Nor-1 with Bcl-2 under physiological conditions. Leptomycin B treatment efficiently blocked the association of Nur77 and Nor-1 with Bcl-2 in PMA/ionomycin-stimulated thymocytes, confirming that the association occurred outside the nucleus. The observation of Nor-1 localization at the mitochondria, along with the protein's ability to bind to Bcl-2, is not surprising. It has been known that Nor-1 and Nur77 share redundancy in function and both exhibit >90% homology at the reported Bcl-2–interacting region, DC1 (14).
The Bcl-2 BH3 domain is exposed in anti-CD3/CD28 or PMA/ionomycin-stimulated thymocytes
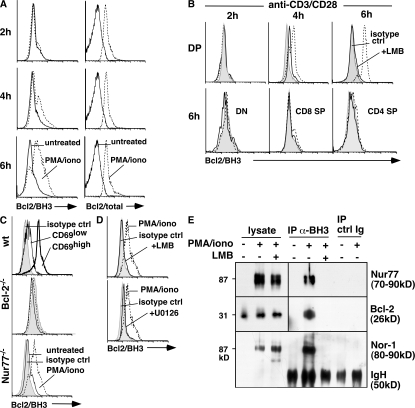
In several cancer cell lines, association of Nur77 with Bcl-2 in mitochondria leads to Bcl-2 conformation change and exposure of its BH3 domain (13–17, 23). We investigated whether this domain exposure also occurs in anti-CD3/CD28 or PMA/ionomycin-treated thymocytes. Thymocytes were cultured for various times with either PMA/ionomycin or anti-CD3/CD28, and then stained with antibodies specific for CD4 and CD8 and intracellularly with antibodies specific for the Bcl-2 BH3 domain. The Bcl-2 BH3 domain is usually buried within the folded Bcl-2 protein and undetectable by the Bcl-2/BH3-specific antibodies (14, 24). Exposure of this domain correlates with the proapoptotic activities of Bcl-2 (14). We measured Bcl-2 BH3 exposure in various thymocyte populations. As expected, unstimulated thymocytes did not stain with the anti–Bcl-2/BH3 antibodies (Fig. 2 A, solid lines). With 1.25 μg/ml PMA and 0.5 μM ionomycin or anti-CD3/CD28 stimulation, however, Bcl-2/BH3 staining was clearly observed in 4- and 6-h–stimulated double positive (DP) but not single positive (SP) or double negative (DN) thymocytes (Fig. 2, A and B). With a higher concentration of PMA/ionomycin (2.5 μg/ml PMA and 0.5 μM ionomycin), Bcl-2/BH3 staining was seen as early as 2 h after stimulation (not depicted). In contrast, intracellular staining with antibodies specific for total Bcl-2 showed the same levels in all times, consistent with the notion that the Bcl-2/BH3 staining is dependent on the conformation change and not on an increase in the total levels of Bcl-2. The Bcl-2/BH3 staining was specific to the CD69high DP population of cells that had just received TCR stimulation (Fig. 2 C). No staining was seen in PMA/ionomycin-stimulated Bcl-2−/− DP thymocytes, confirming the specificity of the antibodies (Fig. 2 C). Interestingly, Bcl-2/BH3 staining was still seen in Nur77−/− DP thymocytes (Fig. 2 C). This could be due to the redundant function of Nor-1, which we have shown is also capable of associating with Bcl-2 at the mitochondria.
Figure 2.
