Abstract
T helper type 17 (Th17) cells play an important pathogenic function in autoimmune diseases; their regulation, however, is not well understood. We show that the expression of a tumor necrosis factor receptor family member, death receptor 3 (DR3; also known as TNFRSF25), is selectively elevated in Th17 cells, and that TL1A, its cognate ligand, can promote the proliferation of effector Th17 cells. To further investigate the role of the TL1A–DR3 pathway in Th17 regulation, we generated a TL1A-deficient mouse and found that TL1A−/− dendritic cells exhibited a reduced capacity in supporting Th17 differentiation and proliferation. Consistent with these data, TL1A−/− animals displayed decreased clinical severity in experimental autoimmune encephalomyelitis (EAE). Finally, we demonstrated that during EAE disease progression, TL1A was required for the optimal differentiation as well as effector function of Th17 cells. These observations thus establish an important role of the TL1A–DR3 pathway in promoting Th17 cell function and Th17-mediated autoimmune disease.
After activation, CD4+ Th cells differentiate into distinct effector subsets that are characterized by their unique cytokine expression and immunoregulatory function (1, 2). A novel Th subset—termed ThIL-17, Th17, or inflammatory Th—has been recently identified as a distinct Th lineage that mediates tissue inflammation (3–5). Th17 differentiation is initiated by TGF-β and IL-6 (4, 6, 7), and reinforced by IL-23 (8) and IL-21 (9–11), in which STAT3 and two orphan nuclear receptors, RORα and RORγ, mediate the lineage specification (8, 12–14). Despite rapid progress in understanding the molecular programs that govern Th17 differentiation, it is not clear how their proliferation, and ultimately function, are regulated in the context of immune responses and diseases.
Th cell activation and differentiation requires not only the recognition of the antigen–MHC class II complex by the cognate TCR but also co-stimulatory signals. Previous experiments have indicated differential co-stimulatory regulation of Th1 and Th2 cell function (15). Over the past decade, our knowledge of how different ligand–receptor pairing interactions contribute to T cell co-stimulation has been steadily expanded. Most of these pairs belong to either the B7-type molecules that bind CD28-like Ig superfamily receptors, or the TNF superfamily ligands that engage their receptor counterparts in the TNFR family (16). Although we have previously shown essential roles of CD28 and inducible co-stimulator in Th17 differentiation (17, 18), the functional contribution of TNF and TNFR family members in regulating Th17 cell function is poorly understood.
Within the TNF family, CD40L, OX-40L, LIGHT, CD27L (CD70), and 4-1BBL have well-established functions in T cell activation and differentiation (16, 19). Recent evidence suggests that another TNF family member, TL1A (Tnfsf15), also plays a role in T cell regulation. TL1A was first identified as a protein expressed on human endothelial cells and up-regulated in response to TNF-α and IL-1α (20), although an earlier study only identified an irregularly spliced transcript. Subsequently, full-length TL1A was cloned, and expression of TL1A by human tissue macrophages, lamina propria T cells and plasma cells, and FcγR-activated peripheral blood (PB) monocytes and monocyte-derived DCs was demonstrated (21–24). TL1A binds death receptor 3 (DR3; also called TNFRSF25), a death domain–containing TNFR family member that signals via NF-κB (25, 26). DR3 expression is significantly increased upon T cell activation and was initially thought to be restricted to lymphocytes (27). However, recent data have expanded the number of cell types that express DR3 to also include NK cells, macrophages, and endothelial cells (22, 28, 29). Although DR3-deficient mice exhibit a partial impairment in the negative selection of thymocytes (30), the impact of DR3 deficiency on immune function has not been further explored. TL1A also binds to DcR3 (20), a decoy receptor for TL1A, LIGHT, and FasL that is present in humans but not mice (31), suggesting additional complexity in the role of this pathway in humans.
Several lines of evidence point to a role for TL1A in modulating T cell activation. Under conditions of suboptimal anti-CD3/CD28 stimulation, TL1A increases IL-2–driven proliferation and IFN-γ and GM-CSF production by human PB T cells, and in vivo administration of recombinant TL1A enhances mouse graft-versus-host reactions (20). Thus, despite the presence of a death domain in DR3, binding of TL1A to DR3 on T cells provides a co-stimulatory signal (20). TL1A also synergizes with IL-12 and IL-18 in stimulating TCR-independent secretion of IFN-γ by human PB CD4+ memory T cells (29, 32). The ability of TL1A to co-stimulate IFN-γ secretion and the association of TL1A expression with areas of active inflammation in Crohn's disease have led to the hypothesis that TL1A acts as a Th1-polarizing cytokine (21, 29, 32). However, it was more recently shown that TL1A acted preferentially to enhance TCR-stimulated proliferation of memory CD4+ T cells (33), which was associated with an increased capacity of memory T cells to express IFN-γ.
In the current study, we found that full-length DR3 expression is selectively elevated in Th17 cells and mediates their development and proliferation. Deficiency in TL1A in DCs substantially reduced their ability in supporting Th17 differentiation and proliferation. Consistently, TL1A KO animals exhibited decreased Th17 generation in vivo and reduced disease severity upon induction of experimental autoimmune encephalomyelitis (EAE). Additionally, the absence of TL1A in recipient mice also resulted in reduced severity of EAE disease induced by adoptive transfer of autoreactive Th17 cells. Collectively, these observations demonstrate that DR3–TL1A interaction is important for the optimal function of Th17 cells and contributes to Th17-mediated autoimmune disease.
RESULTS
Th17 cells highly express DR3
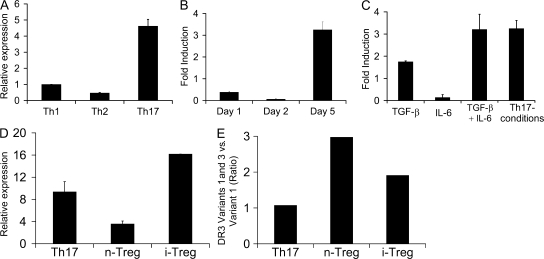
Although considerable progress has been made in identifying the various cytokines and transcriptional factors critical for Th17 differentiation, molecular pathways selectively regulating the expansion and function of this important Th subset remain unclear. To address this, we analyzed gene expression profiles by microarray analysis of Th1, Th2, and Th17 effector cells and discovered that expression of DR3 (TNFRSF25), which binds to TNF family member TL1A, was significantly enhanced in Th17 cells when compared with the other two Th subsets (unpublished data). Because TNF family members regulate cell proliferation and survival, this result suggests the possibility that DR3–TL1A interaction may be involved in the regulation of Th17 cells. To further confirm this observation, CD4+ T cells from OT-II TCR transgenic mice were differentiated under Th1, Th2, or Th17 conditions, and mRNA was prepared on day 5 after restimulation and analyzed for DR3 expression by real-time PCR analysis. As shown in Fig. 1 A, total DR3 expression was greatly increased in the Th17 subset compared with Th1 and Th2 cells, using a primer pair that allows detection of all DR3 isoforms. Furthermore, DR3 expression was not detected during the first 2 d of differentiation but was up-regulated by day 5 (Fig. 1 B), suggesting that DR3 up-regulation occurs at a later stage of Th17 differentiation.
Figure 1.
DR3 expression is greatly enhanced in Th17 cells. Naive OT-II cells were differentiated and DR3 expression was analyzed by real-time PCR analysis. DR3 expression was determined relative to naive CD4+ T cell expression levels and normalized to GAPDH levels in all samples. (A) Comparison of DR3 expression on CD4+ cells differentiated under Th1, Th2, and Th17 conditions. (B) Time-course analysis of DR3 expression in CD4+ T cells differentiating under Th17 conditions. (C) Analysis of DR3 expression in cells differentiated under different combinations of cytokines. Primers used in A–C detect all DR3 isoforms. (D) Analysis of the full-length transmembrane-containing DR3 isoform (variant 1) in Th17 and T reg cells. (E) The ratio of total transmembrane DR3 (variants 1 and 3 combined) and full-length transmembrane DR3 (variant 1) was measured in Th17 and T reg cells. Error bars represent SD.
