Abstract
Most autoimmune diseases are more common in women than in men. This may be caused by differences in sex hormones, sex chromosomes, or both. In this study, we determined if there was a contribution of sex chromosomes to sex differences in susceptibility to two immunologically distinct disease models, experimental autoimmune encephalomyelitis (EAE) and pristane-induced lupus. Transgenic SJL mice were created to permit a comparison between XX and XY within a common gonadal type. Mice of the XX sex chromosome complement, as compared with XY, demonstrated greater susceptibility to both EAE and lupus. This is the first evidence that the XX sex chromosome complement, as compared with XY, confers greater susceptibility to autoimmune disease.
Sex differences in susceptibility to autoimmune diseases have been recognized for decades (1). A variety of sex-related differences in immune responses have been described, with females generally having increased cellular and humoral responses compared with males. Autoimmune diseases characterized by a female preponderance are numerous, including multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus, to name a few. Many experimental models of autoimmune disease also demonstrate a female preponderance (1). Extensive research has been devoted to the role of sex hormones in the sex difference in autoimmune diseases in both humans and animal models, and numerous effects of sex hormones have indeed been shown. However, effects of sex hormones do not rule out a more direct effect of sex chromosomes.
Direct effects of sex chromosomes and indirect effects of sex chromosomes (mediated by sex hormones) are the two major classes of signals that induce sex differences in phenotype. In male mammals, the Y-linked gene Sry is expressed in cells of the undifferentiated gonadal ridges to cause them to differentiate into Sertoli cells, which begins the differentiation of the testes (2). Once the testes have formed, they secrete hormones that are distinct from those of the ovary, and these hormonal differences generate sex differences in many nongonadal tissues, such as the external genitalia, immune system, brain, cardiovascular system, and skeletal system. Indeed, the effects of these hormones account for the majority of sex differences in nongonadal tissues identified to date. However, there are direct genetic differences between males and females arising from the difference in sex chromosome complement that could also contribute to sex differences in phenotype (3, 4). Such possibilities include expression of genes located on the nonrecombining region of the Y chromosome whose role in nongonadal tissues has been understudied, differences in X gene expression that arise from the X chromosome origin (maternal or paternal), and differences in dosage of genes located on the nonpseudoautosomal region of the X chromosome.
Fortunately, a mouse model system has recently been developed to identify effects of the sex chromosome complement without the confounding effects of differences in gonadal type (4). In this study, the testes-determining Sry gene has been deleted from the Y chromosome, producing the Sry-deficient Y−. This results in XX and XY− ovary-bearing mice. Further, when Sry is inserted as a transgene on an autosome, this results in XXSry and XY−Sry testes-bearing mice. This model system allows comparisons between XX and XY− within a female hormonal background, as well as between XXSry and XY−Sry within a male hormonal background (Table S1, available at http://www.jem.org/cgi/content/full/jem.20070850/DC1). We use this model system to present the first evidence of a sex chromosome effect that encompasses two distinct autoimmune disease models.
RESULTS
XX sex chromosome complement confers greater susceptibility to EAE
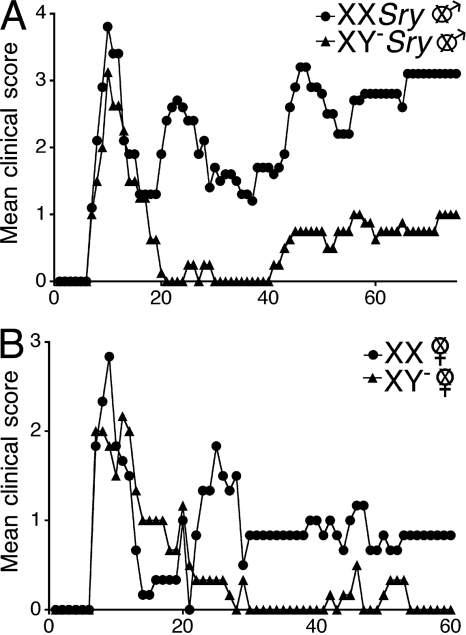
We addressed the role of the sex chromosome complement in the sex difference in EAE using the SJL strain because this strain had previously been shown to demonstrate greater disease susceptibility in females as compared with males (5). We backcrossed the Sry-deficient Y chromosome from original outbred MF1 mice (6) onto the SJL strain to the F16 generation (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070850/DC1). We then gonadectomized both females and males to remove any intercurrent effects of sex hormones that might mask effects of sex chromosomes (7). SJL castrated male mice that were either XXSry or XY−Sry, had active EAE induced with proteolipid protein (PLP) peptide 139–151. Clinical disease course was more severe in XXSry mice, as compared with XY−Sry mice (P = 0.0001, Friedman test including all days; Fig. 1 A and Table I). This difference in disease severity also occurred when comparing ovariectomized female XX versus XY− mice (P = 0.0012, Friedman test including all days; Fig. 1 B and Table I).
Figure 1.
The XX sex chromosome complement, as compared with the XY−, confers greater disease severity to active EAE. (A) Active EAE was induced in castrated XXSry and XY−Sry male mice with autoantigen PLP 139–151. Mean clinical disease course was more severe in castrated male XXSry mice as compared with XY−Sry mice. P < 0.0001. XXSry, •, n = 6; XY−Sry, ▴, n = 5. (B) Active EAE was induced in ovariectomized XX and XY− female mice with autoantigen PLP 139–151. Clinical disease course was more severe in ovariectomized female XX mice compared with XY− mice. P < 0.0001. XX, •, n = 5; XY−, ▴, n = 5. Data are representative of one experiment in males and two independent experiments in females. Graphs show mean clinical score for each group at each time point. Statistical analysis compares mean score for all days from each group. Female symbol with x overlay indicates ovariectomized female; male symbol with x overlay indicates castrated male.
Table I.
Clinical features of active EAE in XX ovariectomized female SJL mice compared with XY− ovariectomized female SJL mice and XXSry castrated male SJL mice compared with XY−Sry castrated male SJL mice
| Genotype | Incidence | Mortality | Mean day of onset | Mean peak | Mean score (all days) |
|---|---|---|---|---|---|
| Experiment 1a | |||||
| XX females | 4/5 | 0/5 | 7.3 | 3.7 | 0.6b |
| XY− females | 3/5 | 0/5 | 8.3 | 2.8 | 0.2 |
| Experiment 2 | |||||
| XX females | 4/4 | 0/4 | 13 | 3.6 | 1.8b |
| XY− females | 3/3 | 0/3 | 16 | 2.3 | 0.8 |
| Experiment 3a | |||||
| XX males | 5/6 | 0/6 | 8 | 3.9b | 1.9b |
| XY− males | 4/5 | 0/5 | 8.5 | 3.1 | 0.6 |
These data are depicted in Fig. 1.