Bcl-2/BH3 exposure was observed in anti-CD3/CD28 and PMA/ionomycin-treated thymocytes. (A) Bcl-2/BH3 and Bcl-2 expression of DP cells from thymocytes cultured for 2, 4, and 6 h with 1.25 ng/ml PMA/0.5 μM ionomycin. Solid lines represent untreated thymocytes, and dotted lines represent treated thymocytes. (B) Bcl-2/BH3 exposure of DP cells from thymocytes cultured for 2, 4, and 6 h with 10 μg/ml anti-CD3/2 μg/ml anti-CD28 (plate-bound) and Bcl-2/BH3 exposure of DN and SP cells treated for 6 h. Here, the shaded area represents isotype control, dotted lines represent treated thymocytes, and solid lines represent treated thymocytes in the presence of leptomycin B. (C) Bcl-2/BH3 exposure of DP cells from Nur77−/− and Bcl-2−/− thymocytes cultured for 4 h with 2.5 ng/ml PMA/0.5 μM ionomycin. Shaded area represents isotype control, solid lines represent untreated thymocytes, and dotted lines represent treated thymocytes. Bcl-2/BH3 expression of CD69high and CD69low DP cells treated with 2.5 ng/ml PMA/0.5 μM ionomycin for 4 h is also shown. The shaded area represents isotype control, the solid line represents CD69low DP cells, and the thick line represents CD69high DP cells. (D) Pretreatment with 20 nM leptomycin B or 25 μM of the ERK5 inhibitor U0126 prevented exposure of the Bcl-2/BH3 domain in DP thymocytes stimulated for 4 h with PMA/ionomycin. Here, the shaded areas represent isotype control, dotted lines represent treated thymocytes, and solid lines represent PMA/ionomycin-treated thymocytes in the presence of leptomycin B or U0126. (E) PMA/ionomycin (PMA/iono) -stimulated thymocytes from lck-Bcl-2 mice were immunoprecipitated with anti-BH3 Bcl-2 antibodies or rabbit IgG, followed by blotting with antibodies specific for Nur77, Bcl-2, or Nor-1. Leptomycin (LMB) was added where indicated (+).
As the MEK5–ERK5 pathway regulates both Nur77 and Nor-1 transcription (25), we used a high concentration of U0126, which can inhibit the ERK5 pathway in all cells (26). Consistent with the MEK5→ERK5→Nur77/Nor-1→Bcl-2/BH3 signal transduction pathway, U0126 blocked the Bcl-2/BH3 conformational change of PMA/ionomycin-stimulated DP thymocytes (Fig. 2 D). We also showed that leptomycin B, which inhibits translocation of Nur77/Nor-1 to mitochondria, completely blocked the Bcl-2/BH3 domain conversion in anti-CD3/CD28 and PMA/ionomycin-stimulated DP thymocytes (Fig. 2, B and D).
To confirm the flow cytometric data further, we performed coimmunoprecipitation studies with the Bcl-2/BH3-specific antibodies. Thymocytes from lck-Bcl-2 transgenic mice were stimulated with PMA/ionomycin for 4 h in the absence or presence of leptomycin B. The immunoprecipitates were then probed for the presence of Nur77, Bcl-2, or Nor-1. Consistent with the flow data, we found Nur77 and Nor-1 solely in the Bcl-2/BH3 immunoprecipitates of stimulated thymocytes (Fig. 2 E). No Nur77 or Nor-1 could be detected in the Bcl-2/BH3 immunoprecipitates of leptomycin B–treated stimulated thymocytes. In addition, Bcl-2 could only be pulled down using these antibodies in apoptotic thymocytes. No Bcl-2 was seen in the immunoprecipitates when we used lysates from either unstimulated or leptomycin B/PMA/ionomycin-treated thymocytes (Fig. 2 E, lanes 4 and 6). Thus, exposure of the Bcl-2 BH3 domain is dependent on TCR-induced proteins (e.g., Nur77 and Nor-1) that are translocated out of the nucleus.
