Abstract
The lethality of pancreatic adenocarcinoma stems from an elevated incidence of tumor cell invasion and metastasis that are mediated by mechanisms not yet understood. Recent studies indicate that the proinvasive integrin α6β4 is highly upregulated in pancreatic adenocarcinomas. To assess the importance of this integrin in pancreatic cancer cell migration and invasion, cell lines were screened for integrin α6β4 expression by immunoblotting and fluorescence-activated cell sorting and their ability to migrate and invade toward hepatocyte growth factor (HGF). We found that cell surface expression of the α6β4 integrin correlated with the cells' ability to migrate and invade toward HGF. When cells expressing high levels of integrin α6β4 were treated with small interfering RNA targeting α6 or β4 integrin subunits, we observed a reduction in cell migration and invasion. Furthermore, the activity of the small GTPase Rac1 was stimulated by α6β4 integrin expression and was necessary for HGF-stimulated chemotaxis. We discovered that expression of the Rac-specific nucleotide exchange factor, Tiam1 (T-lymphoma invasion and metastasis), was upregulated in cells overexpressing the integrin α6β4 and required for the elevated Rac1 activity in these cells. We conclude that the integrin α6β4 promotes the migratory and invasive phenotype of pancreatic carcinoma cells through the Tiam1-Rac1 pathway in part through the upregulation of Tiam1.
Introduction
Pancreatic adenocarcinoma is a highly invasive and metastatic cancer that has the highest mortality rate of all cancers for reasons that are not yet understood [1]. Integrins are involved in multiple aspects of tumor invasion and metastasis, making them good candidates to elucidate the basic mechanisms involved in the metastatic process [2,3]. Our group as well as others has shown that the proinvasive and prometastatic integrin α6β4 is overexpressed in pancreatic adenocarcinomas [4–8] when compared to normal pancreas and chronic pancreatitis. Furthermore, this overexpression occurs at an early stage in pancreatic cancer progression [8]. The early and persistent upregulation of integrin α6β4 suggests that it could actively contribute to pancreatic cancer progression.
Integrins regulate many cellular functions such as cell adhesion to the extracellular matrix and transmission of molecular cues from the outside microenvironment that can influence cell shape, survival, proliferation, gene transcription, protein translation, cell migration and invasion, and tumor development [2]. Their two type I transmembrane α and β subunits can associate in different combinations leading to the formation of at least 25 receptors [9]. The α6β4 integrin is unique among integrins given that the β4 subunit has a cytoplasmic domain that is 1000 amino acids longer than other integrins and it can only associate with the α6 integrin subunit [10]. The α6β4 integrin is primarily expressed on the basal surface of most epithelia where it functions as an adhesion receptor to maintain epithelial structure and integrity through the anchoring of the epithelium to its underlying basement membrane through the formation of hemidesmosomes [11]. The α6β4 integrin is elevated in several types of carcinomas, with increased levels of expression correlating with a highly invasive and motile phenotype [12] as well as reduced patient survival [13]. However, the α6β4 integrin has been suggested to have both tumor suppressing and tumor promoting roles. In the presence of a wild-type p53, the α6β4 can promote apoptosis, but facilitates survival when p53 is mutant or absent [14]. Similarly, the suppressive effects of this integrin on tumor progression can be overcome by mutant K-Ras in a skin tumor model [15]. In squamous carcinomas, the α6β4 integrin has been shown to be released from the hemidesmosomes and associate with the actin cytoskeleton after epidermal growth factor stimulation in a process that is dependent on protein kinase C α-mediated phosphorylation of serine residues in the integrin β4 subunit [16]. Once released from hemidesmosomes, the α6β4 integrin can cooperate and amplify signaling from multiple growth factor receptors to promote various aspects of tumor progression including cell proliferation, migration, invasion, and metastasis [2,12]. The ability of this integrin to signal synergistically with growth factor receptors, such as ErbB-2, ErbB-3 [17,18], and c-Met [19], has been implicated with an invasive phenotype through the activation of signaling intermediates, such as phosphoinositol-3-kinase (PI3-K) [20], Akt [21], and Rac1 [22]. Rac1 is a member of the Rho family of GTPases that regulates actin polymerization leading to the formation of membrane ruffles and lamellae. Rac1 is critical for the migration of most cell types [23] and acts downstream of the PI3-K pathway [24].
The involvement of the α6β4 integrin in the invasive phenotype of multiple carcinomas and upregulation of this integrin in pancreatic adenocarcinomas prompted us to investigate in vitro how the α6β4 integrin may contribute to pancreatic cancer cell migration and invasion. Therefore, the goals of this study were 1) to ascertain if the integrin α6β4 contributes to the migration and invasion phenotype of pancreatic cancer cell lines and 2) to assess mechanisms governing the contributions of integrin α6β4 to the migratory and invasive phenotype.
Materials and Methods
Cell Culture and Antibodies
MiaPaCa-2, Panc-1 (from America Type Culture Collection, ATCC), and Panc-1 subclones were cultured in Dulbecco's modified Eagle's medium (high glucose) with 10% Fetal Plex (Gemini Bio-Products, West Sacramento, CA), 1% l-glutamine, 1% penicillin, and 1% streptomycin (GIBCO BRL, Gaithersburg, MD). Suit-2 (obtained from Dr. Takeshi Iwamura, Miyazaki Medical College, Miyazaki, Japan), ASPC-1, and BXPC-3 (obtained from ATCC) cells were cultured in RPMI 1640 containing 10% Fetal Plex serum plus 1% l-glutamine, 1% penicillin, and 1% streptomycin. Cells were harvested using 0.05% trypsin-EDTA and washed three times with serum-free medium containing 250 µg/ml heat-inactivated BSA (medium/BSA). The antibodies used include anti-β4 integrin subunit 505 rabbit polyclonal (Arthur Mercurio, University of Massachusetts, Worchester, MA), 439-9B rat monoclonal (Chemicon, Temecula, CA), GoH3 rat monoclonal anti-α6 integrin subunit (Santa Cruz Biotechnologies, Santa Cruz, CA), C-28 rabbit polyclonal anti-c-Met (Santa Cruz Biotechnologies), purified mouse monoclonal anti-Rac1 antibody (BD Biosciences, San Jose, CA), rabbit polyclonal anti-Tiam1 antibody (Bethyl Laboratories, Montgomery, TX), MAB1864 rat monoclonal anti-tubulin (Chemicon), and AC-15 mouse monoclonal anti-β-actin (Sigma Aldrich, St. Louis, MO).