A significant difference between groups; P < 0.05.
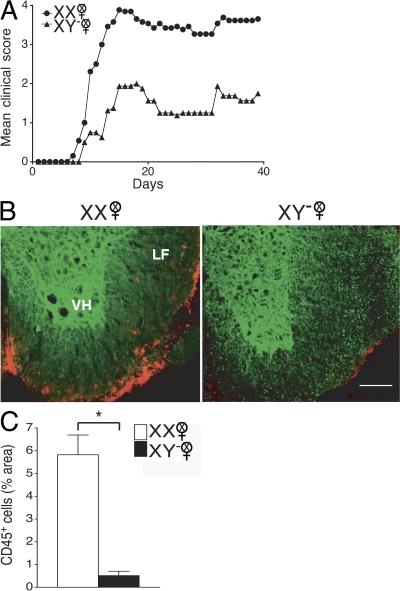
Because the induction phase and the effector phase of EAE occur in the same animal in active EAE, it was unknown whether the difference in active EAE disease severity in XX versus XY− mice was caused by differences in the development of the encephalitogenic immune response or by differences in the target organ, the central nervous system (CNS) in this case. To determine if the sex chromosome effect in active EAE was caused by the influence of the sex chromosome complement on the immune response, we adoptively transferred autoantigen-stimulated LN cells (LNCs) from ovariectomized XX or XY− mice (immunized with PLP 139–151) into WT female mice. LNCs derived from ovariectomized female XX mice, as compared with those derived from XY−, induced more severe clinical disease (P = 0.0001, Friedman test including all days; Fig. 2 A and Table II).
Figure 2.
The XX sex chromosome complement, as compared with the XY−, confers greater disease severity to adoptive EAE. (A) Effect of sex chromosome complement on adoptive EAE. LNCs from ovariectomized female XX and XY− mice (immunized with PLP 139–151) were adoptively transferred into WT females (gonadally intact). Mean clinical disease course was significantly higher in recipients of LNCs derived from XX mice as compared with those derived from XY− mice. P < 0.0001. XX, •, n = 13; XY−, ▴, n = 8. Data are representative of two independent experiments. Graph shows mean clinical score for each group at each time point. Statistical analysis compares mean score for all days from each group. (B) Recipients of XX-derived LNCs had more CNS inflammation than recipients of XY−-derived LNCs. Shown are representative thoracic spinal cord sections of mice with adoptively transferred EAE that were coimmunostained with anti-CD45 (red) and anti–β3-tubulin (green) antibodies. LF, lateral funiculus; VH, ventral horn). EAE mice that received LNCs derived from XX mice (left) had significantly increased CD45+ staining as compared with EAE mice that received LNCs derived from XY− mice (right). Bar, 100 μm. (C) Quantification of EAE neuropathology. Mice that received XX LNCs had a significant increase in CD45+ cells compared with mice that received XY− LNCs. P < 0.001, Student's t test. XX, •, n = 12; XY−, ▴, n = 12. Data are representative of two independent experiments. Histograms show the means and the SEM for mice in each group. Female symbol with x overlay indicates ovariectomized female.
Table II.
Clinical features of adoptive EAE in WT female SJL mice that received PLP 139-151 reactive LN cells from either XX ovariectomized female SJL mice or XY− ovariectomized female SJL mice
| Genotype of donor cells | Incidence | Mortality | Mean day of onset | Mean peak | Mean score (all days) |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| XX females | 12/12 | 7/12 | 9.4 | 4.6a | 3.5a |
| XY− females | 2/11 | 0/11 | 10.5 | 3.3 | 0.2 |
| Experiment 2b | |||||
| XX females | 13/13 | 2/13 | 9.9 | 4.0a | 2.7a |
| XY− females | 5/8 | 1/8 | 11.2 | 3.6 | 1.1 |
A significant difference between groups; P < 0.05.
These data are depicted in Fig. 2.
We then assessed the extent of inflammation in the CNS of mice with adoptive EAE. On day 40 after induction of adoptive EAE, thoracic spinal cord sections of WT females that received either XX or XY− LNCs were coimmunostained with anti-CD45 (an immune cell marker) and anti–β3-tubulin (a neuronal marker). Qualitatively, recipients of XX-derived LNCs had more CNS inflammation than recipients of XY−-derived LNCs (Fig. 2 B). Quantification of CD45+ cell density revealed a significant increase in mice that received XX-derived LNCs versus mice that received XY−-derived LNCs (P = 0.001; Fig. 2 C). Together, these clinical and neuropathology studies demonstrated that the XX sex chromosome complement, as compared with the XY−, confers greater susceptibility to EAE by promoting the development of a more encephalitogenic immune response.
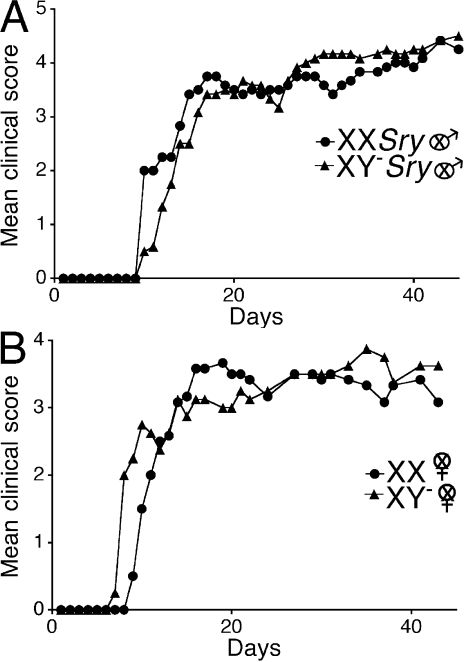
Because it had been previously shown that no gender difference exists in EAE in the C57BL/6 strain of mice (8–10), we then questioned whether an effect of sex chromosomes could be found in this strain. Similar to our work in the SJL, we next backcrossed the Sry-deficient Y chromosome from the original outbred MF1 mice (6) onto the C57BL/6 strain to the F14 generation. We then gonadectomized both females and males to remove any effects of sex hormones that might mask effects of sex chromosomes (7). C57BL/6 castrated male mice that were either XXSry or XY−Sry had active EAE induced with myelin oligodendrocyte protein peptide (MOG) 35–55. In contrast to results in the SJL, clinical disease courses were no different when comparing XXSry mice with XY−Sry mice (Fig. 3 A and Table III). There was also no difference in disease when comparing ovariectomized female XX versus XY− mice (Fig. 3 B and Table III). Together, our data indicated that when a strain is used that is characterized by a female-to-male difference in EAE (the SJL), a sex chromosome effect is present. In contrast, when a strain is used that is not characterized by a female-to-male difference in EAE (the C57BL/6), a sex chromosome effect is not present. The presence of a sex chromosome effect in one strain, but not another, revealed an interaction between sex chromosome complement and genetic background. Notably, effects of sex hormones in EAE have also previously been shown to be dependent on genetic background (9), and some autosomal gene linkages to susceptibility to multiple sclerosis have been identified in one gender, but not the other (11). Thus, in the outbred human population, the genetic background of some, but not all, individuals may be permissive to sex chromosome or sex hormone effects. Notably, because overall there is a gender difference in many autoimmune diseases in humans, the relatively permissive genetic backgrounds are likely to be prevalent, not rare, in occurrence.