Previous studies have shown that TGF-β and IL-6 play essential roles in the differentiation of Th17 cells (4, 6, 7). To identify the cytokine pathways involved in DR3 up-regulation, naive OT-II T cells were activated under different cytokine or Th17 conditions, mRNA was prepared from these cells on day 5 after activation, and total DR3 expression was analyzed by real-time PCR analysis. As shown in Fig. 1 C, TGF-β alone induced DR3 expression, whereas IL-6 was not sufficient for DR3 induction. However, induction of DR3 is further enhanced in the presence of both TGF-β and IL-6 (Fig. 1 C). Additionally, the blocking of IFN-γ and IL-4 in the presence of TGF-β and IL-6 (Th17 conditions) did not further enhance DR3 expression (Fig. 1 C), suggesting that TGF-β and IL-6 alone are sufficient to induce DR3 expression.
Among the three known DR3 isoforms in the mouse, variant 1 is the full-length DR3 that is capable of both ligand binding and intracellular signaling. Variant 2 is a soluble DR3 isoform that likely functions as a decoy receptor. Although variant 3 does contain both transmembrane and cytoplasmic domains, it lacks the fourth cysteine-rich domain in its extracellular region, and its capacity for ligand binding has yet to be determined (30). Therefore, it is critical to determine which isoforms are induced upon Th17 differentiation. Using isoform-specific primers, we found that the full-length, functioning variant 1 was highly up-regulated in Th17 cells (Fig. 1, D and E).
Because TGF-β also induces Foxp3 expression in activated T cells, we examined whether T reg cells expressed DR3. Interestingly, both naturally occurring T reg (n–T reg) and TGF-β–induced T reg (i–T reg) cells up-regulated DR3 expression (Fig. 1, D and E). However, the full-length variant 1 represents a much higher proportion of the total DR3 isoforms expressed in Th17 cells as compared with n–T reg or i–T reg cells, supporting the preferential involvement of TL1A–DR3 interaction in regulating Th17 cell function.
TL1A stimulation regulates Th17 development and proliferation
To establish the potential biological relevance of DR3 expression on Th17 cells, we first tested the effect of recombinant Fc-TL1A protein on Th17 differentiation, proliferation, and cytokine expression. Naive CD4+ T cells were activated with plate-bound anti-CD3/CD28 in the presence or absence of Fc-TL1A and in the presence of TGFβ+IL-6 or Th17 differentiation conditions. In comparison to cells treated with a control Fc-containing protein, Fc-TL1A enhanced IL-2 secretion (Fig. 2 A). It has been recently shown that IL-2 signaling inhibits Th17 differentiation in a STAT5-dependent manner (34). Consistently, percentages of IL-17–producing cells were decreased by 40% in T cells differentiated in the presence of Fc-TL1A (Fig. 2 B). However, upon treatment with an anti–IL-2 antibody, Fc-TL1A enhanced the differentiation of Th17 cells by 35% under TGFβ+IL-6 conditions and 20% under Th17 conditions (Fig. 2 B). These results suggest that in the absence of IL-2 signaling, TL1A–DR3 interaction could function to enhance the differentiation of Th17 cells from naive T cells.
Figure 2.
TL1A enhances the differentiation. (A) The addition of TL1A during naive T cell activation increases IL-2 production. Naive CD4+ T cells were stimulated with plate-bound anti-CD3/CD28 antibody under the indicated conditions. Culture supernatants were analyzed for IL-2 24 h later by ELISA. Data represent the mean of triplicate cultures, and error bars represent SD. Results are representative of three independent experiments with similar results. (B) TL1A regulates Th17 differentiation. Naive CD4+ T cells were stimulated by plate-bound anti-CD3/CD28, in the presence of control Ig or 1 μg/ml Fc-TL1A, and under Th17-differentiating conditions in the absence or presence of anti–IL-2 antibodies. The percentages of IL-17– and IFN-γ–producing cells were measured by intracellular cytokine staining. Data are a representative of three independent experiments with similar results.
Because TL1A has been shown to enhance proliferation and IFN-γ secretion in CD4+ memory T cells (21, 33), we examined the effect of TL1A on in vitro–differentiated Th1 and Th17 cells. Th1 and Th17 cells were incubated with a titrating dose of anti-CD3 and in the presence or absence of TL1A, and proliferation was measured by [3H]thymidine incorporation. Culture supernatants were analyzed for IFN-γ or IL-17 production by ELISA. As shown in Fig. 3 A, TL1A did not affect the proliferation or IFN-γ production by Th1 effector cells. However, addition of TL1A greatly enhanced the proliferation of Th17 cells, especially at lower concentrations of anti-CD3 stimulation (Fig. 3 B). More significantly, in the absence of TCR stimulation, TL1A treatment did not enhance the proliferation of Th1 cells, whereas the proliferation of Th17 cells was greatly increased (Fig. 3, A and B). Despite increased proliferation upon TL1A stimulation, IL-17 production was not increased under these conditions in the culture supernatants (Fig. 3, A and B, bottom) or by intracellular staining (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071364/DC1), suggesting that the primary function of the TL1A–DR3 pathway in Th17 cells may be to enhance cellular proliferation.
Figure 3.
TL1A enhances the proliferation of Th17 effector cells. (A and B) In vitro–differentiated Th1 and Th17 cells were restimulated on day 5 after activation with the indicated doses of anti-CD3 with or without 1 μg/ml of exogenous Fc-TL1A or control human Ig. Proliferation of Th1 cells (A) and Th17 cells (B) was determined by [3H]thymidine incorporation after 24 h of stimulation. In a parallel experiment, culture supernatant was analyzed for IFN-γ (A) and IL-17 (B) expression by ELISA. Data are representative of three independent experiments. (C) In vitro–differentiated Th1 and Th17 cells were restimulated with the indicated doses of exogenous Fc-TL1A. (left) The proliferation of Th1 and Th17 cells was determined by [3H]thymidine incorporation after 24 h of stimulation. (right) The proliferation of Th1 and Th17 cells in the absence or presence of 1 μg/ml Fc-TL1A was represented. Results are representative of three independent experiments. (D) TL1A specifically enhances Th17 cell proliferation. In vitro–differentiated Th1 and Th17 cells, and n–T reg and i–T reg cells were stimulated with control Ig or 1 μg/ml Fc-TL1A for 24 h. Proliferation was determined by [3H]thymidine incorporation during the last 8 h of stimulation. Proliferation in the presence of positive controls was as follows: anti-CD3 for Th1 and Th17 cells (14,000 and 30,000 CPM, respectively), and IL-2 for n–T reg and i–T reg cells (98,000 and 25,000 CPM, respectively). Data are representative of two independent experiments with similar results. Error bars represent SD.
Because TL1A alone induced Th17 cell proliferation, we further analyzed the proliferation of Th17 cells at different concentrations of TL1A. As shown in Fig. 3 C, proliferation of Th17 cells was greatly enhanced with increased concentration of Fc-TL1A, whereas the proliferation of Th1 cells remained unchanged even at the highest concentration (1 μg/ml) of TL1A. Collectively, these results revealed a previously unknown and specific regulation of Th17 cells by the TL1A–DR3 interaction.
Because DR3 expression was observed in both n–T reg and i–T reg cells (Fig. 1, D and E), we also tested whether Fc-TL1A could influence their proliferation. As shown in Fig. 3 D, Fc-TL1A specifically induced the proliferation of Th17 but not n–T reg, i–T reg, or Th1 cells, suggesting that the relative expression levels of different DR3 isoforms may determine the TL1A responsiveness of a given cell type.