Immunoblotting
Cells were lysed with RIPA buffer (150 mM NaCl, 0.5 mM EGTA, 0.1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 50 mM Tris-HCl, pH 7.4) containing 15 µg/ml protease inhibitor cocktail (Sigma Aldrich) and 1 mM PMSF, resolved on a polyacrylamide gel, transferred to a nitrocellulose membrane, blocked, and probed with the indicated primary antibodies. Membranes were washed, incubated with appropriate horseradish peroxidase-Cconjugated secondary antibody and developed using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnologies, Rockford, IL) or Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA).
Fluorescence-Activated Cell Sorting
Suspended cells were incubated with primary antibody or IgG only for 1 hour at 4°C and washed three times with 1x Dulbecco's PBS (pH 7.4) (Gibco, Carlsbad, CA) containing 250 µg/ml heat inactivated BSA (Dulbecco's PBS/BSA). Cells were then incubated with Cy2-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA) and analyzed on a FACS-Scan (Becton-Dickinson, Franklin Lakes, NJ). Relative fluorescence values from the IgG-only samples were subtracted from the samples treated with primary antibody.
Migration and Invasion Assays
The upper and bottom surface of Transwell membranes (6.5-mm diameter, 8-µm pore size; Corning, Corning, NY) were coated with 15 µg/ml of laminin-1 (Trevigen, Gaithersburg, MD) for 30 minutes at 37°C and then washed three times with medium/BSA for chemotaxis studies. For invasion assays, 7.3 µg of Collagen I (Vitrogen/PureCol, Leimuiden, the Netherlands) was diluted in cold water and dried onto the upper surface of each Transwell chamber membrane. Matrix was reconstituted with medium without serum for 1 hour before use. Hepatocyte growth factor (HGF, 50 ng/ml, unless otherwise indicated; Peprotech, Rocky Hill, NJ) diluted in medium/BSA or medium/BSA alone was added to the bottom chamber. Cells (50,000) were added to the upper chamber and allowed to migrate for 4 hours or invade for 6 hours at 37°C. Nonmigrating or noninvading cells were removed with a cotton swab from the top chamber. Cells remaining in the bottom were fixed with 100% methanol, stained with 1% crystal violet in 2% ethanol, and quantified visually from four random fields from each membrane using bright-field optics. Values for triplicate membranes are reported as a mean number of cells per millimeter squared ± the standard deviation of the mean.
RNA Extraction and Real-Time PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using 5 µg of RNA and the SuperScript III First-Strand Synthesis System (Invitrogen) as per the manufacturer's recommendations. Expression of T-lymphoma invasion and metastasis (Tiam1) was assessed by real-time PCR using available probes, reagents, and ABI7000 sequence detector as recommended by the manufacturer (Applied Biosystems, Foster City, CA). Real-time PCR was performed at the real-time PCR core facility, Sealy Center for Cancer Cell Biology, University of Texas Medical Branch. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems). The amounts of targets (2-ΔΔCT) were obtained by normalizing to endogenous reference (18S) and relative to a calibrator (one of the experimental samples).
Small Interfering RNA Electroporation
Suspended cells (3 x 106) were electroporated with or without 200 nM of the indicated small interfering RNA (siRNA, 300 V, 500 µF) with the Gene Pulser Xcell System (BioRad Laboratories, Hercules, CA) and then plated in normal growth medium. Cells were given fresh medium after 24 hours and harvested after 48 hours. Control nontargeting siRNA or siRNAs designed to target the integrin β4, integrin α6, Rac1, and Tiam1 were SMARTPools from Dharmacon (Chicago, IL). For rescue experiments, cells were electroporated as described above, and 48 hours later, cells were transfected with 10 µg of a human Tiam1 full-length construct (John Exton, Vanderbilt University, Nashville, TN) using 30 µl Lipofectamine 2000 (Invitrogen). The following day, cells were assayed for migration and invasion.
Clone Selection
The parental Panc-1 cells were sorted for the integrin β4 subunit by fluorescence-activated cell sorting (FACS) analysis (Becton-Dickinson, Franklin Lakes, NJ). Cell populations with high and low integrin β4 expression were isolated and further subcloned by dilution cloning. Individual clones of the sorted population including Panc-1/2G6 low integrin β4 expresser; Panc-1/3D7, and Panc-1/ZCM high integrin β4 expressers were further identified. To isolate the Panc-1/Δcyt clone, parental Panc-1 cells were transfected with a mutated β4 cDNA that lacked the cytoplasmic domain with the exception of four amino acids distal to the transmembrane sequence (β4-Δcyt [25]) (Arthur Mercurio, University of Massachusetts), using Lipofectamine 2000 (Invitrogen), selected with Genecitin (2 mg/ml) (G418; Invitrogen) and colonies were screened for expression of β4-Δcyt. Expression of integrin β4 in cell lines and Panc-1 subclones were confirmed by immunoblotting and FACS analyses.
Rac Activity Assays
Cells (1 x 106) were plated on laminin-1 (15 µg/ml, Trevigen)-coated plates in medium/BSA and allowed to attach and spread for 1 to 2 hours. Plated cells were then left untreated or treated with HGF (50 ng/ml) for 5 minutes, then harvested with Rac lysis buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 1% Nonidet P-40, 10% glycerol, 2 mM MgCl2, 15 µg/ml protease inhibitor cocktail, and 1 mM PMSF) and analyzed for Rac activity as previously described [26]. Aliquots of total cell extracts and the eluents from the PBD beads were analyzed for bound Rac1 molecules by Western blotting analysis.
Statistical Analysis
All experiments were repeated at least three times and data were reported as mean ± standard deviation. In each case, representative data are shown. The statistical significance of differences between groups was calculated using a two-tailed t test (P < .05 was taken as significant) with the Sigma Stat 2.03 software statistical program (Systat Software, Point Richmond, CA).