Figure 3.
No effect of sex chromosome complement for active EAE in C57BL/6 mice. (A) Active EAE was induced in castrated male XXSry and XY−Sry C57BL/6 mice with autoantigen MOG 35–55. Mean clinical disease course was no different when comparing castrated male XXSry mice to XY−Sry mice. XXSry, •, n = 6; XY−Sry, ▴, n = 6. (B) Active EAE was induced in ovariectomized female XX and XY− C57BL/6 mice with autoantigen MOG 35–55. Mean clinical disease course was also no different when comparing ovariectomized female XX versus XY− mice. XX, •, n = 6; XY−, ▴, n = 4. Data are representative of three independent experiments in males and two independent experiments in females. Graphs show mean clinical score for each group at each time point. Statistical analysis compares mean score for all days from each group. Female symbol with x overlay indicates ovariectomized female; male symbol with x overlay indicates castrated male.
Table III.
Clinical features of active EAE in XX ovariectomized female C57BL/6 mice compared with XY− ovariectomized female C57BL/6 mice and XXSry castrated male C57BL/6 mice compared with XY−Sry castrated male C57BL/6 mice
| Genotype | Incidence | Mortality | Mean day of onset | Mean peak | Mean score (all days) |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| XX females | 7/7 | 0/7 | 11 | 3.4 | 2.8 |
| XY− females | 6/6 | 0/6 | 13 | 3.2 | 2.1 |
| Experiment 2a | |||||
| XX females | 6/6 | 1/6 | 12 | 3.7 | 2.8 |
| XY− females | 4/4 | 1/4 | 9 | 3.9 | 3.0 |
| Experiment 3a | |||||
| XX Sry males | 6/6 | 0/6 | 11 | 4.4 | 3.4 |
| XY− Sry males | 6/6 | 0/6 | 13 | 4.5 | 3.3 |
| Experiment 4 | |||||
| XX Sry males | 5/5 | 0/5 | 14 | 2.9 | 2.3 |
| XY− Sry males | 7/7 | 0/7 | 14 | 3.6 | 2.9 |
| Experiment 5 | |||||
| XX Sry males | 5/5 | 3/5 | 14 | 4.7 | 3.0 |
| XY− Sry males | 7/7 | 4/7 | 12 | 4.4 | 3.0 |
These data are depicted in Fig. 3.
XX sex chromosome complement confers greater susceptibility to lupus
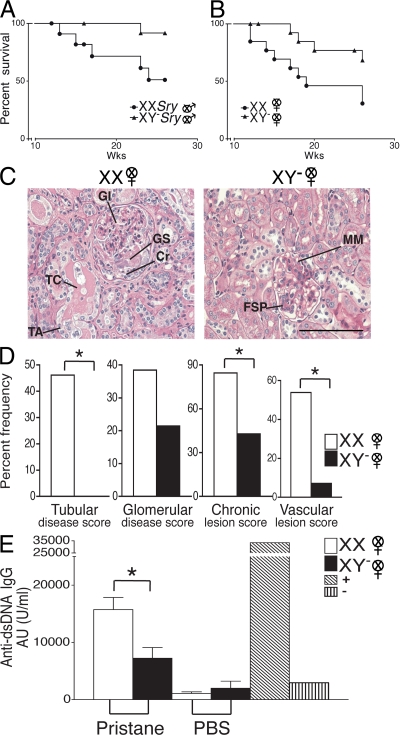
In light of the fact that lupus in humans is characterized by a 9:1 female preponderance, and because we had previously shown that pristane-induced lupus in SJL mice was characterized by greater susceptibility in females as compared with males (12), we next determined whether the effect of sex chromosome complement observed in SJL mice with EAE was unique to this disease or was more pervasive across autoimmune diseases. Thus, we next evaluated the role of sex chromosome complement in pristane-induced lupus. Ovariectomized female XX and XY− SJL mice and castrated male XXSry and XY−Sry mice were injected with pristane and monitored daily for signs of disease (Fig. 4, A and B). By 26 wk, only 30.8% of XX mice had survived, whereas 68.4% of XY− mice had survived (P = 0.036, Kaplan-Meier including all days). This greater disease severity in XX mice also occurred when comparing castrated male XXSry mice with XY−Sry (P = 0.027, Kaplan-Meier including all days).
Figure 4.
The XX sex chromosome complement, as compared with the XY−, confers greater disease severity to lupus. (A) Castrated male XXSry and XY−Sry SJL mice were injected with pristane and monitored daily for signs of disease. By 26 wk, XXSry mice had increased mortality as compared with XY−Sry mice. P = 0.027. XXSry, •, n = 12; XY−Sry, ▴, n = 13. (B) Ovariectomized female XX and XY− SJL mice were injected with pristane and monitored daily for signs of disease. At 26 wk, XX mice had increased mortality as compared with XY− mice. P = 0.036. XX, •, n = 13; XY−, ▴, n = 15. Data are representative of two independent experiments. (C) Kidneys from ovariectomized female XX and XY− female SJL mice injected with pristane were harvested upon death for renal pathology. Representative renal histology from pristane-induced lupus SJL mice showing that ovariectomized female XX mice (left) have more severe nephritis than ovariectomized female XY− mice (right). GI, glomerular infiltration; GS, segmental glomerulosclerosis; Cr, cellular crescent; TC, tubular casts; TA, tubular atrophy; FSP, focal segmental proliferative; MM, mild mesangial matrix. Bars, 50 μm. (D) Lesions from pristane-injected ovariectomized female XX and XY− mice were scored, ranked, and expressed as the percent frequency of mice in each group. A greater percentage of XX mice showed severe tubular disease scores (≥10; P = 0.006), chronic lesion scores (≥3; P = 0.046), and vascular lesion scores (P = 0.013) than their XY− littermates. In addition, a greater percentage of XX mice showed severe glomerular disease scores (≥10; NS). XX, n = 13; XY−, n = 14. XX, white columns; XY−, shaded columns. (E) Effect of sex chromosome complement on autoantibody levels in pristane-induced lupus. Ovariectomized female XX and XY− SJL mice were bled to detect anti-dsDNA antibodies in serum. 16 wk after pristane injection, XX mice had significantly higher levels of anti-dsDNA IgG antibody in sera than XY− mice. P < 0.01. XX, n = 9, white columns; XY−, n = 9, shaded columns; (NZBxNZW)F1 (+control), diagonal-lined columns; BALB/c (−control), vertical-lined columns. Histograms show the means and the SEM for mice in each group from one of three independent experiments. Female symbol with x overlay indicates ovariectomized female; male symbol with x overlay indicates castrated male.