Generation of TL1A-deficient mice
To further understand the function of TL1A–DR3 interaction in regulating Th17 cells in vivo, a TL1A KO mouse was generated by replacing exon 4 of the TL1A (Tnfsf15) locus with a neomycin cassette (Fig. 4 A). Exon 4 encodes amino acids 103–252 of TL1A, encompassing the TNF homology domain that is essential for TL1A function. Lack of TL1A expression was confirmed by RT-PCR of kidney tissues (Fig. 4 B), which contain high levels of TL1A mRNA (20, 35). TL1A KO mice appeared phenotypically normal, and similar immune cell numbers and proportions were observed in the lymph nodes, spleen, thymus, and bone marrow (Fig. 4 C; and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071364/DC1). These results suggest that TL1A is likely not critical for the development of lymphocytes and peripheral lymphocytes.
Figure 4.
TL1A targeting strategy and lymphocyte characterization from TL1A KO mice. (A) Restriction map of the mouse Tnfsf15 locus and the thymidine kinase (TK)– and neomycin (neo)-containing targeting construct derived from it. Restriction enzyme sites indicated are as follows: Ba, BamHI; Bg, BglII; E, EcoRI; and X, XbaI. Exons are represented as black boxes, and arrows indicate the direction of transcription. (B) RT-PCR analysis of TL1A mRNA in TL1A KO and WT kidneys. (C) Surface phenotype of naive WT (white bars) and TL1A KO (gray bars) lymph node cells. (left) Percentages of total lymphocytes positive for the indicated markers are plotted. (right) Percentages of CD4+ or CD8+ T cells positive for the indicated marker combinations are plotted. Data represent means of eight animals per group, and error bars are SEM.
TL1A-deficient DCs exhibit reduced potency to induce Th17 differentiation and proliferation
Because Fc-TL1A enhanced the differentiation and proliferation of Th17 cells, we tested the impact of TL1A deficiency in APCs on the differentiation of naive T cells into Th17 cells. LPS-stimulated bone marrow–derived DCs (BMDCs) from WT and TL1A KO mice were pulsed with OT-II peptide and used for activating naive OT-II CD4 T cells under Th1 and Th17 differentiation conditions. The development of effector cells was monitored by intracellular cytokine staining for IL-17 and IFN-γ on day 6 after stimulation. As shown in Fig. 5, differentiation of Th1 cells was not affected because of TL1A deficiency; however, when OT-II CD4 T cells were differentiated with KO BMDCs, we observed a modest, yet reproducible, decrease in the percentage of Th17 cells generated (Fig. 5). These results are in agreement with our earlier in vitro results on the differentiation of naive CD4+ T cells with recombinant Fc-TL1A (Fig. 2), which indicate that TL1A expressed on APCs may promote the differentiation of Th17 cells. The effect on Th17 cells appears quite specific, as exogenous TL1A did not have any effect on T reg cell differentiation induced by TGF-β (Fig. S3 A, available at http://www.jem.org/cgi/content/full/jem.20071364/DC1), even though these cells also up-regulate DR3 expression. Conversely, although OT-II CD4 T cells activated by TL1A KO BMDCs exhibited reduced Th17 differentiation based on intracellular IL-17A staining, Foxp3 expression was not reciprocally increased (Fig. S3 B).
Figure 5.
Decreased differentiation of Th17 cells in response to stimulation by TL1A KO DCs. Naive OT-II CD4+ T cells were stimulated with either WT or TL1A KO BMDCs under polarized Th1- and Th17-differentiating conditions. Cells were harvested on day 6 and restimulated with PMA plus ionomycin, followed by intracellular cytokine staining for IFN-γ and IL-17. Dot plots represent the IFN-γ and IL-17 expression among CD4+ cells (percentages are shown). Data are representative of three independent experiments.
Because Fc-TL1A promoted Th17 cell proliferation, we also assessed the impact of TL1A deficiency in APCs on the expansion of differentiated Th17 cells. In vitro–differentiated Th1 and Th17 cells were either left alone or incubated with WT or TL1A-deficient BMDCs, and their proliferation was measured by [3H]thymidine incorporation at 24 h. As shown in Fig. 6 A, the proliferation of Th1 cells was not affected by the presence of either WT or TL1A KO BMDCs. In contrast, in the absence of TCR signaling, WT BMDCs greatly enhanced the proliferation of Th17 cells, and this enhancement effect was significantly diminished when TL1A-deficient BMDCs were used (Fig. 6 A). Importantly, the addition of exogenous Fc-TL1A to the latter culture condition restored the enhancement effect (Fig. 6 B), clearly demonstrating that the ability of WT BMDCs to promote antigen-independent proliferation of Th17 cells requires TL1A expression. Even in the presence of OVA peptide, the proliferation of Th1 cells stimulated by WT and TL1A-deficient BMDCs was similar, whereas the proliferation of Th17 cells was once again reduced using TL1A-deficient BMDCs (Fig. 6 C). Interestingly, the reduced proliferation of Th17 cells in the absence of TL1A was observed only when lower concentrations of OVA peptide were used (Fig. 6 C). These data are consistent with our earlier results that Fc-TL1A enhanced the proliferation of Th17 cells only under conditions of suboptimal TCR stimulation (Fig. 3 B).
Figure 6.
Reduced proliferation of Th17 cells upon restimulation by TL1A KO BMDCs. (A) In vitro–differentiated Th1 and Th17 cells were left unstimulated or restimulated on day 5 with WT or TL1A KO BMDCs for 24 h. Proliferation was measured by [3H]thymidine incorporation during the last 8 h of stimulation. (B) In vitro–differentiated Th17 cells were stimulated with WT or TL1A KO BMDCs with and without Fc-TL1A for 24 h. Proliferation was measured by [3H]thymidine incorporation during the last 8 h of stimulation. (C) In vitro–differentiated Th1 and Th17 cells were stimulated with WT or TL1A KO BMDCs pulsed with a titrating dose of OT-II peptide. The proliferation of Th1 and Th17 cells was determined at 24 h of stimulation by [3H]thymidine incorporation. The horizontal dashed line represents the baseline proliferation of Th1 or Th17 cells alone. Data are representative of three independent experiments. Error bars represent SD.
TL1A deficiency results in decreased severity of EAE disease
To determine the biological significance of the TL1A–DR3 pathway in vivo, we used a Th17-mediated autoimmune disease model, myelin oligodendrocyte glycoprotein (MOG)-induced EAE (18, 36). In four independent experiments, TL1A KO and WT animals exhibited similar timing of disease onset, but TL1A KO mice consistently showed a significantly reduced disease severity, as manifested by both a lower maximal disease score and lower cumulative scores throughout the course of the disease (Fig. 7 A and Table I). Although no statistical significance was achieved, a consistent trend toward lower disease incidence was observed in TL1A KO mice (unpublished data).
Figure 7.
TL1A deficiency protects mice from EAE. (A) EAE clinical course in TL1A KO animals. EAE disease course in TL1A KO and WT mice. Mice were immunized with MOG35-55 and pertussis toxin, as described in Materials and methods. Values represent the mean clinical score for each group. The disease course is representative of four independent experiments (n = 7–10 animals per group). (B) Histological analysis. Spinal cord analysis at day 27 after immunization for TL1A KO, WT, and unimmunized WT (WT-untr) animals. Representative images are shown. Spinal cord cross sections were paraffin embedded and stained with the indicated histochemical stains or the anti-CD3 antibody. Bar, 500 μm. (C) Higher magnification of the Luxol fast blue staining is shown. Bar, 200 μm. (D) Quantification of the number of CD3-positive cells per cross section in TL1A KO and WT animals on day 27 after immunization. Images were analyzed as described in Materials and methods. Results are shown for seven individual animals in each group and one unimmunized WT animal (untr). Symbols represent the number of CD3-positive cells per cross section; horizontal bars indicate means. (E and F) Kinetic analysis of CNS-infiltrating T cells. CNS-infiltrating leukocytes were isolated as described in Materials and methods. (E) Representative FACS plots of CD45 and CD4 staining (gated on CD45+ cells) are shown for the WT CNS untreated (left) and at day 17 after immunization (right), with equal numbers of events plotted (percentages are shown). (F) Percentage of CD45+CD4+ cells in the CNS of WT (white bars) and TL1A KO (gray bars) mice at the indicated days after immunization. Cells are gated as in Fig. 7 E. Five to seven animals per group are shown; the black bar represents untreated WT mice. The reduction seen in the KO mice is statistically significant for TL1A KO over the course of the experiment (P = 0.021, as determined by two-way analysis of variance). Error bars represent SD. (G) A reduced number of cytokine-expressing CD4+ T cells in TL1AKO CNS after MOG-EAE induction. CNS cell isolates of WT and TL1A KO mice (pools from six animals for each group) were analyzed on day 17 after disease induction. The absolute numbers of cytokine-positive CD4+ cells were determined by intracellular cytokine staining. Results are representative of two independent experiments.