The Bcl-2 BH3 domain is exposed in apoptotic thymocytes from two TCR transgenic mouse models
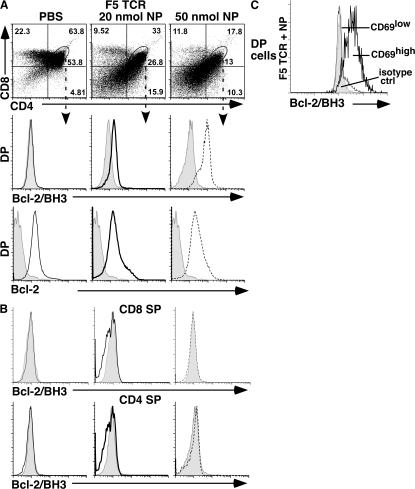
To see if Nur77/Nor-1–mediated Bcl-2 BH3 conversion also occurs during negative selection, we examined two TCR transgenic mouse models: F5 and HY (27, 28). In the F5 TCR transgenic model, the majority of thymocytes express a receptor containing Vβ4 and Vβ11 TCR segments specific for the influenza nucleocapsid protein (NP 366–374) in the context of H-2Db. Negative selection is induced by intraperitoneal injection of the NP peptide 366–374 (27). To analyze Bcl-2/BH3 domain conversion, F5 TCR transgenic mice were injected intraperitoneally with PBS alone or with 20 or 50 nmol of NP peptide. After 15h, thymocytes were analyzed by flow cytometry for Vβ11, CD4, CD8, and Bcl-2/BH3 expression. In PBS-injected F5 TCR transgenic mice, DP thymocytes that expressed Vβ11were negative for BH3 exposure (Fig. 3). An injection of 20 or 50 nmol of NP peptide resulted in a decrease in DP thymocytes, as reported previously (27). DP cells undergoing selection showed high expression of the BH3-exposed form of Bcl-2 (Fig. 3 A). Staining was specific for CD69high DP cells, as CD69low DP cells and CD4 or CD8 SP thymocytes were all negative for Bcl-2/BH3 signals (Fig. 3, B and C).
Figure 3.
Flow cytometric analysis of CD4, CD8, Bcl-2/BH3, and Bcl-2 staining of thymocytes from F5 TCR transgenic mice. (A) Bcl-2/BH3 and Bcl-2 expression of DP cells from F5 TCR transgenic mice 15 h after injection of PBS or 20 or 50 nmol of nucleocapsid peptide. Here, the shaded area represents isotype control, solid lines represent DP cells from PBS-injected mice, thick solid lines represent DP cells from mice injected with 20 nmol NP peptide, and dotted lines represent DP cells from mice injected with 50 nmol NP peptide. (B) Bcl-2/BH3 exposure was not observed in DN and SP cells from F5 TCR transgenic mice after injection of PBS or 20 or 50 nmol of nucleocapsid peptide. (C) Bcl-2/BH3 expression on CD69high and CD69low DP cells from a F5 TCR transgenic animal 10 h after injection with 50 nmol NP peptide. Here, the shaded area represents isotype control, the solid line represents CD69low DP cells, and the thick solid line represents CD69high DP cells. Five independent experiments have been done with similar results. All staining of F5 TCR transgenic mice is on Vβ11+ thymocytes.
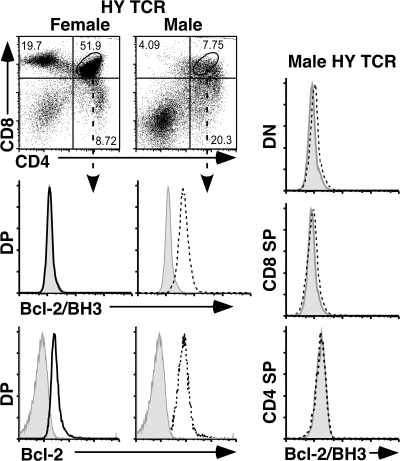
To see if the same results can be extended to another negative selection model, we analyzed the HY TCR transgenic mice. The HY TCR transgenic mice express a TCR that specifically recognizes the HY peptide derived from the Y chromosome in the context of the class I MHC molecule Db (28). During thymic development, thymocytes from male but not female transgenic mice undergo massive apoptosis due to negative selection of transgenic DP cells that recognize the male-specific antigens in the thymus (24). Staining of DP cells from female and male HY TCR transgenic mice showed the exposure of the Bcl-2/BH3 domain in male but not female DP thymocytes (Fig. 4). Although male transgenic animals expressed more Bcl-2 in all thymocyte populations, BH3 exposure was restricted to DP cells and was undetectable in DN, CD8 SP, or CD4 SP thymocytes (Fig. 4). Thus, only negatively selecting cells in vivo expose the Bcl-2 BH3 domain.