Upon reaching death or terminal moribund state, mice were evaluated for kidney pathology. The representative photomicrograph of a kidney from an XX female demonstrates diffuse glomerular infiltration (GI), segmental glomerulosclerosis, a cellular crescent, tubular casts, and tubular atrophy, whereas the photomicrograph of a kidney from an XY− female shows only focal segmental proliferative nephritis and mild mesangial matrix deposition with relatively intact tubules (Fig. 4 C). Further, kidney lesions from pristane-injected XX and XY− mice were scored, ranked, and expressed as percent frequency of mice in each group. A greater percentage of XX mice showed more severe tubular disease scores (≥10; P = 0.006), chronic lesion scores (≥3; P = 0.046), and vascular lesion scores (P = 0.013) than their XY− littermates. In addition, a greater percentage of XX mice showed severe glomerular disease scores (≥10), but this effect did not reach significance (Fig. 4 D).
To assess possible sex chromosome complement effects on humoral autoimmunity, sera from ovariectomized female XX and XY− SJL mice injected with pristane were assessed for the level of anti-dsDNA antibodies. 16 wk after pristane injection, XX mice had significantly higher levels of anti-dsDNA IgG antibodies in sera than XY− mice (P < 0.01; Fig. 4 E). To determine whether the sex chromosome effect on autoantibody production was related to disease induction or was inherent in healthy SJL mice, levels of anti-dsDNA IgG antibodies were also determined in sera from age-matched XX and XY− mice injected with PBS. At 16 wk, antibody levels in XX and XY− mice injected with PBS were low and similar to levels from normal BALB/c-negative controls, and there was no difference between the groups in these low levels. Together, these studies demonstrated that the XX sex chromosome complement, as compared with the XY−, confers greater susceptibility to pristane-induced lupus.
Finally, we and others have previously reported that sex hormones and sex chromosomes have interactions, whereby their effects are synergistic in some organ systems and antagonistic in other organ systems (7, 13). Although the goal of this paper was to study the effect of sex chromosomes in a pure design that was not confounded by sex hormone effects, we nevertheless repeated the lupus experiment using gonadally intact mice. Similar to results in gonadectomized mice, differences were observed in survival when comparing gonadally intact XX versus XY− phenotypically female mice. Specifically, at 38 wk after disease induction, there was 27.3% survival in XX (n = 11), whereas there was 66.7% survival in XY− mice (n = 9; P = 0.05, Kaplan-Meier from week 28–38). Disease onset was relatively later in gonadally intact as compared with the gonadectomized mice, thus survival was no different between groups earlier in the course of disease (week 0–28). In gonadally intact XXSry versus XY−Sry phenotypically male mice, no significant mortality was observed in either group for the entire duration of observation. This was likely caused by the protective effect of endogenous testosterone (14–16).
Immune response differences in XX versus XY− mice
Because we had shown that the disease-promoting effect of the XX sex chromosome complement as compared with the XY− was present in both EAE and lupus, it suggested an effect of sex chromosomes on the immune system. Data of a sex chromosome complement effect on the induction phase of EAE using the adoptive transfer EAE model were consistent with this hypothesis. Although these data did not rule out additional sex chromosome effects on the target organs (i.e., brain or kidneys) of these two diseases, they did provide a rationale for assessing whether there were sex chromosome effects on the peripheral immune response. To this end, ovariectomized female XX and XY− SJL mice were immunized with PLP 139–151 and draining LNs were stimulated ex vivo with autoantigen and assessed for cytokine production. The Th2 cytokines IL-13 and -5 were significantly higher in XY− females than in XX females (P < 0.05; Fig. 5, top and middle). In addition, there was also a trend for higher production of the Th2 cytokine IL-10 in XY− mice than in XX mice, but this did not reach statistical significance (unpublished data). There were also trends for higher levels of Th1 cytokines, namely IFN-γ and TNFα, in XY− mice, but these also did not reach significance. There were no differences in IL-1β, -2, -6, -17, -23, -27, or TGFβ when comparing the two groups of mice with different sex chromosome complements. IL-4, -12p40, and -12p70 were at the lower limits of detection and were also no different between the two groups. There were also no significant differences in the proportions and numbers of the different cell subsets in the spleens of PLP-immunized SJL mice when comparing the two sex chromosome complements (Table S2, available at http://www.jem.org/cgi/content/full/jem.20070850/DC1).
Figure 5.
Effect of sex chromosome complement on Th2 cytokine levels. Ovariectomized female XX and XY− SJL mice were immunized with PLP 139–151, and after 10 d, draining LNCs were stimulated with PLP 139–151 and assessed for cytokine levels in supernatants. IL-13 (top) and IL-5 (middle) were significantly increased in XY− females compared with XX females. *, P < 0.05. XX, •, n = 4; XY−, ▴, n = 3. Ovariectomized female XX and XY− SJL mice were injected with pristane, and after 10 d, splenocytes were stimulated with anti-CD3ε/anti-CD28 and cultured for cytokine production. IL-4 levels in supernatant were significantly higher in XY− females as compared with XX females. *, P < 0.05. XX, •, n = 3; XY−, ▴, n = 3. All data are representative of at least three independent experiments. Graphs show the means and the SEM of mice in each group for each concentration. Female symbol with x overlay indicates ovariectomized female.
When ovariectomized female XX and XY− SJL mice were injected with pristane and splenocytes were cultured with anti-CD3 and -CD28 antibodies, results similar to those in PLP-immunized mice were observed. Namely, IL-13 and -5 were each higher in XY−compared with XX mice (unpublished data). Additionally, unlike PLP-immunized mice, IL-4 was detectable after pristane injection. XY− mice, as compared with XX, were found to have significantly higher levels of IL-4 as well (Fig. 5, bottom). No significant differences were observed in other cytokines as above or in spleen cell subsets from pristane-injected mice when comparing the two sex chromosome complements (Table S2).
As a control experiment, to assess whether in vivo disease induction was or was not a prerequisite to observing sex chromosome–related differences in the Th2 cytokines (IL-13, -5, and -4), ovariectomized female XX and XY− SJL mice were injected with saline, and splenocytes were assessed for cytokine production. In contrast to the results observed after PLP immunization and pristane injection, there were no differences observed in cytokine production in XX versus XY− SJL mice injected with saline (unpublished data). This demonstrated that in vivo disease activation was necessary to drive the Th2 cytokine differences observed when comparing immune responses in mice of the two sex chromosome complements.