Table I.
Statistical parameters for MOG-EAE
| WT | KO | |
|---|---|---|
| Maximal score | 3.7 | 2.6 (P = 0.014)a |
| Cumulative score | 56.9 | 37.6 (P = 0.002) |
| Day of onsetb | 12 | 14.2 |
| Incidence | 9/9 (100%) | 7/9 (78%) |
Values are for the experiment in Fig. 7.
p-values are shown for TL1A KO versus WT groups based on the Mann-Whitney nonparametric test.
Day of onset was calculated for diseased animals only.
Histological examination of the spinal cords was performed to determine whether the difference in clinical symptoms observed between TL1A KO and WT mice correlated with the degree of inflammatory infiltration and demyelination in the central nervous system (CNS). TL1A KO mice exhibited fewer mononuclear infiltrates and demyelination foci than WT control animals at day 27 after immunization (Fig. 7, B and C). These results indicate that the reduced clinical scores observed in the KO animals were likely caused by decreased inflammation and tissue damage in the CNS. Because our in vitro studies suggest that the TL1A–DR3 pathway may be involved in the generation and/or function of pathogenic MOG-specific T cells during the course of EAE, the levels of T cell infiltration were further quantified by image analysis of anti-CD3 staining. As shown in Fig. 7 D, TL1A KO animals had fewer CD3+ cells per spinal cord cross section than WT counterparts.
Several distinct factors could potentially influence the degree of CNS T cell infiltration in the context of EAE. To rule out the possibility that the lesser T cell infiltration seen in the spinal cords of TL1A KO animals was caused by a reduced ability of their T cells to survive in the CNS, Tdt-mediated dUTP-biotin nick-end labeling and anti-activated caspase-3 staining were performed to assess the extent of apoptosis in TL1A KO and WT CNS. The level of apoptosis in the WT spinal cord was low on days 21 or 27 after immunization. Furthermore, no increase in apoptotic cells was observed in the KO spinal cords (unpublished data), suggesting that T cell apoptosis is unlikely to be a contributing factor underlying the reduced infiltrating T cell numbers observed in the CNS of TL1A-deficient animals with EAE.
To determine whether the reduction in CNS T cell frequency seen on day 27 was caused by different temporal kinetics between WT and KO animals, levels of CD4+ T cells in the CNS (spinal cord and cerebellum) were assessed at multiple time points by flow cytometry. CD4+ T cells start accumulating in the CNS of WT mice 1 or 2 d before the onset of clinical symptoms, and their levels peak at days 5–7 after disease onset (unpublished data) (37, 38). We found that the percentage of CD45+CD4+ cells in the CNS of TL1A KO mice was consistently reduced as compared with WT animals over the course of the study (Fig. 7, E and F). Absolute numbers of CD45+CD4+ cells were also examined and showed a similar trend (unpublished data). These observations show that the effect of TL1A deficiency in reducing CD4 T cell levels in the CNS is evident early in and maintained throughout the disease course. In addition, there were dramatic reductions in the total numbers of recovered CD4+ T cells expressing proinflammatory cytokines such as IL-17, IFN-γ, IL-6, and TNF-α in day 17 CNS cell isolates (Fig. 7 G).
Function of TL1A in the generation, expansion, and effector function of autoreactive T cells
By signaling through its receptor DR3, TL1A could potentially contribute to the pathogenesis of autoimmune diseases at multiple levels. We first tested whether TL1A deficiency affects T cell priming in EAE. WT and TL1A KO mice were immunized with MOG peptide in CFA, and lymph node and spleen cells were analyzed for IL-17 and IFN-γ expression on day 10. As shown in Fig. 8, the percentages of IL-17– and IFN-γ–expressing populations were significantly decreased in TL1A KO mice. More impressively, a population of CD4+ T cells expressing both IL-17 and IFN-γ was observed in WT mice, and was most consistently and dramatically reduced in the KO animals. Similar results were obtained with MOG restimulation (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20071364/DC1). The frequency of Foxp3+ CD4+ cells was also measured after MOG restimulation of lymph node and spleen cells, with no significant difference found between WT and TL1A KO mice (1.74 vs. 1.92% FoxP3+ CD4+ cells, respectively; unpublished data). These results are consistent with our in vitro differentiation results (Fig. 2 A and Fig. 5) and suggest that TL1A deficiency impairs the priming of MOG-specific CD4+ T cells, which may contribute to decreased EAE severity in TL1A KO mice.
Figure 8.
Reduced T cell Priming in MOG-immunized TL1A KO mice. WT and TL1A KO mice were primed with MOG-CFA, and draining lymph nodes and spleen were harvested on day 10. Single-cell suspensions were stimulated with PMA plus ionomycin with Golgi Plug for 4 h, stained, and analyzed by flow cytometry. Percentages of IL-17– and/or IFN-γ–secreting cells among CD4+ cells are shown. Values shown are means ± SEM (n = 12 mice per group). Results are compiled from two experiments with similar results. Differences between WT and KO mice are significant for each of the cytokine-expressing populations (IL-17+, P = 0.004; IFN-γ+, P = 0.002; IL-17+IFN-γ−, P = 0.019; IFN-γ+IL-17−, P = 0.011; and IL-17+IFN-γ+, P = 0.005, as determined by the Student's t test).
Because TL1A promoted the proliferation and differentiation of Th17 cells in vitro, we sought to test whether TL1A can also contribute to disease progression by expanding autoreactive T cells and/or potentiating their pathogenic effects in the context of EAE using an adoptive transfer model. Splenocytes and draining lymph node cells from MOG-immunized mice were expanded in vitro with MOG peptide and 5 ng/ml of suboptimal IL-23 in the presence of control Ig or Fc-TL1A. On day 10 after expansion, purified CD4+ T cells were counted, and 10 million cells were adoptively transferred into recipient mice to induce EAE. Our results showed that not only were more cells recovered with Fc-TL1A treatment, but more importantly, when transferred with the same number of CD4+ T cells, mice receiving Fc-TL1A–expanded cells developed EAE disease with 100% disease incidence and a peak clinical score of 2 (Fig. S5 A, available at http://www.jem.org/cgi/content/full/jem.20071364/DC1), whereas mice receiving control Ig–expanded cells did not show any disease symptoms. Furthermore, when CD4+ T cells from MOG-immunized mice were expanded without IL-23, only a portion of the recipients of CD4+ T cells cultured in the presence of MOG peptide and Fc-TL1A showed minimal signs of EAE disease development, with a mean clinical score of 1.33 in affected mice with 60% disease incidence, whereas recipients of CD4+ T cells cultured with MOG peptide and control Ig did not show any disease symptoms (Fig. S5 B). Therefore, these results indicate that in addition to participating in the efficient priming of autoreactive T cells, TL1A may further contribute to T cell–mediated autoimmunity through promoting the proliferation of Th17 cells, as well as maximizing their pathogenic potential.