Results
Expression of Integrin α6β4 Correlates with the Ability of Pancreatic Cancer Cell Lines to Chemotax toward HGF
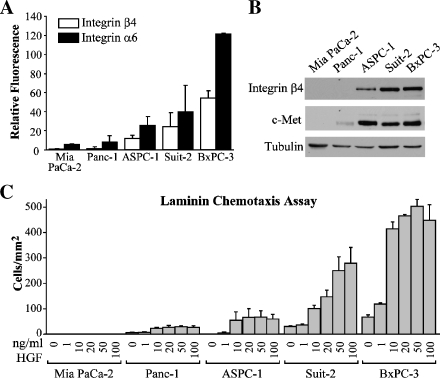
Integrin α6β4 expression is positively correlated with the invasive and migratory phenotype of several types of carcinomas including colon, thyroid, breast, gastric, bladder, and squamous carcinomas [12]. Due to the high tumor-dissemination rate and early overexpression of integrin α6β4 associated with pancreatic adenocarcinomas [8], we hypothesized that pancreatic cancer cell lines with increased levels of integrin α6β4 expression would migrate at a higher rate than cells with low levels of integrin α6β4. To test this hypothesis, we assessed the cell surface expression of both the integrin α6 and β4 subunits by FACS analysis in five pancreatic cancer cell lines and determined how this expression related to their migratory rate toward HGF. For these experiments, we used HGF as the chemoattractant because its receptor c-Met is known to cooperate with the integrin α6β4 to increase motility and invasion of cells in other tumor models [19,27] and is recognized as an important factor in pancreatic tumor progression [28]. As shown in Figure 1A, we found that MiaPaCa-2 and Panc-1 cells contained very low levels of cell surface α6β4 integrin, whereas ASPC-1, Suit-2, and BXPC-3 cells each had increasingly high levels. These results were mirrored to a lesser extent in the immunoblot analysis of total protein. Furthermore, all cell lines except the MiaPaCa2 cells express appreciable amount of c-Met (Figure 1B). Next, Transwell chemotaxis assays were performed. Here, we found that the expression level of integrin α6β4, rather than c-Met, correlated well with the ability of cells to chemotax toward HGF on laminin-1, a ligand of integrin α6β4 (Figure 1C). Moreover, increasing concentrations of HGF (from 0 to 100 ng/ml) resulted in a steady increase in motility of Suit-2 and BXPC-3 cells rather than the plateau observed with the less aggressive cell lines or bell-shaped curve characteristic of most chemoattractants. Overall, these results indicate that integrin α6β4 expression positively correlates with the ability of pancreatic cancer cells to chemotax toward HGF on a laminin substrate.
Figure 1.
Integrin β4 expression in pancreatic cancer cell lines correlates with chemotactic efficiency toward HGF. (A) Select pancreatic cell lines, as noted, were assessed for the α6 and β4 integrin subunit cell surface expression by FACS. Values represent average mean fluorescence and standard deviation of triplicate determinations. (B) Total cell lysates (80 µg) from pancreatic cancer cell lines were immunoblotted and probed for expression of integrin β4 subunit, c-Met, and tubulin (loading control). (C) Pancreatic cancer cell lines were assayed for chemotactic migration using a Transwell chamber assay toward varying concentrations of HGF as described in the Materials and Methods section. Values reported represent the mean number of cells migrated per millimeter squared ± standard deviation obtained from triplicate determinations.
Downregulation of α6β4 Integrin Expression Decreases HGF-stimulated Chemotactic Migration and Invasion of Pancreatic Cancer Cells
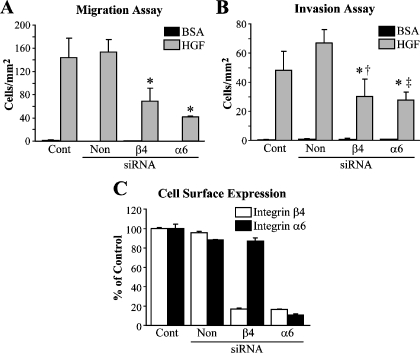
To directly assess the importance of α6β4 integrin in pancreatic cancer cell motility, we used synthetic siRNAs designed to target the α6 and β4 subunits to reduce expression of the α6β4 integrin in ASPC-1 cells. We then assessed the effectiveness of siRNA to downregulate protein expression by FACS as the plasma membrane-associated integrins are the species that are expected to function in cell signaling and adhesion events. After 48 hours of siRNA treatment against the α6 or β4 subunit, the cell surface expression level of the α6β4 integrin was decreased and resulted in reduced HGF-stimulated chemotactic migration and invasion (Figure 2). Similar results were obtained with Suit-2 cells (data not shown).
Figure 2.
Downregulation of integrin α6β4 decreases chemotactic migration and invasion of ASPC-1 cells toward HGF. ASPC-1 cells (3 x 106) were electroporated-only control (Cont) or electroporated with 200 nM siRNA specific for the integrin subunits β4 or α6, or a nontargeting control (Non) as indicated and analyzed 48 hours after siRNA treatment for chemotactic migration (A) (*P < .001 compared to Cont and Non) and invasion (B) (*P < .001 compared to Non; †P = .02 compared to Cont; ‡P = .004 compared to Cont) toward medium/BSA only or 50 ng/ml HGF. (C) Effects of siRNA treatment on integrin α6β4 cell surface expression determined by FACS as performed in Figure 1A. Values expressed as percent of electroporated-only controls.