Figure 4.
Flow cytometric analysis of CD4, CD8, Bcl-2/BH3, and Bcl-2 staining of thymocytes from HY TCR transgenic mice. (A) Bcl-2/BH3 and Bcl-2 expression of DP cells from female and male HY TCR transgenic mice. The shaded area represents isotype control, solid lines represent female DP cells, and the dotted lines represent male DP cells. (B) Male HY TCR mice show no exposure of the BH3 domain of Bcl-2 in DN or SP cells. Five independent experiments have been done with similar results. All staining of HY TCR transgenic mice is on HY TCR+ thymocytes.
Negative selection is one of the most important processes in thymocyte development. It is critical that potentially dangerous thymocytes are eliminated. Thus, in addition to up-regulating and/or activating proapoptotic proteins, antiapoptotic proteins must be effectively inhibited. In various negative selection models, Nur77 and Bim have been consistently found to be key regulators of thymocyte cell death (29, 30). In contrast to Bim, the effector mechanism of Nur77-mediated apoptosis has yet to be clearly defined. The transcriptional activity of Nur77 was initially thought to be responsible for its apoptotic activity. This conclusion was based on characterization of transgenic mice expressing C-terminal truncation variants of Nur77 in the thymus (11), and evidence showing an N-terminal truncation of the transcriptional activation domain abolishes Nur77-dependent apoptosis (6, 7). However, C-terminally truncated Nur77 may also abolish Nur77's ability to export from the nucleus, as this region contains an export signal. Our data here are consistent with the notion that Nur77-induced cell death in thymocytes can be mediated by its translocation to the mitochondria where it binds to Bcl-2, converting the antiapoptotic molecule to a proapoptotic molecule during negative selection. This is consistent with a recent datum showing translocation of Nur77 to mitochondria in ionomycin-treated thymocytes (20). Although others have previously reported that Nur77 resides solely in the nucleus of immature DP thymocytes (18), the discrepancy is most likely due to the fact that thymocytes are extremely fragile and their organelles can be lysed easily with even a brief sonication or pestle homogenization. In this study, we used a method with minimal manipulations and simply lysed thymocytes in a hypotonic solution. This protocol allowed us to consistently detect Nur77 and Nor-1 in mitochondria in an activation- and nuclear export–dependent fashion.
Our findings suggest that negative selection may work through two effector molecules that converge at the mitochondria via their interaction with Bcl-2. While Bim antagonizes Bcl-2 (1), Nur77 converts Bcl-2 to a killer form. This hypothesis could explain the disparity between the effects of Bim deficiency and Bcl-2 overexpression on negative selection. Although negative selection is defective in Bim−/− mice, it is only inefficiently blocked by overexpression of Bcl-2 (5, 31, 32). Overexpression of Bcl-2 was sufficient to block most other Bim-mediated apoptosis (irradiation, glucocorticoid, and death by neglect) but was unable to efficiently inhibit negative selection to any significant degree. Induction of Nur77/Nor-1 in negatively selected thymocytes and their relocalization to mitochondria followed by association with Bcl-2 can lead Bcl-2 to promote instead of block apoptosis in negatively selecting thymocytes. Although expressed at low levels in DP thymocytes, Bcl-2 has been shown to be induced during negative selection (29). Thus, in the presence of Nur77, Bcl-2 is a proapoptotic protein. Together, Nur77 and Bim converge at the mitochondria to initiate apoptosis during negative selection. Additional experimentation, including that with transgenic mice, will be necessary to test and refine this model further.
MATERIALS AND METHODS
Antibodies and reagents.