To definitively demonstrate that Th17 cells indeed require TL1A signaling to completely exert their disease-driving capacities in vivo, we again took advantage of the discussed adoptive transfer model of EAE, except in this case the pathogenic Th17 cells were differentiated and expanded in vitro under optimal IL-23 concentrations. The majority (∼66–86%) of the resulting MOG-specific CD4+ T cells appeared to be IL-17 producing, based on intracellular cytokine staining, and expressed DR3, including the full-length variant 1 isoform, as confirmed by real-time PCR analysis (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20071364/DC1). The in vivo role of TL1A in regulating the pathogenic functions of these largely differentiated Th17 cells was then assessed by examining EAE disease severities observed after adoptive transfer into either WT or TL1A KO mice. Fig. 9 clearly shows that in comparison to WT recipients, the severity of EAE disease was significantly reduced in TL1A KO mice, indicating that continuous TL1A signaling is in fact required in vivo for these transferred pathogenic T cells to achieve their maximal disease-driving potential. Consistently, the numbers of IL-17, IFN-γ, and double-producing cells were all greatly reduced in the CNS infiltrates of TL1A KO mice (Fig. 9 B). These findings thus confirm the in vivo contribution of the TL1A–DR3 pathway in regulating Th17 cell function and in promoting Th17-mediated autoimmune disease pathologies.
Figure 9.
EAE induced by adoptive transfer of MOG-specific Th17 cells is dependent on TL1A. CD4+ T cells from MOG-primed WT animals were expanded in vitro in the presence of MOG peptide and IL-23 and adoptively transferred into WT and TL1A KO mice. (A) EAE clinical course in WT and TL1A KO mice (n = 11–12 mice per group). Results shown are compiled from two independent experiments. Disease severity was significantly reduced in KO mice (WT vs. KO mean cumulative score, P = 0.04; and mean maximal score, P = 0.05, as determined by the Mann Whitney nonparametric test), with no difference in disease incidence (WT, 9 out of 11 mice; KO, 10 out of 12 mice). Disease onset tended to be delayed (P = 0.06, as determined by the Student's two-tailed t test). (B) Mononuclear infiltrating cells from the CNS of WT and TL1A KO mice (pools of five animals) at day 24 after adoptive transfer were restimulated with PMA plus ionomycin and analyzed by intracellular cytokine staining. The absolute number of IL-17– and/or IFN-γ–expressing CD4+ T cells are shown.
DISCUSSION
The recent identification and characterization of Th17 lineage cells have significantly advanced our understanding of the pathogenic mechanisms underlying several inflammatory and autoimmune diseases (3–5, 39). Compared with the considerable progress on the various pathways important for the differentiation of Th17 cells from naive CD4+ T cells, little is known regarding the regulatory mechanisms governing the proliferation and function of effector Th17 cells. In this study, we have identified the TNF family ligand–receptor pairing of TL1A–DR3 as a novel regulator of Th17 cells.
TL1A, a member of the TNF superfamily, exerts its effects by binding and signaling through DR3 (19). Previous studies have shown that recombinant TL1A enhances the IL-2 responsiveness of TCR-activated human CD4+ T cells and promotes IFN-γ secretion, and synergizes with IL-12 and IL-18 in stimulating TCR-independent secretion of IFN-γ by human PB CD4+ memory T cells (20, 32). Additionally, mouse memory CD4+ T cells were reported to proliferate in response to TL1A stimulation, whereas naive CD4+ T cells were unresponsive (33). However, the effect of TL1A on Th17 cells was not investigated. Our results show that DR3 expression is enhanced in Th17 cells compared with Th1 and Th2 cells. Although differentiation of naive CD4+ T cells into Th17 cells is promoted by the combinatorial action of TGF-β and IL-6 (4, 7), our results reveal a novel role for TL1A in regulating Th17 differentiation. Although TL1A protein inhibited Th17 differentiation in vitro via increased IL-2 production, it promoted Th17 differentiation in the absence of IL-2 signaling. Consistently, lack of TL1A on DCs resulted in decreased Th17 differentiation by OT-II T cells. In these experiments, TL1A may act during the early stages of Th17 commitment and/or promote the expansion of Th17 lineage cells during differentiation. It has been proposed that Th17 survival and expansion are dependent on IL-23 (7, 39), and that IL-23 maintains their pathogenic potential (40); the precise function of IL-23 remains to be determined. Our observations suggest that TL1A expands Th17 cells and enhances the pathogenic potential of these cells when cultured with IL-23 at suboptimal levels. TL1A may thus synergize with IL-23 signaling in promoting the optimal effector functions of pathogenic T cells; the lack of this regulation may thus have contributed to reduced severity of EAE disease in the TL1A KO mice.
TL1A plays a specific role in expanding Th17 effector cells even in the absence of TCR stimulation, whereas IL-17 secretion was not increased upon TL1A stimulation, suggesting that TL1A primarily supports the proliferation of Th17 cells. It is possible that IL-17 secretion upon TL1A stimulation may require additional signaling pathways, e.g., from TCR or IL-23R. In contrast, TL1A did not affect expansion or IFN-γ secretion by in vitro–differentiated effector Th1 cells. Moreover, our data indicate that Th17 but not Th1 cell proliferation is supported by mature DCs independent of TCR stimulation. The significance of this finding is unclear at this stage, but this regulation may result in the expansion of Th17 cell numbers in the inflammatory tissues, which is important for early host defense against certain infection or inflammatory autoimmune diseases such as EAE. The homeostatic proliferation of Th17 cells in response to mature DCs appears to be dependent on TL1A–DR3 interaction, as expansion of Th17 effector cells is selectively impaired in the presence of TL1A KO BMDCs. Our results suggesting the specific role of TL1A in Th17 effector cell expansion differ from previous reports that TL1A promotes expansion and IFN-γ secretion by Th1 cells. However, previous studies used mouse and human memory T cells that were generated in vivo. In addition, they did not examine the effect of TL1A on IL-17 or Th17 cells, nor did they demonstrate a role for endogenous TL1A in regulating IFN-γ production (33). At this point, it is possible that TL1A regulation of effector and memory cells differs. Additionally, TL1A-mediated effects on Th1 cells may require the synergistic action of additional signaling pathways such as IL-12 or IL-18 cytokines, whereas TL1A alone is sufficient to induce Th17 proliferation (33). Further experiments are needed to explore the differences in the action of TL1A on Th17 and Th1 effector and memory CD4+ T cells, which may shed additional light on the novel function of TL1A.
DR3 expression is also up-regulated in both n–T reg and i–T reg cells, consistent with the observation that TGF-β signaling primarily up-regulates DR3. In contrast to Th17 cells, T reg cells also up-regulate a DR3 isoform in which there is a deletion of cysteine-rich region IV in the extracellular domain (variant 3), as compared with full-length DR3 (variant 1). TL1A stimulation did not enhance the proliferation T reg cells. Our data also demonstrate that differentiation of T reg cells is not affected by TL1A recombinant protein or enhanced in the absence of TL1A signaling in vitro. In addition, we found that the percentages of FoxP3+ CD4+ cells in the lymph nodes and spleens of MOG-immunized mice were similar in WT and TL1A KO mice (unpublished data). Further studies are required to understand the function of different isoforms of DR3 in T reg cells.
The physiological relevance of our findings was addressed using TL1A-deficient mice. TL1A KO mice exhibit a greatly decreased percentage of IL-17+CD4+ T cells, as well as a significant decrease in the percentages of IFN-γ+CD4+ T cells and IL-17+ IFN-γ+ double-producing cells after MOG immunization in vivo. Although TL1A does not regulate Th1 cell proliferation or differentiation in vitro, TL1A deficiency appears to affect IFN-γ expression in vivo. Future studies are needed to investigate how TL1A is regulating these various cytokine producing subsets in vivo. It is possible that TL1A may regulate initial IL-2 expression and the extent of T cell proliferation. Alternatively, a defect in IL-17 expression may affect Th1 differentiation, as we recently observed reduced IFN-γ production in IL-17–deficient mice (unpublished data).