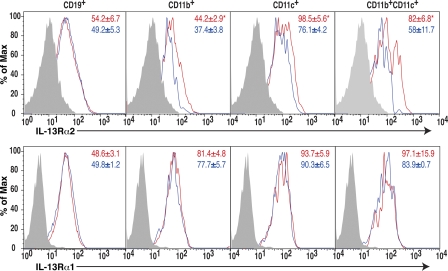
Finally, the IL-13Rα2 gene is on the X chromosome (17), thereby representing a candidate gene underlying immune differences between XX and XY− sex chromosome complements. IL-13Rα2 is also known to be a decoy receptor with the ability to limit Th2 responses (18–21). Because we had observed relatively lower Th2 responses in XX compared with XY− mice, we hypothesized that this could potentially be caused by increased expression of IL-13Rα2 in XX. Ovariectomized SJL XX mice and XY− mice were immunized with autoantigen (PLP 139–151) and expression of IL-13Rα2 and -13Rα1 was assessed on freshly isolated spleen cells. Both IL-13Rα2 and -13Rα1 were detected on dendritic cells, macrophages, and B cells in spleens of immunized animals (Fig. 6). Consistent with our hypothesis, IL-13Rα2 expression was higher on macrophages (CD11b+) and dendritic cells (CD11c+), particularly on the myeloid subset (CD11c+CD11b+) of XX mice as compared with these cells from XY− mice (Fig. 6 A). In contrast, IL-13Rα1 expression was no different between XX and XY− mice (Fig. 6 B). In summary, increased expression of the decoy IL-13Rα2 on spleen cells derived from XX, as compared with XY−, mice was consistent with lower levels of IL-13 and other Th2 cytokines in XX mice.
Figure 6.
Effect of sex chromosome complement on IL-13Rα2 expression. Freshly isolated splenocytes from PLP 139–151 immunized SJL mice of the XX or the XY− sex chromosome complement were analyzed for the expression of IL-13Rα2 (top) and IL-13Rα1 (bottom) on B cells (CD19+), macrophages (CD11b+), dendritic cells (CD11c+), and myeloid dendritic cells (CD11c+CD11b+) by flow cytometry. XX, thick red lines; XY−, blue lines; shaded area, negative control. The mean fluorescent intensities are indicated on FACS plots (red, XX; blue, XY−). IL-13Rα2 expression was significantly higher on macrophages, dendritic cells, and myeloid dendritic cells in XX mice than in XY− mice. *, P < 0.05; n = 3 each. Results represent two independent experiments.
DISCUSSION
The goal of this article was to use a uniquely informative model system to determine whether sex chromosome complement may contribute to the gender difference in susceptibility to EAE and lupus. Our data have indicated that the XX sex chromosome complement is disease promoting compared with the XY− complement. Has the possibility been ruled out that long-term effects of developmental hormones may have had an influence in our system? Regarding a potential effect of hormonal differences present before gonadectomy at 4 wk, the comparisons made in our manuscript are between XX versus XY− femice, with both groups having had ovaries during development. Analogously, comparisons between XXSry versus XY−Sry male mice are between groups that have both had testes during development. Moreover, our laboratory (7) and another (22) have assessed testosterone levels in 6–8-wk-old XXSry versus XY−Sry male mice, as well as in XX versus XY− female mice, and have found no difference between groups. Although it is impossible to state that hormones were equivalently secreted from XXSry versus XY−Sry testes, or from XX versus XY− ovaries, at every day of development, when phenotypic measures in the CNS that are known to undergo organizational effects mediated by gonadal hormones were previously compared between XXSry and XY−Sry males, as well as between XX and XY− females, no differences were found (23). Together, these previously published results provide strong evidence against a gonadal hormone difference between our comparisons of mice that differ in sex chromosome complement although they have the same gonadal type. Finally, the fact that differences similar to those observed when comparing XXSry versus XY−Sry in autoimmune disease outcomes in this article were observed when comparing XX versus XY−suggests that this difference in autoimmune disease outcomes did not require a particular history of a difference in ovarian or testicular secretions. Thus, we conclude that the female sex chromosome complement, as compared with the male sex chromosome complement, has a direct effect on promoting susceptibility to two distinct autoimmune diseases, which is unlikely to be mediated by indirect effects of differences in gonadal hormones.
In addressing what immune mechanisms might underlie the disease-promoting effect of the XX as compared with the XY− sex chromosome complement, we found clear differences in Th2 cytokine production, with higher levels from cells derived from XY− mice. These Th2 cytokines (IL-13, -4, and -10) have been previously associated with protection from disease in EAE (24–28). Therefore, increased Th2 cytokine production in XY− mice compared with that in XX mice could underlie the decreased EAE disease severity in XY− mice compared with XX mice. Further, although late stages of murine lupus characterized by renal fibrosis have been considered a Th2-mediated disease (29), initiation of events in the early phase of disease have been reduced by treatment with Th2 cytokines such as IL-4 and -10 (30–33). Consistently, in vivo blockade of IL-4 has been shown to result in increases in serum IgG anti-DNA antibody levels (29). Therefore, as in EAE, relatively higher levels of Th2 cytokines early during disease induction in XY− as compared with XX mice could underlie the decreased severity of pristane-induced lupus in XY− mice.
Our finding of increased IL-13Rα2 expression in spleen cells derived from XX mice, as compared with XY−, was particularly interesting because the IL-13Rα2 gene is on the X chromosome (17) and because not all X genes undergo X inactivation (34, 35). Thus, increased expression of IL-13Rα2 could be caused by an X dosage effect in XX as compared with XY−. Relatively higher expression of IL-13Rα2 in XX could in turn act as a decoy receptor to limit Th2 responses in the XX (18–21), which is consistent with lower levels of Th2 cytokines in XX as compared with XY−. Alternatively, cytokine differences could be the cause, not the effect, of differences in IL-13Rα2. This is however less likely because cytokines such as IL-13 and -10, which up-regulate IL-13Rα2 expression, were not increased in XX. Furthermore, expression of another IL-13 receptor, IL-13Rα1, was no different between XX and XY− mice. Such a selective increase in IL-13Rα2, but not IL-13Rα1, has been described in other models of immunoinflammatory diseases, such as in oxazolone-induced colitis and bleomycin-induced inflammatory disease (36–38). Ongoing experiments will further investigate the contribution of IL-13Rα2 in mediating the enhanced susceptibility of XX mice to increased autoimmune diseases in females.