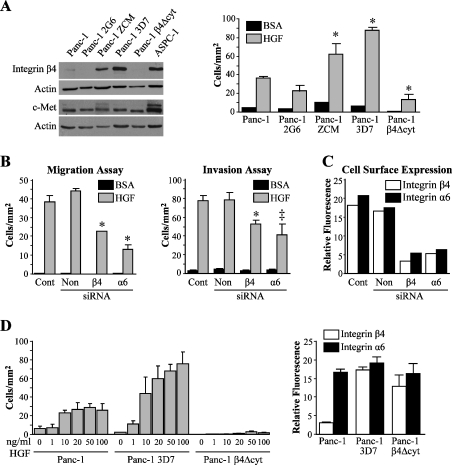
To further confirm the positive role of integrin α6β4 in the motile and invasive phenotype, we isolated clones from the parental Panc-1 cell line, which endogenously expresses low (Panc-1/2G6) or high levels (Panc-1/ZCM and Panc-1/3D7) of integrin α6β4 but have similar levels of c-Met (Figure 3A). We then assessed the ability of these cells to migrate on laminin-1 toward HGF and found that the levels of expression of the integrin α6β4 correlated with motility (Figure 3A, right panel). Next, we treated the Panc-1/3D7 cells with siRNAs targeting the α6 and β4 integrin subunits. We found that migration and invasion toward HGF were effectively downregulated by the reduction in α6β4 integrin expression (Figure 3, B and C) to a similar extent as seen with the ASPC-1 cells (Figure 2). To test the effects of signaling transduced from the integrin α6β4 cytoplasmic domain on motility, we stably transfected the Panc-1 parental cell line with cDNA containing a mutant β4, which lacked the cytoplasmic domain except for the four amino acids distal to the transmembrane sequence [25] (Panc-1/β4-Δcyt). This construct acts as a dominant negative [29]. Expression of this construct was determined by FACS (Figure 3, A and D, right panels) because the antibody used in this study for immunoblot analysis recognizes the cytoplasmic domain of integrin β4. As shown in Figure 3, A and D, cells expressing the β4-Δcyt construct had substantially slower migration rate toward HGF than other Panc-1 clones, whereas it had minimal effect on the overall cell surface level of the integrin. In addition, we show that the Panc-1/3D7 cells have a steady increase in motility with increasing concentration of HGF as seen with the Suit-2 and BXPC-3 cells (Figure 1C). Taken together, these results further confirm the involvement of the integrin α6β4 in the motile phenotype of pancreatic cancer cell lines.
Figure 3.
Integrin α6β4 expression is required for the migration and invasion of pancreatic cancer cell lines in vitro. (A) Total cell lysates (80 µg) from indicated Panc-1 cell lines were immunoblotted and probed for integrin β4 subunit, c-Met, and actin (loading control) expression (left panel). Pancreatic cancer cell lines were assayed for migration toward medium/BSA only or 50 ng/ml HGF (right panel). (*P < .001 compared to Panc-1). (B) Panc-1/3D7 cells (3 x 106) were treated with siRNA as described in Figure 2 and assayed for chemotactic migration (*P < .001 compared to Cont and Non) and invasion toward medium/BSA or 50 ng/ml HGF (*P = .002 compared to Cont and P = .006 compared to Non; †P < .001 compared to Cont and P = .002 compared to non). (C) Integrin α6β4 cell surface expression determined by FACS to confirmed downregulation levels from (B). (D) Panc-1, Panc-1/3D7 (high integrin β4 expression), and Panc-1 β4-Δcyt cells were assayed for migration toward increasing concentrations of HGF (left panel). The level of integrin α6β4 expression from these cells was confirmed by FACS (right panel).
Rac1 Activity Is Required for HGF-Stimulated Migration and Invasion of Pancreatic Cancer Cells
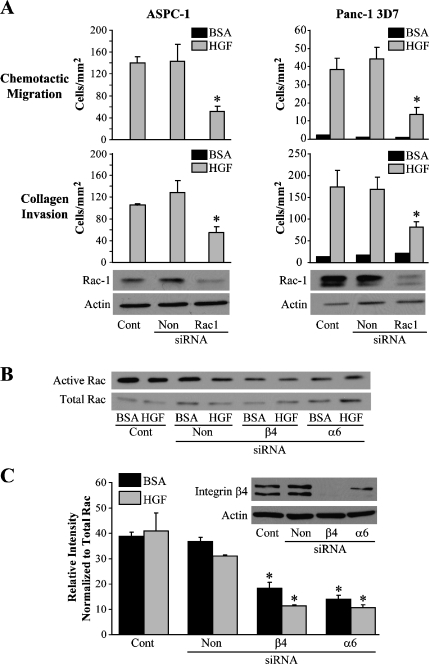
The integrin α6β4 cooperates with growth factor receptors and affects multiple signaling components that contribute to the increased migratory and invasive phenotype of cancer cells [2,3,12,18]. Among the molecules activated by the integrin α6β4 are PI3-K [20] and the small GTPase Rac1 [22]. Using PI3-K inhibitors wortmannin and LY294002, we determined that PI3-K activity is critical for the migration and invasion of the various pancreatic carcinoma cell lines toward HGF (Figure W1). We further found that HGF substantially induced the activation of PI3-K activation as revealed by the phosphorylation status of Akt and the p85 regulatory subunit of PI3-K. However, we were unable to link the α6β4 integrin to the activation of PI3-K through either integrin clustering (data not shown) or reduction of the integrin α6β4 expression by siRNA alone or in conjunction with HGF treatment (Figure W2). These observations suggest that the activation of PI3-K was solely dependent on HGF stimulation. Therefore, we turned our attention to Rac1. Rac1 can enhance tumor cell motility and invasion in culture [30] and is overexpressed in 70% of pancreatic cancer tumor samples [31]. To test the involvement of Rac1 in pancreatic cancer cell motility and invasion, ASPC-1 and Panc1/3D7 cells were treated with Rac1-specific siRNAs to downregulate the expression of Rac1 before assessing the cells' ability to migrate toward and invade HGF. We found that Rac1 siRNA treatment reduced the migration and invasiveness of both cell lines toward HGF (Figure 4A). Next, we assessed Rac1 activity using a PAK domain glutathione S-transferase pull-down assay after Panc-1/3D7 cells were treated with integrin α6 and β4 subunit siRNAs. For these experiments, cells were plated on laminin for 2 hours and then left untreated or treated with HGF for 5 minutes. Our results indicate that basal levels of Rac1 activity in the absence of HGF stimulation were high in these cells and therefore difficult to detect any increase of Rac activity after HGF stimulation (Figure 4B). However, upon treatment with α6 or β4 siRNAs, Rac1 activity was decreased under basal and HGF-stimulated conditions (Figure 4, B and C). Similar results were obtained with the ASPC-1 cell line (data not shown). Together these data suggest that Rac1 is required for motility and invasion of pancreatic cancer cells and that expression of the integrin α6β4 promotes activation of Rac1.