Although Th1 cells and IFN-γ have long been implicated in inflammatory autoimmune diseases such as EAE and collagen-induced arthritis, recent studies have shown that Th17 cells and IL-23 play pivotal roles in promoting inflammation and mediating disease pathogenesis. Our results indicate that TL1A-deficient mice show reduced disease severity and decreased accumulation of T cells in the CNS. Furthermore, TL1A plays an important role in the EAE disease pathogenesis induced by adoptively transferred Th17 cells from MOG-immunized mice. These data are consistent with the proinflammatory function of Th17 cells in EAE and support a role of TL1A in the regulation of Th17 cells in vivo. TL1A may function in draining lymph nodes where T cell priming occurs, and/or in CNS tissue where Th17 cells initiate tissue inflammation. Reduced Th17 cells in the spleen and lymph nodes in TL1A KO mice after MOG immunization support the former. Of note, DCs have been shown recently to present antigens and selectively regulate Th17 cells in the CNS (41). Reduced cytokine-expressing CD4+ cells in the CNS of TL1A KO mice after disease induction supports reduced Th17 expansion in the tissue, and thereby reduced IL-17–induced chemokine production (18).
In summary, we show in this paper that TL1A expressed by mature DCs regulates Th17 differentiation, effector cell expansion, and function in EAE disease through its receptor DR3, which is selectively up-regulated in Th17 cells. This analysis reveals a novel and unique regulation of Th17 cells. Because TL1A and DR3 have been implicated in other inflammatory diseases (41), targeting this pathway may be beneficial in patients with Th17-mediated diseases.
MATERIALS AND METHODS
Mice.
TL1A KO mice were generated on the 129/Sv background by a targeted deletion of exon 4 of the TL1A locus on chromosome 4C1. TL1A deficiency was confirmed by RT-PCR on kidney tissues previously shown to express high levels of TL1A mRNA (20, 35). Mice were maintained at Biogen Idec or the M.D. Anderson Cancer Center animal facility in compliance with Institutional Animal Care and Use Committee (IACUC) guidelines. TL1A-deficient mice were backcrossed onto the C57BL/6 background for 5–10 generation for these experiments. Mice were genotyped for TL1A deficiency using a PCR-based assay on genomic DNA from tail biopsy samples. EAE experiments were conducted with mice backcrossed to the C57BL/6 strain for 6 or 10 generations with similar results. All animal experiments were performed based on the protocol approved by the IACUCs at M.D. Anderson Cancer Center or Biogen Idec.
Gene expression analysis.
Differentiating CD4+ T cells were harvested at the times indicated in the figures, RNA was prepared using TRIzol reagent (Invitrogen), and cDNA was prepared as described previously (10, 42). Naive CD4+ T cell cDNA was used for normalization of DR3 expression. Expression of DR3 was analyzed by real-time PCR analysis using the SYBR green PCR kit (Bio-Rad Laboratories). Expression of GAPDH was used as normalization control. Primer sequences are as follows: total DR3, (sense) 5′-ACACCCTCTTGGCACCTCCAAG-3′ and (antisense) 5′-GGCTCCAATTTCCGCTTCCC-3′; total transmembrane DR3 (variants 1 and 3), (sense) 5′-GTGACCCTTGAGAACTGCTCGGC-3′ and (antisense) 5′-GGAACGCGACTCCTAGAAGCACC-3′; full-length DR3 (variant 1), (sense) 5′-GTGACCCTTGAGAACTGCTCGGC-3′ and (antisense) 5′-GCCTTAGGGCAAACGCTGCTG-3′; and GAPDH, (sense) 5′-GAGAACTTTGGCATTGTGG-3′ and (antisense) 5′-ATGCAGGGATGATGTTCTG-3′. R. Nurieva and G. Martinez (M.D. Anderson Cancer Center, Houston, TX) provided Th17 cDNA and T reg cDNA, respectively.
Generation of BMDCs.
Mouse hind limbs were dissected, and tibia and fibula were flushed with warm RPMI 1640 medium. Red blood cells were lysed, and a single-cell suspension was cultured in RPMI 1640 medium containing GM-CSF for 7 d. Media was replaced every 2 d with fresh RPMI 1640 medium containing GM-CSF. On day 7, immature BMDCs were stimulated with 1 μg/ml LPS overnight before being used as APCs. Percentages of BMDCs before and after activation were confirmed by staining for CD11c, MHC class II, CD80, and CD86.
T cell differentiation and inducible T reg cell preparation.
Naive OT-II CD4+ T cells were purified from RAG KO/OT-II mice by magnetic separation using anti-CD4 magnetic beads, and a cell purity of >97% was routinely obtained. In some experiments, CD4+CD62LhiCD25− naive T cells or CD4+CD62LhiCD44lowCD25+ve n–T reg cells were sorted from single-cell suspensions of spleens and lymph nodes by FACS. 0.5 × 106 OT-II cells were cultured at a 20:1 ratio with BMDCs pulsed with 10 μg/ml OT-II peptide, or were cultured with 10 μg/ml OT-II peptide–pulsed irradiated splenocytes at a 1:1 ratio. Additionally, for differentiating Th1 cells, 20 ng/ml IL-12 and 5 μg/ml anti–IL-4 (clone 11B11) were added to the culture. For Th17 differentiation, 10 ng/ml mIL-1β, 5 ng/ml hTGF-β, 20 ng/ml mIL-6, 10 μg/ml anti–IFN-γ (clone XMG1.2), 5 μg/ml anti–IL-4 (clone 11B11), and 20 ng/ml mIL-23 were added. For generating Th2 cells, 20 ng/ml IL-4 and 10 μg/ml anti–IFN-γ were added. For generating T reg cells, 5ng/ml hTGF-β (PeproTech), and 10 μg/ml anti–IFN-γ and anti–IL-4 were added. Percentages of T reg cells were analyzed by intracellular staining using the mouse FoxP3 staining kit according to the manufacturer's recommendations (eBioscience). All cytokines used were purchased from PeproTech. Human Fc-TL1A, as previously described (42), or control human Ig (Sigma-Aldrich) were added as indicated in the figures at 1 μg/ml.
Proliferation and cytokine measurement.
In vitro–differentiated cells were restimulated on day 5 with a titrating dose of anti-CD3 with or without exogenous human Fc-TL1A, as described previously (43), or control human Ig. For anti-CD3 stimulation, 96-well plates were coated with the concentration of anti-CD3ε (clone 2C11) indicated in the figures, and 1.5–2 × 105 cells were added per well. For restimulation of differentiated cells with BMDCs, OVA peptide was titrated and incubated with BMDCs, and 1.5 × 105 cells T cells were added at a 20:1 ratio with DCs. To measure proliferation, including naive and T reg cell experiments, 1 μCi [3H]thymidine was added per well during the last 8 h of stimulation and harvested, and radioactive incorporation was measured by a β-scintillation counter (PerkinElmer). For cytokine measurement, culture supernatant was harvested at 24 h after stimulation and analyzed by ELISA, as previously described (18).
Intracellular cytokine staining of in vitro–differentiated Th cells.
Differentiated Th cells were harvested on the day indicated in the figures, and restimulated with 50 ng PMA and 500 ng/ml ionomycin for 6 h in the presence of Golgi Plug (1 μg/ml Brefeldin A; BD Biosciences). Cells were harvested and stained for CD4 (clone RM4-4) and fixed with 4% paraformaldehyde. Cells were then permeabilized with Cytoperm solution (BD Biosciences), and stained with anti–mouse IL-17 (clone TC11-18H10.1) and anti–mouse IFN-γ. At least 50,000 live events were acquired on a FACSVantage system (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.)
EAE induction.