Our data demonstrating an increase in IL-13Rα2 expression in cells derived from XX mice do not preclude the possibility of increased expression of other candidate genes on X. These other candidate genes are numerous and include CD40 ligand, FoxP3, and Toll-like receptor 7, to name a few. Consistent with a potential X dosage effect in our lupus model, XXY men and XX women have a similarly high risk of developing lupus, whereas the incidence of lupus in XO females is very low (39). Because these sex chromosome differences in humans are confounded by differences in sex hormones during both development and adulthood, one may never be able to distinguish between a sex chromosome effect versus a sex hormone effect in humans. In contrast, our experimental murine system offers distinct advantages for investigating direct effects of X and Y genes. Future studies using XO mice will determine whether the XX versus XY− differences observed in this study are caused by an X dosage effect or an effect of a gene on the Y chromosome.
Previous descriptions of an effect of a gene on a sex chromosome on lupus exist, but our findings are distinct from those results. It is well known that the BXSB strain develops lupus with a reversed sex bias, with males having accelerated disease. This was reported to be caused by a gene duplication event, whereby the Yaa region containing Toll-like receptor 7 was duplicated on the Y chromosome (40). Although this was an elegant demonstration of how gene duplication can localize to a sex chromosome to affect autoimmunity, our results are unique. Specifically, the previous study described a gene duplication event in the pseudoautosomal region, whereas our comparisons between XX versus XY− revealed effects from differences in the nonpseudoautosomal region because mice compared in this study had the same pseudoautosomal region (4). Second, lupus disease acceleration caused by Yaa on the Y chromosome in the BXSB strain is opposite from the known enhanced susceptibility of females to lupus in humans, whereas our finding of enhanced susceptibility in XX mice are consistent with the known female bias. Lastly, Yaa is disease accelerating in lupus, whereas it is protective in EAE (41). Thus, the Yaa effect is disease specific. In contrast, our finding of enhanced susceptibility in XX exists in both EAE and lupus. Thus, our data are the first to describe a sex chromosome effect that is consistent with the known female sex bias and is present across immunopathologically distinct autoimmune diseases.
MATERIALS AND METHODS
Mice.
SJL and C57BL/6 mice were obtained from The Jackson Laboratory. MF1 XY−Sry males (Y− chromosome of 129 origin) (6) were backcrossed with WT SJL females for over 16 generations to obtain litters consisting of the following genotypes: female XX, female XY−, male XXSry, and male XY−Sry (4). Animals were housed under guidelines set by the National Institutes of Health, and experiments were conducted in accordance with the University of California Los Angeles Chancellor's Animal Research Committee and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Gonadectomies.
Gonadectomies were performed at 4 wk of age, 8 wk before disease induction. Methods for ovariectomy and castration have been previously described (9).
EAE induction.
Active EAE induction ensued with s.c. injection of an emulsion containing the autoantigen PLP peptide, aa 139–151 (200 μg/mouse) for SJL mice, or MOG peptide, aa 35–55 (200 μg/mouse; Mimotopes) for C57BL/6 mice, in combination with Mycobacterium tuberculosis (200 μg/mouse) in complete Freund's adjuvant (CFA). SJL mice were immunized 1 time, on day 0, whereas C57BL/6 mice were immunized twice, on day 0 and 7. Pertussis toxin (500 ng/mouse; List Biological Laboratories) was injected i.p. on day 0 and 2. Mice were monitored daily for EAE disease severity and scored using the standard EAE grading scale, as previously described (5). For adoptive EAE, SJL mice were immunized with autoantigen PLP peptide, aa 139-151 (200 μg/mouse) in CFA. After 10 d, LNCs were harvested, cultured with autoantigen, and transferred to gonadally intact recipient mice, as previously described (5).
Neuropathology.
Thoracic spinal cord sections of mice with adoptively transferred EAE were coimmunostained with anti-CD45 (an immune cell marker; Millipore) and anti–β3-tubulin (a neuronal marker; Sigma-Aldrich) antibodies. Density of infiltrating CD45+ cells was assessed by ImageJ (http://rsb.info.nih.gov/ij/) in the entire delineated white matter (including dorsal, lateral, and ventral funiculi) and presented as the percentage of area, as previously described (42). A total of four T1-T5 sections were analyzed per mouse, with 3 mice in each group, yielding a total of 12 sections analyzed per group.
Induction of pristane-induced lupus.
Mice were injected i.p. once with 0.5 ml of pristane (2,6,10,14-tetramethyl-pentadecane; Sigma-Aldrich) at 12 wk of age. Mice were monitored daily for signs of disease (lethargy, ruffled fur, and distended abdomen) and survival rates were attained. In some cases, mice required euthanasia before natural death, caused by their moribund state. For survival graphs, death was assumed to occur 2 wk after euthanasia.
Assessment of nephritis.
Upon sacrifice, kidneys were collected for pathological studies. Kidney sections were stained with standard hematoxylin and eosin, Masson's trichrome, and periodic acid-Schiff. Sections were scored by two observers for active and chronic kidney lesions (glomerular cellularity and necrosis, glomerulosclerosis, interstitial infiltration, tubular atrophy, interstitial fibrosis, and vasculitis) as follows: glomerular activity was scored on a scale of 0–21; tubular disease was scored on a scale of 0–15; chronic lesions were scored on a scale of 0–18; and vascular (arterial or arteriolar) lesions were scored on a scale of 0–3, as previously described (29, 43).
Detection of autoantibodies.
16 wk after pristane injection, serum samples were assessed for anti-dsDNA IgG antibody by ELISA, as previously described (44). Values were expressed as attributed units per ml using a reference-positive standard of pooled serum.
Immune response.
Immunizations took place at least 8 wk after gonadectomy. For autoantigen-specific immune responses, PLP peptide 139–151 (American Peptide) emulsified in complete Freund's adjuvant was used during immunization. After 10 d, draining LNCs were cultured with 6, 12.5, or 25 μg/ml PLP peptide 139–151. For lupus immune response, mice were injected i.p. once with 0.5 ml of pristane (2,6,10,14-tetramethyl-pentadecane). After 10 d, splenocytes were cultured with either 0.2 μg/ml anti-CD3ε and anti-CD28 or 1 μg/ml anti-CD3ε and 2.5 μg/ml anti-CD28. Production of IL-13 and -10 cytokines in supernatants was determined at 12, 24, and 48 h using SearchLight Sample Testing Service (Thermo Fisher Scientific) or Mouse Inflammation Cytometric Bead Array kit according to manufacturer's instructions (BD Biosciences), as previously described (45).
Flow cytometry.
Biotinylated anti–mouse IL-13Rα2 antibody (R&D Systems) raised against the extracellular domain of the receptor was used to detect IL-13Rα2. Biotinylated IL-13α2 antibody was conjugated either with streptavidin FITC or streptavidin Cy5. IL-13Rα1 expression was detected using FITC-conjugated antibody H300 (Santa Cruz Biotechnology). The following antibodies were used to assess expression in each cell type: PE-conjugated anti-CD11b (M1/70), FITC- or PE-conjugated anti-CD11c (HL3), and FITC-conjugated antiCD19 (MB19-1). Isotype controls were used as negative controls. Unless otherwise indicated, all antibodies were purchased from (BD Biosciences).