Figure 4.
Rac1 is required for the chemotactic migration and invasive potential toward HGF. ASPC-1 and Panc-1/3D7 cells (3 x 106) were electroporated only (Cont) or electroporated with 200 nM siRNA specific for Rac1 or a nontargeting control (Non) as indicated and analyzed 48 hours after siRNA treatment for chemotactic migration and invasion (A) toward medium/BSA or 50 ng/ml HGF; *P < .001 compared to Cont and Non. Immunoblot of cell lysates was used to confirm downregulation of Rac1 for the ASPC-1 cells (lower left) and Panc-1/3D7 cells (lower right). (B) Panc-1/3D7 cells (3 x 106) were electroporated only (Cont), treated with 200 nM siRNA specific for the integrin subunits β4 and α6 or a nontargeting control (Non) as indicated. After 48 hours of siRNA treatment, cells (3 x 106) were plated on laminin-1 and left untreated or treated for 5 minutes with 50 ng/ml HGF. Cell lysates were then analyzed for Rac1 activity using the PBD binding assay as described in the Materials and Methods section. (C) Densitometric analysis of three different exposures of immunoblots from (B) of activated (PAK-associated) Rac1. Data are reported as values for activated Rac1 divided by value for total cellular Rac1. *P < .001 compared to Cont and Non, BSA, and HGF. Inset shows immunoblot of integrin β4 to confirm target downregulation and actin as loading control. Data are representative of three independent experiments.
Tiam1 Is Upregulated by the Integrin α6β4 and Is Required to Activate Rac1 and Promote Migration and Invasion of Pancreatic Cancer Cells
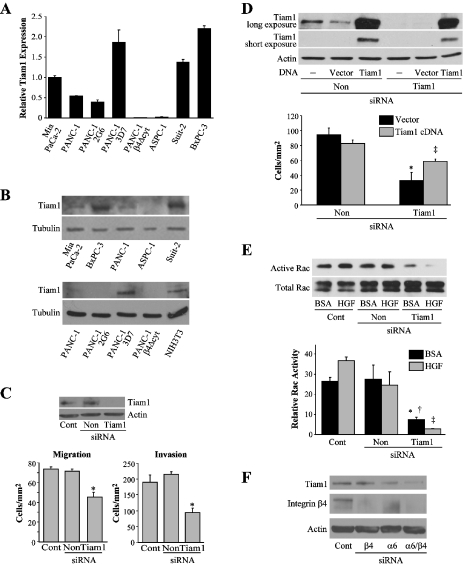
Having established a link between the expression of integrin α6β4 and Rac1 activation, we next sought to determine molecules that could connect them to promote increased motility and invasion. To gain insight into possible candidate molecules, we performed Affymetrix gene chip analysis on the Panc-1 clones. For this analysis, RNA from two low α6β4 expressing clones and two high α6β4 expressing clones were prepared and processed for Affymetrix gene chip analysis. The results were then processed using the statistical package S-Plus Array Analyzer (S-PLUS). Differential expression testing was determined using the local pooled-error test to determine genes differentially expressed as a consequence of α6β4 expression to a 99% confidence level. Among these results, we found that the high α6β4 integrin-expressing clones expressed significantly more Tiam1, a Rac-specific guanine nucleotide exchange factor (GEF) that has been implicated in cancer cell migration, invasion, and tumor progression [32,33]. To validate these results, we investigated the expression of Tiam1 in various pancreatic cancer cell lines. Using real-time PCR and immunoblot analysis, we found that Tiam1 was increased in three of the four pancreatic cancer cell lines (Panc-1/3D7, Suit-2, and BXPC-3) that overexpressed the α6β4 integrin. In addition, we discovered that the Panc-1/Δcyt cells, which are unable to signal through the β4 subunit, did not express Tiam1 (Figure 5, A and B).
Figure 5.
Tiam1 expression is required for Rac1 activity, migration, and invasion toward HGF of most pancreatic cancer cell lines that have high levels of integrin α6β4. (A) Pancreatic cancer cell lines were assessed for Tiam1 mRNA expression with real-time PCR. Data were normalized to 18S and reported relative to MiaPaCa2. (B) Lysates from the indicated cell lines were immunoblotted for Tiam1 (top panels) and tubulin as a loading control (bottom panels). (C) Panc-1/3D7 cells (3 x 106) were electroporated only (Cont), or electroporated with 200 nM siRNA that is nontargeting (Non) or specific for Tiam1, as indicated, and analyzed 48 hours after siRNA treatment for chemotactic migration on laminin-1 or collagen invasion toward 50 ng/ml of HGF; *P < .001 compared to Cont and Non-treated cells. Immunoblot of Tiam1 for siRNA target confirmation and actin as loading control (top panel). (D) Panc-1/3D7 cells were either treated with 200 nM siRNA specific for Tiam1 or with a nontargeting control (Non) as indicated. After 48 hours of siRNA treatment, cells were left untreated (-) or transfected with vector or a full-length Tiam1 construct. After 48 hours, cells were assessed for their ability to migrate toward HGF (bottom panel) as in (C). Immunoblot analysis demonstrates Tiam1 levels after Tiam1 transfection and siRNA ablation with actin as loading control (top panel). *P < .001 compared to Non/vector and Non/Tiam1 FL; ‡P = .001 compared to Tiam1/vector. (E) Panc-1/3D7 cells from (C) were assessed for Rac1 activity as performed in Figure 4C. Graph in (E) shows densitometry analysis from two different blot exposures; *P < .001 compared to Cont/BSA; †P = .003 compared to Non/BSA; ‡P < .001 compared to Cont and Non/HGF. Data are representative of three independent experiments. (F) Panc-1/3D7 cells (3 x 106) were electroporated only (Cont), treated with 200 nM siRNA specific for the integrin subunits β4 or α6, alone or in combination, as indicated. After 48 hours of siRNA treatment, cells (3 x 106) were lysed and probed for the presence of Tiam1, integrin β4, and actin.