8–12-wk-old female C57BL/6 TL1A KO and WT animals were immunized subcutaneously with 200 μg MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) emulsified in CFA (Chrondex) with 200 μg Mycobacterium tuberculosis. 50 ng of pertussis toxin (List Biological) in PBS was given i.p. at the time of immunization, and 7–10 animals were used for each experimental group. For adoptive transfer, C57BL/6 WT mice were immunized subcutaneously with MOG peptide, as described, with 400 μg M. tuberculosis, and 7 d later the draining lymph nodes and spleen cells from MOG-primed animals were restimulated in vitro with 25 μg/ml MOG and 20 ng/ml rIL-23 for 7 d. 10 million CD4+ T cells purified by negative selection using magnetic beads (purity >80%) were adoptively transferred into WT or TL1A KO recipients by i.p. injection, followed by 50 ng of pertussis toxin per mouse i.p. at the time of transfer. In some experiments, cells were expanded using 25 μg/ml MOG, and either in the presence or absence of suboptimal 5 ng/ml rIL-23 or in the presence or absence of Fc-TL1A for 10 d. CD4+ T cells were then purified and adoptively transferred into C57BL/6 recipient mice i.v., followed by 500 ng/ml of pertussis toxin. Animals were monitored for weight loss and development of EAE symptoms. Disease severity was scored as follows: 0, no sign of impairment; 1, limp tail; 2, hind limb weakness; 3, hind limb paresis, or paresis of one side of the body and rolling; 4, hind limb paralysis; and 5, moribund or dead (44).
Histological evaluation.
Animals were killed, and spinal cords were flushed out with PBS and fixed in 4% paraformaldehyde for 72 h. Tissue was paraffin embedded and cross sectioned using standard protocols. Sections were stained for hematoxylin and eosin, Luxol fast blue for myelin, CD3, F4/80, Ki-67, Tdt-mediated dUTP-biotin nick-end labeling, and anti-active caspase-3 using standard procedures. Tissues were examined microscopically, and the CD3 staining was quantified using the MetaMorph image analysis software (MDS Analytical Technologies). In brief, a threshold for positive brown staining was established, and the total thresholded area was determined for each spinal cord cross section (at 10× magnification). The area was divided by the mean area of brown staining corresponding to one CD3-positive cell to calculate the approximate number of CD3-positive cells per spinal cord cross section. Six spinal cord sections were analyzed per animal.
Intracellular cytokine expression in the spleen and lymph nodes after MOG immunization.
8–12-wk-old female C57BL/6 TL1A KO and WT animals were immunized subcutaneously with 200 μg MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) emulsified in CFA with 400 μg M. tuberculosis. 10 d later, the spleen and lymph nodes were collected from each individual and processed into a single-cell suspension, and stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of 1 μg/ml Golgi Plug for 5 h, or restimulated overnight with 50 μg/ml of MOG peptide with Golgo Plug added during the last 5 h. Cells were treated with Fc-blocking mAbs and surface stained with anti-CD4–allophycocyanin, followed by intracellular staining with PE-conjugated mAb to IL-17 and FITC-conjugated mAb to IFN-γ, or isotype control mAbs according to the manufacturer's instructions (BD Biosciences). Data were acquired using an LSRII instrument (Becton Dickinson) and analyzed using FlowJo FACS analysis software. Percentages of cytokine-expressing cells within the CD4+ population were determined after subtracting the percentages obtained with isotype control mAbs.
Isolation and analysis of CNS-infiltrating cells.
At the desired time points after EAE induction or adoptive transfer of EAE, animals were killed and perfused through the left ventricle with room temperature Ames medium (Sigma-Aldrich) or PBS to remove blood leukocytes. Spinal cords and cerebellum or whole brains were collected into ice-cold HBSS with 10% fetal bovine serum. Tissue was disrupted through a 100-μm strainer, washed 1× with HBSS/serum, and resuspended in 5 ml of 37% Percoll (GE Healthcare) in PBS. Gradients were underlayered with 2.5 ml of 70% Percoll in PBS and centrifuged at 2,000 g for 20 min. The mononuclear cells were collected from the 37/70 interface, washed with HBSS/serum, and processed for FACS staining. For kinetic analysis of CNS-infiltrating T cells, cells were stained with BD Bioscience antibodies for anti-CD45–PerCP, CD11b-allophycocyanin, and anti-CD4–PE. For the analysis of intracellular cytokine staining, CNS-isolated WT and KO cells (pools of five to six individual mice per group) were for the last 5 h with 50 ng/ml of PMA and 500 ng/ml of ionomycin in the presence of 1 μg/ml Golgi Plug. Cells were treated with Fc-blocking mAbs and surface stained with anti-CD45–PerCP and anti-CD4–allophycocyanin (BD Biosciences), followed by intracellular cytokine staining with PE-conjugated mAbs to IL-17, IFN-γ, IL-6, and TNF, a PE-conjugated mAb to IL-17 and an FITC-conjugated mAb to IFN-γ, or isotype control mAbs, according to the manufacturer's instructions (BD Biosciences). Data were acquired on the LSRII instrument and analyzed using the FlowJo FACS analysis software. Absolute numbers of cytokine-expressing CD4+ cells were calculated.
Statistical analysis.
Statistical analysis was performed using Prism software (GraphPad), and analysis methods used are indicated in the respective figure legends.
Online supplemental material.
Fig. S1 shows that TL1A-Ig does not induce IL-17 expression by effector Th17 cells. Fig. S2 provides an analysis of lymphocyte subpopulations and mononuclear cell suspensions in TL1A KO and WT spleen, lymph node, thymus, and bone marrow. Fig. S3 describes the differentiation of naive CD4+ T cells in the presence of WT or TL1A KO BMDCs into Th17 and i–T reg cells. Fig. S4 describes the cytokine profile of in vitro restimulation of CD4+ T cells from MOG-primed WT and TL1A KO mice. Fig. S5 studies the effect of TL1A on the pathogenicity of MOG-specific T cells upon in vitro expansion and adoptive transfer. In Fig. S6, DR3 isoform expression was calculated by real-time PCR analysis in MOG-specific Th17 cells and compared with n–T reg and i–T reg cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20071364/DC1.
Supplementary Material
Acknowledgments
We thank Bruz Marzolf for his assistance in the microarray analysis, the Biogen Idec animal facility for animal maintenance, Tom Crowell and the histology facility for tissue processing, Juanita Campos and the FACS facility for sample analysis, Leila Rieder and Norm Allaire for TaqMan PCR, and Dr. Ben Hahm and Yi Luo for technical assistance and helpful discussions.
This work was supported in part by research grants from the National Institutes of Health (to C. Dong). B.P. Pappu is an Odyssey Fellow supported by the Odyssey Program and the Theodore N. Law Endowment for Scientific Achievement at the University of Texas M.D. Anderson Cancer Center. C. Dong is a Cancer Research Institute Investigator, an American Lung Association Career Investigator, and an M.D. Anderson Cancer Center Trust Fellow.
A. Borodovsky, T. Zheng, P. Wu, S. Weng, B. Browning, M. Scott, L. Su, and L.C. Burkly are present or former employees of and own stock in Biogen Idec. The rest of the authors declare no competing financial interests.
Abbreviations used: BMDC, bone marrow–derived DC; CNS, central nervous system; DR3, death receptor 3; EAE, experimental autoimmune encephalomyelitis; i–T reg cell, TGF-β–induced T reg; MOG, myelin oligodendrocyte glycoprotein; n–T reg cell, naturally occurring T reg cell; PB, peripheral blood.
B.P. Pappu and A. Borodovsky contributed equally to this study.