Freshly isolated spleen cells from PLP 139–151 immunized mice were washed in FACS buffer and incubated with the above antibodies for 30 min on ice in the dark. Stained cells were acquired on FACSCalibur and analyzed with Cell Quest (BD Biosciences) or FlowJo (Tree Star, Inc.) software, as previously described (46, 47).
Statistics.
For EAE, mean clinical scores, calculated from all mice induced with EAE, were compared between groups (genotype) over all time points measured using the nonparametric Friedman test. Mean peak disease scores, calculated from all affected mice, were also compared between groups (genotype) using Student's t tests. For pristane-induced lupus, survival curves between groups (genotype) were computed using the Kaplan-Meier method and compared using the log-rank test. Fisher's exact test was used to compare renal scores. Antibody and cytokine levels were compared using GraphPad Prism software. Student's t tests were used if data followed a normal distribution; otherwise, the Mann-Whitney test was used. All graphs show the mean and SEM for mice in each group.
Online supplemental material.
Fig. S1 shows the breeding strategy for backcrossing MF1Y−Sry mice onto the SJL background to yield the informative four core genotypes to dissociate sex chromosome complement from gonadal sex. Table S1 demonstrates the genotypes of the phenotypic male and phenotypic female mice of the four core genotypes. Table S2 indicated the cellular composition of spleen cells in mice of the XX and XY− sex chromosome complement which were injected with either PLP or pristane. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20070850/DC1.
Supplementary Material
Acknowledgments
Thanks to Rebecca Watkins in the Arnold laboratory for assistance.
This investigation was supported by National Institutes of Health (NIH) grants AI50839 (R.R. Voskuhl), AI070306 (R.R. Voskuhl), NS043196 (A.P. Arnold), AR50797 (R.R. Singh) and AR47322 (R.R. Singh), and National Multiple Sclerosis Society grant CA1028 (R.R. Voskuhl). D.L. Smith-Bouvier was supported by an NIH T32 Training Grant. J. King was supported by an NIH T32 grant 1T32 AR053463. A. Divekar was supported by an NIH Re-entry award AR055057-01S1.
The authors have no conflicting financial interests.
Abbreviations used: CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; LNC, LN cell; MOG, myelin oligodendrocyte glycoprotein; PLP, proteolipid protein.
References
- 1.Whitacre, C.C., S.C. Reingold, and P.A. O'Looney. 1999. A gender gap in autoimmunity. Science. 283:1277–1278. [DOI] [PubMed] [Google Scholar]
- 2.Capel, B., K.H. Albrecht, L.L. Washburn, and E.M. Eicher. 1999. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech. Dev. 84:127–131. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, A.P. 2004. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 5:701–708. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, A.P., and P.S. Burgoyne. 2004. Are XX and XY brain cells intrinsically different? Trends Endocrinol. Metab. 15:6–11. [DOI] [PubMed] [Google Scholar]
- 5.Voskuhl, R.R., H. Pitchekian-Halabi, A. MacKenzie-Graham, H.F. McFarland, and C.S. Raine. 1996. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann. Neurol. 39:724–733. [DOI] [PubMed] [Google Scholar]
- 6.Mahadevaiah, S.K., T. Odorisio, D.J. Elliott, A. Rattigan, M. Szot, S.H. Laval, L.L. Washburn, J.R. McCarrey, B.M. Cattanach, R. Lovell-Badge, and P.S. Burgoyne. 1998. Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum. Mol. Genet. 7:715–727. [DOI] [PubMed] [Google Scholar]
- 7.Palaszynski, K.M., D.L. Smith, S. Kamrava, P.S. Burgoyne, A.P. Arnold, and R.R. Voskuhl. 2005. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 8:3280–3285. [DOI] [PubMed] [Google Scholar]
- 8.Okuda, Y., M. Okuda, and C.C. Bernard. 2002. Gender does not influence the susceptibility of C57BL/6 mice to develop chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Immunol. Lett. 81:25–29. [DOI] [PubMed] [Google Scholar]
- 9.Palaszynski, K.M., K.K. Loo, J.F. Ashouri, H. Liu, and R.R. Voskuhl. 2004. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 146:144–152. [DOI] [PubMed] [Google Scholar]
- 10.Papenfuss, T.L., C.J. Rogers, I. Gienapp, M. Yurrita, M. McClain, N. Damico, J. Valo, F. Song, and C.C. Whitacre. 2004. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J. Neuroimmunol. 150:59–69. [DOI] [PubMed] [Google Scholar]
- 11.Kantarci, O.H., A. Goris, D.D. Hebrink, S. Heggarty, S. Cunningham, I. Alloza, E.J. Atkinson, M. de Andrade, C.T. McMurray, C.A. Graham, et al. 2005. IFNG polymorphisms are associated with gender differences in susceptibility to multiple sclerosis. Genes Immun. 6:153–161. [DOI] [PubMed] [Google Scholar]
- 12.Smith, D.L., X. Dong, S. Du, M. Oh, R.R. Singh, and R.R. Voskuhl. 2007. A female preponderance for chemically induced lupus in SJL/J mice. Clin. Immunol. 122:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vries, G.J. 2004. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 145:1063–1068. [DOI] [PubMed] [Google Scholar]
- 14.Melez, K.A., W.A. Boegel, and A.D. Steinberg. 1980. Therapeutic studies in New Zealand mice. VII. Successful androgen treatment of NZB/NZW F1 females of different ages. Arthritis Rheum. 23:41–47. [DOI] [PubMed] [Google Scholar]
- 15.Roubinian, J.R., N. Talal, J.S. Greenspan, J.R. Goodman, and P.K. Siiteri. 1978. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J. Exp. Med. 147:1568–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg, A.D., K.A. Melez, E.S. Raveche, J.P. Reeves, W.A. Boegel, P.A. Smathers, J.D. Taurog, L. Weinlein, and M. Duvic. 1979. Approach to the study of the role of sex hormones in autoimmunity. Arthritis Rheum. 22:1170–1176. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson, D.D., M.J. Whitters, L.J. Fitz, T.Y. Neben, H. Finnerty, S.L. Henderson, R.M. O'Hara Jr., D.R. Beier, K.J. Turner, C.R. Wood, and M. Collins. 1998. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J. Immunol. 161:2317–2324. [PubMed] [Google Scholar]