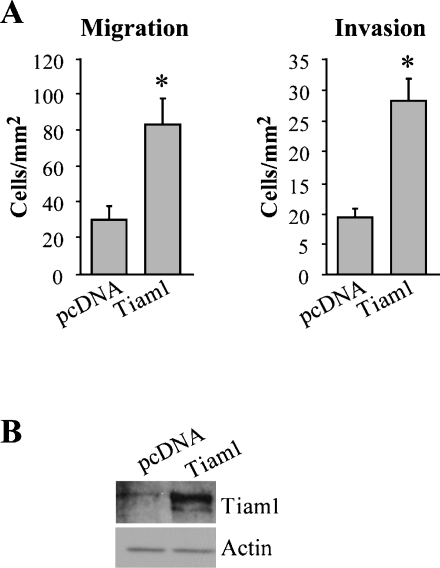
Next, we investigated if Tiam1 expression facilitated HGF-stimulated chemotactic migration and invasion and if Tiam1 was responsible for promoting Rac1 activity in cells with increased expression levels of integrin α6β4. After 48 hours of treatment with Tiam1-specific siRNAs, we found that Tiam1 siRNA reduced the migration and invasion of Panc-1/3D7 cells toward HGF (Figure 5C). Moreover, the migratory phenotype that was suppressed with Tiam1 siRNA treatment was rescued by the transfection of a full-length construct of Tiam1 (Figure 5D). Next, we investigated whether increased Tiam1 levels contributed to the high levels of Rac1 activity using siRNA. We found that Tiam1-specific siRNAs lead to a substantial decrease in Rac1 activity (Figure 5E), similar to that observed with α6 or β4 integrin-specific siRNAs (Figure 4). Additionally, cells treated with integrin β4 and α6 siRNAs, alone or in combination, displayed a decrease in Tiam1 expression (Figure 5F). Notably, the combination of α6β4 siRNAs was most efficacious in reducing Tiam1 expression. Therefore, we hypothesized that if the upregulation of Tiam1 was instrumental to the enhanced migration and invasion seen with high α6β4 integrin expression then the exogenous expression of Tiam1 would be able to mimic elevated levels of α6β4 integrin. For these experiments, we transiently transfected the low integrin β4 expressing Panc-1/2G6 clone with a full-length Tiam1 construct and assessed their migratory and invasive ability toward HGF. We noted that the exogenous Tiam1 expression increased the migration and invasion of the Panc-1/2G6 subclone compared to vector-transfected cells (Figure 6). Together, these data indicate that Tiam1 expression correlates well with α6β4 integrin expression and that this exchange factor promotes the activation of Rac1 as well as HGF-induced chemotactic migration and invasion of the majority of pancreatic cancer cells tested.
Figure 6.
Exogenous Tiam1 promotes migration and invasion of pancreatic cancer cells. (A) Panc-1 2G6 cells (low integrin β4 expression) were transiently transfected with a full-length construct of Tiam1 and then assessed for chemotactic migration on laminin-1 and collagen invasion toward 50 ng/ml of HGF. *P < .001 compared to pcDNA transfected cells. (B) Immunoblot of Tiam1 to confirm expression of full-length Tiam1 and actin as loading control. Transfection efficiency was approximately 60%.
Discussion
The integrin α6β4 is associated with carcinoma progression and subsequent metastasis through its ability to enhance motility and invasiveness of cancer cells [3]. However, the role of this integrin in pancreatic cancers has remained underexplored. We and others have shown that the integrin α6β4 is overexpressed in pancreatic cancers when compared to normal pancreas and chronic pancreatitis [4–8], which occurs early in pancreatic adenocarcinoma tumor progression [8]. In the current study, we extended our observations of the role of α6β4 integrin in pancreatic cancers to show that there is a good correlation of integrin α6β4 expression and the migratory potential of pancreatic carcinoma cells. Using siRNA technology to limit expression of the integrin α6 and β4 subunits, we establish that this promigratory and proinvasive integrin facilitates HGF-stimulated motility and invasion of pancreatic cancer cells in vitro. Moreover, our data support other studies demonstrating that signaling through the α6β4 integrin and c-Met cooperate to promote motility and invasion of cancer cells [19,27]. One study suggests that the full oncogenic potential of c-Met requires the α6β4 integrin during tumorigenesis [34]. We further demonstrate that α6β4 integrin expression is required to promote Rac1 activation through the upregulation of specific Rac GEFs.
Rac1 is a Rho family GTPase that enhances tumor cell migration and invasion in culture [30] and is activated downstream of integrin α6β4 to induce invasion of breast cancer cells that expressed integrin α6β4 [20,22]. Similarly, we report that Rac1 is required for pancreatic cancer cells to migrate and invade toward HGF and that expression of the integrin α6β4 promotes activation of Rac1. The activity of Rac is tightly regulated by GEFs and GTPase-activating proteins that transition GTPases between an inactive state (GDP-bound) and active state (GTP-bound). Several Rac GEFs have been shown to act downstream of β1 integrin signaling [35]; however, little is known about their association with the α6β4 integrin. In this study, we highlight a novel mechanism by which integrin α6β4 affects activation of Rac1 through the upregulation of the Rac1-specific GEF, Tiam1.
Tiam1 was originally identified as an invasion and metastasis-inducing gene by proviral tagging in combination with in vitro selection for invasiveness in T-lymphoma cells [33] and subsequently found to selectively activate the Rho family GTPase Rac [36]. The role of Tiam1 in cell migration, invasion, and metastasis is not limited to T lymphoma, as this exchange factor is involved in promoting tumor progression in other cancers such as colorectal, breast, and lung, as well as Ras-induced skin tumors [32]. In this study, we found that expression of Tiam1 was required for pancreatic cancer cells to activate Rac1 and correlated with HGF-induced migration and invasion in an integrin α6β4-dependent manner. We also demonstrated that the cytoplasmic tail of the integrin α6β4, known to be responsible for the signaling transduced from this integrin [19,20], is required for expression of Tiam1 because the Panc-1/Δcyt clone lacked expression of Tiam1. In cells that are less motile and invasive and contain low expression levels of both integrin α6β4 and Tiam1, exogenous expression of Tiam1 aided HGF-induced migration and invasion (Figure 6). Interestingly, we found that exogenous expression of Tiam1 did not increase the high migratory levels of cells with endogenous expression of integrin α6β4 and Tiam1 (Figure 5D). One reason for this observation may be due to a role of Tiam1 as a rate-limiting step during HGF-mediated migration but not basal migration. Alternatively, integrin α6β4 could also regulate other properties of Tiam1, such as its activity and/or localization, two mechanisms that are critical for the function of this protein to activate Rac. Certainly, the activation of Tiam1 downstream of the α6β4 integrin does not appear to require the PI3K pathway because PI3K is not activated in the absence of HGF stimulation and HGF does not promote the activation of Rac. These observations are in accordance with the fact that Tiam1 activation, unlike that of many other Rac GEFs, does not necessarily require PI3K [37]. Although the activation of Tiam1 downstream of HGF has recently been established in vascular endothelial permeability studies [38,39], our work highlights a previously unrecognized connection between Tiam1, the motility promoting functions of HGF/c-Met, and pancreatic cancer.