Ionomycin and phorbol myristate acetate were obtained from EMD. The nuclear export inhibitor leptomycin B, the ERK5 inhibitor U0126, and dexamethasone were obtained from Sigma-Aldrich. The antibodies used in this study include anti–Bcl-2 BH3 (Abgent), anti–mouse Bcl-2 (eBioscience), and anti-CD4, CD8, CD69, HY TCR, cytochrome c, Nur77, and Vβ11 (all from BD Biosciences). The rabbit IgG antibody was purchased from Jackson ImmunoResearch Laboratories. Nor-1–specific rabbit antibodies were manufactured in-house (9). The HSP 60 and lamin B antibodies were purchased from Santa Cruz Biotechnology, Inc. and Abcam, respectively.
Thymocyte culture.
4 × 106/ml thymocytes were cultured in RPMI 1640, supplemented with 10% fetal calf serum, and stimulated with 1.25 or 2.5 ng/ml PMA plus 0.5 μM ionomycin or 10 μg/ml plate-bound anti-CD3 and 2 μg/ml anti-CD28. A 2-h pretreatment with 20 nM leptomycin B or 25 μM U0126 was used where indicated.
Intracellular staining for Bcl-2 BH3 and Bcl-2.
2 × 106 thymocytes were washed twice with PBS, stained for their surface markers, and permeabilized using a cytofix/cytoperm kit (BD Biosciences). In brief, thymocytes were resuspended in the cytofix/cytoperm solution (4% paraformaldehyde) on ice for 10 min, washed in the cytoperm/wash buffer, and incubated with 5% goat serum. To detect intracellular levels of the Bcl-2 BH3 domain and full-length Bcl-2, cells were stained with the anti-BH3 domain antibody (Abgent) and anti–mouse Bcl-2 (eBioscience) or anti–human Bcl-2 (Dako). Thymocytes were stained with the following antibodies from BD Biosciences: CD4, CD8, CD69, and TCR.
Transient transfection.
4 × 105 293T cells were seeded overnight in six-well plates. Cells were transfected with a total of 3.5 μg DNA using 6 μl FuGENE 6 (Roche) according to the manufacturer's protocol. 24 h after transfection, leptomycin B was added and luciferase activity was determined 6 h later using the Dual Luciferase Assay kit (Promega).
Cell fractionation.
Thymocytes from 4–6-wk-old mice were isolated and stimulated with 2.5ng PMA/0.5 μM ionomycin or 10 μg/ml anti-CD3 and 2 μg/ml anti-CD28 (plate-bound) for 2 and 6 h. In brief, after washing 6 × 107 thymocytes with PBS, cells were resuspended in 175 μl Solution A (10 mM Hepes-KOH, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.2 mM PMSF, 1 mM DTT, and 0.6% Nonidet P-40). They were incubated on ice for 45 min and spun down briefly. The pellet was resuspended in 20 μl Solution B (20 mM Hepes-KOH, pH 7.9, 400 mM NaCl 20% glycerol, 0.2 mM EDTA, 0.2 mM PMSF, 1 mM DTT, and 0.6% Nonidet P-40) and referred to as the nuclear fraction. The supernatant was transferred to a fresh tube and centrifuged at 700 g for 10 min. This was followed by a higher-speed centrifugation (25,000 g) for 25 min. The resulting supernatant contained the cytosolic fraction, and the pellet (mitochondria) was resuspended in 10 μl Solution A. Protein was quantified using BCA reagents (Bio-Rad Laboratories), and 30 μg of protein was loaded onto a 12% SDS-PAGE gel.
Immunoprecipitation.
Thymocytes (75 × 106 cells) were treated with 2.5 ng/ml PMA and 0.5 μM ionomycin for 4 h. Cells were then washed once in PBS and lysed in 50 μl buffer containing 1% Nonidet P-40 and the protease inhibitor cocktail (Sigma-Aldrich). 200 μl of cell lysates was incubated with 2 μg of immunoprecipitating antibody (Nur77, Nor-1, or control rabbit IgG) overnight at 4°C and then incubated with 1% BSA-cleared protein G–agarose beads (Thermo Fisher Scientific) for 2 h at 4°C. The protein–bead complex was then washed in lysis buffer and collected by centrifugation, and samples were boiled in loading buffer, run on 12% SDS-PAGE gels, probed with the antibody of interest, and processed for Western blotting. For Bcl-2/BH3 coimmunoprecipitation, we treated lck-Bcl-2 thymocytes with PMA/ionomycin for 4 h and used 2 μg of Bcl-2/BH3 antibodies.