- 18.Chiaramonte, M.G., M. Mentink-Kane, B.A. Jacobson, A.W. Cheever, M.J. Whitters, M.E. Goad, A. Wong, M. Collins, D.D. Donaldson, M.J. Grusby, and T.A. Wynn. 2003. Regulation and function of the interleukin 13 receptor α2 during a T helper cell type 2-dominant immune response. J. Exp. Med. 197:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie, A.N., and P.G. Fallon. 2003. Decoy receptors in the regulation of T helper cell type 2 responses. J. Exp. Med. 197:675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mentink-Kane, M.M., and T.A. Wynn. 2004. Opposing roles for IL-13 and IL-13 receptor alpha 2 in health and disease. Immunol. Rev. 202:191–202. [DOI] [PubMed] [Google Scholar]
- 21.Wood, N., M.J. Whitters, B.A. Jacobson, J. Witek, J.P. Sypek, M. Kasaian, M.J. Eppihimer, M. Unger, T. Tanaka, S.J. Goldman, et al. 2003. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor α2. J. Exp. Med. 197:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatewood, J.D., A. Wills, S. Shetty, J. Xu, A.P. Arnold, P.S. Burgoyne, and E.F. Rissman. 2006. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci. 26:2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vries, G.J., E.F. Rissman, R.B. Simerly, L.Y. Yang, E.M. Scordalakes, C.J. Auger, A. Swain, R. Lovell-Badge, P.S. Burgoyne, and A.P. Arnold. 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 22:9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettelli, E., M.P. Das, E.D. Howard, H.L. Weiner, R.A. Sobel, and V.K. Kuchroo. 1998. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 161:3299–3306. [PubMed] [Google Scholar]
- 25.Cash, E., A. Minty, P. Ferrara, D. Caput, D. Fradelizi, and O. Rott. 1994. Macrophage-inactivating IL-13 suppresses experimental autoimmune encephalomyelitis in rats. J. Immunol. 153:4258–4267. [PubMed] [Google Scholar]
- 26.Offner, H., S. Subramanian, C. Wang, M. Afentoulis, A.A. Vandenbark, J. Huan, and G.G. Burrows. 2005. Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J. Immunol. 175:4103–4111. [DOI] [PubMed] [Google Scholar]
- 27.Racke, M.K., A. Bonomo, D.E. Scott, B. Cannella, A. Levine, C.S. Raine, E.M. Shevach, and M. Röcken. 1994. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 180:1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young, D.A., L.D. Lowe, S.S. Booth, M.J. Whitters, L. Nicholson, V.K. Kuchroo, and M. Collins. 2000. IL-4, IL-10, IL-13, and TGF-beta from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J. Immunol. 164:3563–3572. [DOI] [PubMed] [Google Scholar]
- 29.Singh, R.R., V. Saxena, S. Zang, L. Li, F.D. Finkelman, D.P. Witte, and C.O. Jacob. 2003. Differential contribution of IL-4 and STAT6 vs STAT4 to the development of lupus nephritis. J. Immunol. 170:4818–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi, T., K. Hasegawa, Y. Sasaki, T. Mori, C. Adachi, and K. Maeda. 2007. Systemic administration of interleukin-4 expressing plasmid DNA delays the development of glomerulonephritis and prolongs survival in lupus-prone female NZB x NZW F1 mice. Nephrol. Dial. Transplant. 22:3131–3138. [DOI] [PubMed] [Google Scholar]
- 31.Santiago, M.L., L. Fossati, C. Jacquet, W. Muller, S. Izui, and L. Reininger. 1997. Interleukin-4 protects against a genetically linked lupus-like autoimmune syndrome. J. Exp. Med. 185:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theofilopoulos, A.N., S. Koundouris, D.H. Kono, and B.R. Lawson. 2001. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 3:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin, Z., G. Bahtiyar, N. Zhang, L. Liu, P. Zhu, M.E. Robert, J. McNiff, M.P. Madaio, and J. Craft. 2002. IL-10 regulates murine lupus. J. Immunol. 169:2148–2155. [DOI] [PubMed] [Google Scholar]
- 34.Disteche, C.M. 1995. Escape from X inactivation in human and mouse. Trends Genet. 11:17–22. [DOI] [PubMed] [Google Scholar]
- 35.Disteche, C.M. 1999. Escapees on the X chromosome. Proc. Natl. Acad. Sci. USA. 96:14180–14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fichtner-Feigl, S., W. Strober, K. Kawakami, R.K. Puri, and A. Kitani. 2006. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 12:99–106. [DOI] [PubMed] [Google Scholar]
- 37.Jakubzick, C., E.S. Choi, B.H. Joshi, M.P. Keane, S.L. Kunkel, R.K. Puri, and C.M. Hogaboam. 2003. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J. Immunol. 171:2684–2693. [DOI] [PubMed] [Google Scholar]
- 38.Matsushita, M., T. Yamamoto, and K. Nishioka. 2004. Upregulation of interleukin-13 and its receptor in a murine model of bleomycin-induced scleroderma. Int. Arch. Allergy Immunol. 135:348–356. [DOI] [PubMed] [Google Scholar]
- 39.Scofield, R., G. Bruner, J. Kelly, n. B., and J. Harley. 2004. Evidence from XXY, XX and XY for a gene dose effect from the X chromosome in human systematic lupus erythematosus. Lupus. 13:757. [Google Scholar]
- 40.Pisitkun, P., J.A. Deane, M.J. Difilippantonio, T. Tarasenko, A.B. Satterthwaite, and S. Bolland. 2006. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 312:1669–1672. [DOI] [PubMed] [Google Scholar]
- 41.Teuscher, C., R. Noubade, K. Spach, B. McElvany, J.Y. Bunn, P.D. Fillmore, J.F. Zachary, and E.P. Blankenhorn. 2006. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc. Natl. Acad. Sci. USA. 103:8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales, L.B., K.K. Loo, H.B. Liu, C. Peterson, S. Tiwari-Woodruff, and R.R. Voskuhl. 2006. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J. Neurosci. 26:6823–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, J.Q., A.K. Singh, M.T. Wilson, M. Satoh, A.K. Stanic, J.J. Park, S. Hong, S.D. Gadola, A. Mizutani, S.R. Kakumanu, et al. 2003. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J. Immunol. 171:2142–2153. [DOI] [PubMed] [Google Scholar]
- 44.Fan, G.C., and R.R. Singh. 2002. Vaccination with minigenes encoding VH-derived major histocompatibility complex class I–binding epitopes activates cytotoxic T cells that ablate autoantibody-producing B cells and inhibit lupus. J. Exp. Med. 196:731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari-Woodruff, S., L.B. Morales, R. Lee, and R.R. Voskuhl. 2007. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER){alpha } and ERbeta ligand treatment. Proc. Natl. Acad. Sci. USA. 104:14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soldan, S.S., A.I. Retuerto, N.L. Sicotte, and R.R. Voskuhl. 2003. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J. Immunol. 171:6267–6274. [DOI] [PubMed] [Google Scholar]
- 47.Yang, J.Q., X. Wen, H. Liu, G. Folayan, X. Dong, M. Zhou, L. Van Kaer, and R.R. Singh. 2007. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 56:1219–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.