It is interesting to note that ASPC-1 cells express high levels of integrin α6β4 and have high Rac1 activity, but lack expression of Tiam1. These data imply that an additional exchange factor could be responsible for Rac1 activation by the integrin α6β4 to promote the migratory and invasive phenotype observed. One candidate is Vav1, as it was shown to play a role in pancreatic cancer tumorigenesis [40], but others such as Tiam2, Trio, Sos, and Pix could also be involved, as these GEFs are known to activate Rac [41,42]. One possibility for the discrepancy in Tiam1 expression could be the origin from which these pancreatic cancer cell lines were derived because the ASPC-1 cells were isolated from a metastasis to the ascites [43] and the Panc-1 cells were isolated from a primary tumor of the head of the pancreas [44]. In support of the idea that GEF expression could be cell type specific, in breast cancer cell lines GEFs other than Tiam1 are elevated by the α6β4 integrin to promote Rac activity (M. Chen and K. L. O'Connor, unpublished observation). The concept that the α6β4 integrin can affect similar changes, such as the activation of Rac, through the cell type-specific upregulation of specific factors is intriguing and may help to explain why the α6β4 integrin can promote the migratory and invasive phenotype of many types of cells. However, there remains much to be learned about the mechanisms of how integrin α6β4 affects migration and invasion of cancer cells, especially how the α6β4 integrin can affect GEF expression and its subsequent activation.
The genesis of activating mutations in K-Ras is one of the first events in pancreatic adenocarcinoma progression that occurs at a frequency of around 85% [45]. Interestingly, signaling induced from this type of oncogenic Ras has been reported to have differential effects on Tiam1 expression, which can, in turn, affect tumor development and progression. For example, the overexpression of Tiam1 in normal polarized epithelial cells has been shown to tighten cell-cell junctions thereby inhibiting HGF-mediated scattering [46]. This inhibition can be reversed by oncogenic Ras signaling, which promotes a decrease in Tiam1 expression [47]. However, effective initiation of chemically induced epidermal tumors by oncogenic Ras in vivo requires Tiam1 expression and Rac signaling [48]. Therefore, if oncogenic Ras signaling has the potential to downregulate Tiam1 expression, then other GEFs must be used to induce migration and invasion of tumor cells. Alternatively, other signaling pathways could be activated to increase Tiam1 and compensate for the Ras-induced Tiam1 loss. In the chemical induction model for epidermal tumors, for example, the integrin α6β4 is required for Ras to promote the formation of human squamous cell carcinomas [49]. Because our results show that integrin α6β4 can upregulate Tiam1 expression and this integrin is overexpressed at an early stage during pancreatic adenocarcinoma progression [8], we suggest that increased expression of integrin α6β4 could restore Tiam1 expression after its downregulation by oncogenic Ras signaling in tumors. Taken together, these observations suggest a possible mechanism involving the upregulation of Tiam1 and the subsequent activation of Rac for the cooperation between integrin α6β4 and Ras signaling in pancreatic adenocarcinomas that could contribute to its aggressive nature.
In summary, this study reports that integrin α6β4 cell surface expression in pancreatic cancer cells correlates with increased HGFinduced chemotactic motility. In addition, we show that integrin α6β4 expression is required to activate Rac1 and promote HGF-induced motility and invasion. This activation of Rac is mediated by the upregulation of the Rac-specific GEF, Tiam1. Our findings are consistent with the scenario that the α6β4 integrin cooperates with c-Met and further demonstrates that one level of cooperation lies within the Tiam1-Rac1 pathway. Our study in pancreatic cancer extends findings in other cancer systems that highlight the important contribution of the integrin α6β4 expression and downstream signaling events to induce tumor cell migration and invasion.
Supplementary Material
Acknowledgments
We gratefully acknowledge Arthur Mercurio, John H. Exton, and Takeshi Iwamura for reagents and Karen Martin for graphics assistance. We also thank B. Mark Evers, Sarita Sastry, Lisa Elferink, Adriana Paulucci, and Min Chen for helpful discussions as well as L. Nicole Towers and Huiping Guo for technical assistance.
Abbreviations
- FACS
fluorescence-activated cell sorting
- GEF
guanine nucleotide exchange factor
- HGF
hepatocyte growth factor
- PI3-K
phosphoinositol-3-kinase
- siRNA
small interfering RNA
- Tiam1
T-lymphoma invasion and metastasis
Footnotes
This study was supported by National Institutes of Health, National Cancer Institute, grants R21 CA102125 and R01 CA109136 (K.L.O.) and F31 CA106201 (Z.C.M.).