A. Borodovsky's present address is Alnylam, Cambridge, MA 02142.
M. Scott's present address is Merck Research Laboratories, Boston, MA 02115.
References
- 1.Dong, C., and R.A. Flavell. 2000. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glimcher, L.H., and K.M. Murphy. 2000. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14:1693–1711. [PubMed] [Google Scholar]
- 3.Dong, C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 6:329–333. [DOI] [PubMed] [Google Scholar]
- 4.Weaver, C.T., L.E. Harrington, P.R. Mangan, M. Gavrieli, and K.M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688. [DOI] [PubMed] [Google Scholar]
- 5.Bettelli, E., M. Oukka, and V.K. Kuchroo. 2007. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 7.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 8.Yang, X.O., A.D. Panopoulos, R. Nurieva, S.H. Chang, D. Wang, S.S. Watowich, and C. Dong. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282:9358–9363. [DOI] [PubMed] [Google Scholar]
- 9.Korn, T., E. Bettelli, W. Gao, A. Awasthi, A. Jager, T.B. Strom, M. Oukka, and V.K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 448:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nurieva, R., X.O. Yang, G. Martinez, Y. Zhang, A.D. Panopoulos, L. Ma, K. Schluns, Q. Tian, S.S. Watowich, A.M. Jetten, and C. Dong. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483. [DOI] [PubMed] [Google Scholar]
- 11.Zhou, L., I.I. Ivanov, R. Spolski, R. Min, K. Shenderov, T. Egawa, D.E. Levy, W.J. Leonard, and D.R. Littman. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Z., A. Laurence, Y. Kanno, M. Pacher-Zavisin, B.M. Zhu, C. Tato, A. Yoshimura, L. Hennighausen, and J.J. O'Shea. 2006. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 103:8137–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 14.Yang, X.O., B.P. Pappu, R. Nurieva, A. Akimzhanov, H.S. Kang, Y. Chung, L. Ma, B. Shah, A.D. Panopoulos, K.S. Schluns, et al. 2008. T Helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 28:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong, C., and R.A. Flavell. 2001. Th1 and Th2 cells. Curr. Opin. Hematol. 8:47–51. [DOI] [PubMed] [Google Scholar]
- 16.Croft, M. 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3:609–620. [DOI] [PubMed] [Google Scholar]
- 17.Dong, C., and R.I. Nurieva. 2003. Regulation of immune and autoimmune responses by ICOS. J. Autoimmun. 21:255–260. [DOI] [PubMed] [Google Scholar]
- 18.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watts, T.H. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23–68. [DOI] [PubMed] [Google Scholar]
- 20.Migone, T.S., J. Zhang, X. Luo, L. Zhuang, C. Chen, B. Hu, J.S. Hong, J.W. Perry, S.F. Chen, J.X. Zhou, et al. 2002. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 16:479–492. [DOI] [PubMed] [Google Scholar]
- 21.Bamias, G., C. Martin III, M. Marini, S. Hoang, M. Mishina, W.G. Ross, M.A. Sachedina, C.M. Friel, J. Mize, S.J. Bickston, et al. 2003. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J. Immunol. 171:4868–4874. [DOI] [PubMed] [Google Scholar]
- 22.Kang, Y.J., W.J. Kim, H.U. Bae, D.I. Kim, Y.B. Park, J.E. Park, B.S. Kwon, and W.H. Lee. 2005. Involvement of TL1A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 29:229–235. [DOI] [PubMed] [Google Scholar]
- 23.Prehn, J.L., S. Mehdizadeh, C.J. Landers, X. Luo, S.C. Cha, P. Wei, and S.R. Targan. 2004. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin. Immunol. 112:66–77. [DOI] [PubMed] [Google Scholar]
- 24.Prehn, J.L., L.S. Thomas, C.J. Landers, Q.T. Yu, K.S. Michelsen, and S.R. Targan. 2007. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J. Immunol. 178:4033–4038. [DOI] [PubMed] [Google Scholar]
- 25.Bodmer, J.L., K. Burns, P. Schneider, K. Hofmann, V. Steiner, M. Thome, T. Bornand, M. Hahne, M. Schroter, K. Becker, et al. 1997. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95). Immunity. 6:79–88. [DOI] [PubMed] [Google Scholar]
- 26.Chinnaiyan, A.M., K. O'Rourke, G.L. Yu, R.H. Lyons, M. Garg, D.R. Duan, L. Xing, R. Gentz, J. Ni, and V.M. Dixit. 1996. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 274:990–992. [DOI] [PubMed] [Google Scholar]
- 27.Screaton, G.R., X.N. Xu, A.L. Olsen, A.E. Cowper, R. Tan, A.J. McMichael, and J.I. Bell. 1997. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc. Natl. Acad. Sci. USA. 94:4615–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Lamki, R.S., J. Wang, S. Thiru, N.R. Pritchard, J.A. Bradley, J.S. Pober, and J.R. Bradley. 2003. Expression of silencer of death domains and death-receptor-3 in normal human kidney and in rejecting renal transplants. Am. J. Pathol. 163:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadakis, K.A., J.L. Prehn, C. Landers, Q. Han, X. Luo, S.C. Cha, P. Wei, and S.R. Targan. 2004. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J. Immunol. 172:7002–7007. [DOI] [PubMed] [Google Scholar]
- 30.Wang, E.C., J. Kitson, A. Thern, J. Williamson, S.N. Farrow, and M.J. Owen. 2001. Genomic structure, expression, and chromosome mapping of the mouse homologue for the WSL-1 (DR3, Apo3, TRAMP, LARD, TR3, TNFRSF12) gene. Immunogenetics. 53:59–63. [DOI] [PubMed] [Google Scholar]
- 31.Sung, H.H., J.H. Juang, Y.C. Lin, C.H. Kuo, J.T. Hung, A. Chen, D.M. Chang, S.Y. Chang, S.L. Hsieh, and H.K. Sytwu. 2004. Transgenic expression of decoy receptor 3 protects islets from spontaneous and chemical-induced autoimmune destruction in nonobese diabetic mice. J. Exp. Med. 199:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadakis, K.A., D. Zhu, J.L. Prehn, C. Landers, A. Avanesyan, G. Lafkas, and S.R. Targan. 2005. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J. Immunol. 174:4985–4990. [DOI] [PubMed] [Google Scholar]
- 33.Bamias, G., M. Mishina, M. Nyce, W.G. Ross, G. Kollias, J. Rivera-Nieves, T.T. Pizarro, and F. Cominelli. 2006. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc. Natl. Acad. Sci. USA. 103:8441–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurence, A., C.M. Tato, T.S. Davidson, Y. Kanno, Z. Chen, Z. Yao, R.B. Blank, F. Meylan, R. Siegel, L. Hennighausen, E.M. Shevach, and J.J. O'Shea. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26:371–381. [DOI] [PubMed] [Google Scholar]
- 35.Zhai, Y., J. Ni, G.W. Jiang, J. Lu, L. Xing, C. Lincoln, K.C. Carter, F. Janat, D. Kozak, S. Xu, et al. 1999. VEGI, a novel cytokine of the tumor necrosis factor family, is an angiogenesis inhibitor that suppresses the growth of colon carcinomas in vivo. FASEB J. 13:181–189. [DOI] [PubMed] [Google Scholar]
- 36.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carboni, S., F. Aboul-Enein, C. Waltzinger, N. Killeen, H. Lassmann, and C. Pena-Rossi. 2003. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J. Neuroimmunol. 145:1–11. [DOI] [PubMed] [Google Scholar]
- 38.Juedes, A.E., P. Hjelmstrom, C.M. Bergman, A.L. Neild, and N.H. Ruddle. 2000. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J. Immunol. 164:419–426. [DOI] [PubMed] [Google Scholar]
- 39.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 40.McGeachy, M.J., and D.J. Cua. 2007. T cells doing it for themselves: TGF-beta regulation of Th1 and Th17 cells. Immunity. 26:547–549. [DOI] [PubMed] [Google Scholar]
- 41.Bailey, S.L., B. Schreiner, E.J. McMahon, and S.D. Miller. 2007. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol. 8:172–180. [DOI] [PubMed] [Google Scholar]
- 42.Chung, Y., X. Yang, S.H. Chang, L. Ma, Q. Tian, and C. Dong. 2006. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 16:902–907. [DOI] [PubMed] [Google Scholar]
- 43.Bossen, C., K. Ingold, A. Tardivel, J.-L. Bodmer, O. Gaide, S. Hertig, C. Ambrose, J. Tschopp, and P. Schneider. 2006. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 281:13964–13971. [DOI] [PubMed] [Google Scholar]
- 44.Fuller, K.G., J.K. Olson, L.M. Howard, J.L. Croxford, and S.D. Miller. 2004. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease. Methods Mol. Med. 102:339–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.