Mice.
C57BL/6 strain was used as the wild-type mice in most of the biochemical experiments. When analyzing transgenic mice, the nontransgenic littermates were used as the wild-type controls. Genotyping of lck-Bcl-2 (22), TCR F5 (27), TCR HY (28), Bcl-2−/−, and Nur77−/− mice was done using PCR analysis. All experimental protocols involving animals were approved by the Animal Care and Use Committee.
Acknowledgments
We thank Sue Sohn and Gavin Lewis for mice, Hector Nolla for help with flow cytometry, and Stephanie Osborn and Allen Wensky for reading the manuscript.
This work is supported by a grant from the National Institutes of Health (CA66236).
The authors have no conflicting financial interests.
References
- 1.Strasser, A., L. O'Connor, and V.M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217–245. [DOI] [PubMed] [Google Scholar]
- 2.Mathis, D., and C. Benoist. 2007. A decade of AIRE. Nat. Rev. Immunol. 7:645–650. [DOI] [PubMed] [Google Scholar]
- 3.Starr, T.K., S.C. Jameson, and K.A. Hogquist. 2003. Positive and negative selection of T cells. Annu. Rev. Immunol. 21:139–176. [DOI] [PubMed] [Google Scholar]
- 4.Sohn, S.J., J. Thompson, and A. Winoto. 2007. Apoptosis during negative selection of autoreactive thymocytes. Curr. Opin. Immunol. 19:510–515. [DOI] [PubMed] [Google Scholar]
- 5.Bouillet, P., J.F. Purton, D.I. Godfrey, L.C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J.M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926. [DOI] [PubMed] [Google Scholar]
- 6.Zhou, T., J. Cheng, P. Yang, Z. Wang, C. Liu, X. Su, H. Bluethmann, and J.D. Mountz. 1996. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J. Exp. Med. 183:1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calnan, B., S. Szychowski, F.K.M. Chan, D. Cado, and A. Winoto. 1995. A role of the orphan steroid receptor Nur77 in apoptosis accompaying antigen-induced negative selection. Immunity. 3:273–282. [DOI] [PubMed] [Google Scholar]
- 8.Winoto, A., and D.R. Littman. 2002. Nuclear hormone receptors in T lymphocytes. Cell. 109:S57–S66. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, L.E., F.K. Chan, D. Cado, and A. Winoto. 1997. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T cell apoptosis. EMBO J. 16:1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajpal, A., Y.A. Cho, B. Yelent, P.H. Koza-Taylor, D. Li, E. Chen, M. Whang, C. Kang, T.G. Turi, and A. Winoto. 2003. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 22:6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuang, A.A., D. Cado, and A. Winoto. 1999. Nur77 transcription activity correlates with its in vivo apoptotic function. Eur. J. Immunol. 29:3722–3728. [DOI] [PubMed] [Google Scholar]
- 12.Newton, K., A.W. Harris, M.L. Bath, K.G.C. Smith, and A. Strasser. 1998. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 17:706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, K.W., L. Ma, X. Yan, B. Liu, X.K. Zhang, and P. Cohen. 2005. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J. Biol. Chem. 280:16942–16948. [DOI] [PubMed] [Google Scholar]
- 14.Lin, B., S.K. Kolluri, F. Lin, W. Liu, Y.H. Han, X. Cao, M.I. Dawson, J.C. Reed, and X.K. Zhang. 2004. Conversion of Bcl-2 from protection to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 116:527–540. [DOI] [PubMed] [Google Scholar]
- 15.Wilson, A.J., D. Arango, J.M. Mariadason, B.G. Heerdt, and L.H. Augenlicht. 2003. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 63:5401–5407. [PubMed] [Google Scholar]