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 3.Lipscomb EA, Mercurio AM. Mobilization and activation of a signaling competent α6β4 integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev. 2005;24:413–423. doi: 10.1007/s10555-005-5133-4. [DOI] [PubMed] [Google Scholar]
- 4.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 5.Crnogorac-Jurcevic T, Missiaglia E, Blaveri E, Gangeswaran R, Jones M, Terris B, Costello E, Neoptolemos JP, Lemoine NR. Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol. 2003;201:63–74. doi: 10.1002/path.1418. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Furukawa Y, Nakagawa H, Tsunoda T, Ohigashi H, Murata K, Ishikawa O, Ohgaki K, Kashimura N, Miyamoto M, et al. Genome-wide cDNA microarray analysis of gene expression profiles in pancreatic cancers using populations of tumor cells and normal ductal epithelial cells selected for purity by laser microdissection. Oncogene. 2004;23:2385–2400. doi: 10.1038/sj.onc.1207392. [DOI] [PubMed] [Google Scholar]
- 7.Gleason B, Adley B, Rao MS, Diaz LK. Immunohistochemical detection of the β4 integrin subunit in pancreatic adenocarcinoma. J Histochem Cytochem. 2005;53:799–801. doi: 10.1369/jhc.4B6522.2005. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Monserrate Z, Qiu S, Evers BM, O'Connor KL. Upregulation and redistribution of integrin alpha6beta4 expression occurs at an early stage in pancreatic adenocarcinoma progression. Mod Pathol. 2007;20:656–667. doi: 10.1038/modpathol.3800782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 10.Hemler ME, Crouse C, Sonnenberg A. Association of the VLA alpha 6 subunit with a novel protein. A possible alternative to the common VLA beta 1 subunit on certain cell lines. J Biol Chem. 1989;264:6529–6535. [PubMed] [Google Scholar]
- 11.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 12.Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion—lessons from the α6β4 integrin. Semin Cancer Biol. 2001;11:129–141. doi: 10.1006/scbi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 13.Grossman HB, Lee C, Bromberg J, Liebert M. Expression of the α6β4 integrin provides prognostic information for bladder cancer. Oncol Rep. 2000;7:13–16. [PubMed] [Google Scholar]
- 14.Bachelder RE, Marchetti A, Falcioni R, Soddu S, Mercurio AM. p53 inhibits α6β4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Biol Chem. 1999;274:20733–20737. [Google Scholar]
- 15.Raymond K, Kreft M, Song JY, Janssen H, Sonnenberg A. Dual role of alpha6beta4 integrin in epidermal tumor growth: tumor-suppressive versus tumor-promoting function. Mol Biol Cell. 2007;18:4210–4221. doi: 10.1091/mbc.E06-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146:1147–1159. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between α6β4 integrin and ErbB2 receptor is required to promote phosphatidylinositol 3-kinase-dependent adhesion. J Biol Chem. 2000;275:10604–10610. doi: 10.1074/jbc.275.14.10604. [DOI] [PubMed] [Google Scholar]
- 18.Folgiero V, Bachelder RE, Bon G, Sacchi A, Falcioni R, Mercurio AM. The alpha6beta4 integrin can regulate ErbB-3 expression: implications for alpha6beta4 signaling and function. Cancer Res. 2007;67:1645–1652. doi: 10.1158/0008-5472.CAN-06-2980. [DOI] [PubMed] [Google Scholar]
- 19.Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LM, Rabinovitz I, Wang HH-F, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 21.Bachelder RE, Ribick MJ, Marchetti A, Falcioni R, Soddu S, Davis KR, Mercurio AM. p53 inhibits α6β4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J Cell Biol. 2003;163:1397–1407. doi: 10.1083/jcb.200302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz AR, Parsons JT. Cell migration—movin' on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Clarke AS, Lotz MM, Chao C, Mercurio AM. Activation of the p21 pathway of growth arrest and apoptosis by the β4 integrin cytoplasmic domain. J Biol Chem. 1995;270:22673–22676. doi: 10.1074/jbc.270.39.22673. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor KL, Mercurio AM. Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. J Biol Chem. 2001;276:47895–47900. doi: 10.1074/jbc.M107235200. [DOI] [PubMed] [Google Scholar]
- 27.Chung J, Yoon SO, Lipscomb EA, Mercurio AM. The Met receptor and α6β4 integrin can function independently to promote carcinoma invasion. J Biol Chem. 2004;279:32287–32293. doi: 10.1074/jbc.M403809200. [DOI] [PubMed] [Google Scholar]
- 28.Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- 29.Shaw LM, Chao C, Wewer UM, Mercurio AM. Function of the integrin α6β1 in metastatic breast carcinoma cells assessed by expression of a dominant-negative receptor. Cancer Res. 1996;56:959–963. [PubMed] [Google Scholar]
- 30.Keely JP, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3) K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 31.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, Jones M, Tyson K, Bassi C, Scarpa A, et al. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 32.Minard ME, Kim LS, Price JE, Gallick GE. The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res Treat. 2004;84:21–32. doi: 10.1023/B:BREA.0000018421.31632.e6. [DOI] [PubMed] [Google Scholar]
- 33.Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 34.Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res. 2005;65:10674–10679. doi: 10.1158/0008-5472.CAN-05-2827. [DOI] [PubMed] [Google Scholar]
- 35.Yron I, Deckert M, Reff ME, Munshi A, Schwartz MA, Altman A. Integrin-dependent tyrosine phosphorylation and growth regulation by Vav. Cell Adhes Commun. 1999;7:1–11. doi: 10.3109/15419069909034388. [DOI] [PubMed] [Google Scholar]
- 36.Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 37.Fleming IN, Gray A, Downes CP. Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem J. 2000;351:173–182. doi: 10.1042/0264-6021:3510173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 2007;21:2776–2786. doi: 10.1096/fj.06-7660com. [DOI] [PubMed] [Google Scholar]
- 39.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JGN. CD44 regulates hepatocyte growth factor-mediated vascular integrity: role of c-Met, Tiam1/Rac1, dynamin 2 and cortactin. J Biol Chem. 2007 doi: 10.1074/jbc.M702573200. Aug 16 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Chiu CY, Leng S, Martin KA, Kim E, Gorman S, Duhl DM. Cloning and characterization of T-cell lymphoma invasion and metastasis 2 (TIAM2), a novel guanine nucleotide exchange factor related to TIAM1. Genomics. 1999;61:66–73. doi: 10.1006/geno.1999.5936. [DOI] [PubMed] [Google Scholar]
- 42.Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, Giardina G, Di Fiore PP. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 2000;19:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen WH, Horoszewicz JS, Leong SS, Shimano T, Penetrante R, Sanders WH, Berjian R, Douglass HO, Martin EW, Chu TM. Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites. In Vitro. 1982;18:24–34. doi: 10.1007/BF02796382. [DOI] [PubMed] [Google Scholar]
- 44.Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975;15:741–747. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- 45.Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994;54:3568–3573. [PubMed] [Google Scholar]
- 46.Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 47.Zondag GCM, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–781. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 49.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.