- 16.Wu, Q., S. Liu, X.F. Ye, Z.W. Huang, and W.J. Su. 2002. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 23:1583–1592. [DOI] [PubMed] [Google Scholar]
- 17.Li, H., S.K. Kolluri, J. Gu, M.I. Dawson, X.H. Cao, P.D. Hobbs, B.Z. Lin, G.Q. Chen, L.S. Lu, F. Lin, et al. 2000. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 289:1159–1164. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham, N.R., S.C. Artim, C.M. Fornadel, M.C. Sellars, S.G. Edmonson, G. Scott, F. Albino, A. Mathur, and J.A. Punt. 2006. Immature CD4+CD8+ thymocytes and mature T cells regulate Nur77 distinctly in response to TCR stimulation. J. Immunol. 177:6660–6666. [DOI] [PubMed] [Google Scholar]
- 19.Bunin, A., F.W. Khwaja, and G.J. Kersh. 2005. Regulation of Bim by TCR signals in CD4/CD8 double-positive thymocytes. J. Immunol. 175:1532–1539. [DOI] [PubMed] [Google Scholar]
- 20.Stasik, I., A. Rapak, W. Kalas, E. Ziolo, and L. Strzadala. 2007. Ionomycin-induced apoptosis of thymocytes is independent of Nur77 NBRE or NurRE binding, but is accompanied by Nur77 mitochondrial targeting. Biochim. Biophys. Acta. 1773:1483–1490. [DOI] [PubMed] [Google Scholar]
- 21.Crews, C.M., and U. Splittgerber. 1999. Chemical genetics: exploring and controlling cellular processes with chemical probes. Trends Biochem. Sci. 24:317–320. [DOI] [PubMed] [Google Scholar]
- 22.Linette, G.P., M.J. Grusby, S.M. Hedrick, T.H. Hansen, L.H. Glimcher, and S.J. Korsmeyer. 1994. Bcl-2 is upregulated at the CD4+ CD8+ stages during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1:197–205. [DOI] [PubMed] [Google Scholar]
- 23.Kolluri, S.K., N. Bruey-Sedano, X. Cao, B. Lin, F. Lin, Y.H. Han, M.I. Dawson, and X.K. Zhang. 2003. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell. Biol. 23:8651–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng, X., F. Gao, T. Flagg, J. Anderson, and W.S. May. 2006. Bcl2's flexible loop domain regulates p53 binding and survival. Mol. Cell. Biol. 26:4421–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasler, H.G., J. Victoria, O. Duramad, and A. Winoto. 2000. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol. Cell. Biol. 20:8382–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mody, N., J. Leitch, C. Armstrong, J. Dixon, and P. Cohen. 2001. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 502:21–24. [DOI] [PubMed] [Google Scholar]
- 27.Mamalaki, C., J. Elliott, T. Norton, N. Yannoutsos, A.R. Townsend, P. Chandler, E. Simpson, and D. Kioussis. 1993. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev. Immunol. 3:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teh, H.S., H. Kishi, B. Scott, P. Borgulya, H. von Boehmer, and P. Kisielow. 1990. Early deletion and late positive selection of T cells expressing a male-specific receptor in T-cell receptor transgenic mice. Dev. Immunol. 1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz, I., L.K. Clayton, and E.L. Reinherz. 2003. Gene expression analysis of thymocyte selection in vivo. Int. Immunol. 15:1237–1248. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin, T.A., and K.A. Hogquist. 2007. Transcriptional analysis of clonal deletion in vivo. J. Immunol. 179:837–844. [DOI] [PubMed] [Google Scholar]
- 31.Sentman, C.L., J.R. Shutter, D. Hockenbery, O. Kanagawa, and S.J. Korsmeyer. 1991. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 67:879–888. [DOI] [PubMed] [Google Scholar]
- 32.Strasser, A., A.W. Harris, and S. Cory. 1991. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 67:889–899. [DOI] [PubMed] [Google Scholar]