Abstract
We determined whether phosphorylated epidermal growth factor receptor (EGFR) expressed on tumor-associated endothelial cells is a primary target for therapy with EGFR tyrosine kinase inhibitors (TKIs). Human colon cancer cells SW620CE2 (parental) that do not express EGFR or human epidermal growth factor receptor 2 (HER2) but express transforming growth factor α (TGF-α) were transduced with a lentivirus carrying nontargeting small hairpin RNA (shRNA) or TGF-α shRNA. The cell lines were implanted into the cecum of nude mice. Two weeks later, treatment began with saline, 4-[R]-phenethylamino-6-[hydroxyl] phenyl-7H-pyrrolo [2,3-d]-pyrimidine (PKI166), or irinotecan. Endothelial cells in parental and nontargeting shRNA tumors expressed phosphorylated EGFR. Therapy with PKI166 alone or with irinotecan produced apoptosis of these endothelial cells and necrosis of the EGFR-negative tumors. Endothelial cells in tumors that did not express TGF-α did not express EGFR, and these tumors were resistant to treatment with PKI166. The response of neoplasms to EGFR antagonists has been correlated with EGFR mutations, HER2 expression, Akt activation, and EGFR gene copy number. Our present data using colon cancer cells that do not express EGFR or HER2 suggest that the expression of TGF-α by tumor cells leading to the activation of EGFR in tumor-associated endothelial cells is a major determinant for the susceptibility of neoplasms to therapy by specific EGFR-TKI.
Introduction
The major cause of death from cancer is due to metastases that are resistant to conventional therapies. The genetic instability of tumor cells in general and metastatic cells in particular is responsible for generating biologic heterogeneity in metastatic lesions which is a major cause for the failure of systemic antitumor therapy [1,2]. Because the progressive growth and survival of all neoplasms are dependent on the development of an adequate vascular supply [3], targeting the tumor vasculature can be an effective approach for therapy for primary tumors in general and metastases in particular.
Growth factors and their receptors play a central role in the progressive growth of neoplasms. Overexpression of the epidermal growth factor receptor (EGFR) and its ligands, transforming growth factor α (TGF-α)/EGF by many cancers has been correlated with poor prognosis [4–6]. Colon cancer cells secrete TGF-α in response to hypoxia and the ligand signals, the cell surface EGFR, to initiate a sequence of cell survival programs [7]. This activation of the EGFR signaling pathways contributes to cell proliferation and survival by triggering downstream signaling molecules, such as Akt and mitogen-activated protein kinase [8–10]. The close association between coexpression of TGF-α/EGF and EGFR in tumor cells and stroma cells [11–13] with resistance to chemotherapy and hence poor survival has advanced EGFR as a logical target for therapy.
Small-molecule EGFR tyrosine kinase inhibitors (TKIs) have been studied in multiple clinical trials against relapsed non-small cell lung cancer. However, only a small percentage of the patients responded to EGFR antagonists given as a single agent [14–16]. Whether the sensitivity to EGFR-TKI is correlated to the expression level of EGFR on tumor cells has been controversial [17–19]. Several studies reported that the response to EGFR-TKI is associated with specific mutation in the tyrosine domain of EGFR [20–23] or with a high EGFR gene copy number [17,24]. Later studies, however, indicated that mutations in the tyrosine domain of EGFR were also found in nonresponding tumors [18,19], suggesting that the response to therapy may be due to other mechanisms.
We have recently reported that in multiple carcinomas, EGFR was phosphorylated not only on tumor cells but also on tumor-associated endothelial cells. The phosphorylation of EGFR on tumor-associated endothelial cells, however, was only found in the vasculature of tumors that produced TGF-α/EGF [25–27]. In nude mice implanted with human carcinoma cells (pancreas, colon, renal, prostate, ovarian) into the relevant orthotopic organs, treatment with specific EGFR-TKI produced apoptosis of tumor cells and tumor-associated endothelial cells [28]. On the basis of these findings, we hypothesized that a major determinant for neoplastic sensitivity to EGFR-TKI is the production of TGF-α/EGF by tumor cells and activation of EGFR on tumor-associated endothelial cells. To test this hypothesis, we used the SW620CE2 human colon cancer cells. These cells do not express EGFR or human epidermal growth factor receptor 2 (HER2) but do express TGF-α [29]. The cells were transduced with lentivirus small hairpin RNA (shRNA) or lentivirus TGF-α shRNA. The three different SW620CE lines were implanted into the cecal wall of nude mice, and 2 weeks later, treatment with a specific EGFR-TKI began. Only tumors producing TGF-α were sensitive to the therapy. Because none of the tumor cells expressed EGFR, the data identified the EGFR expressed by tumor-associated endothelial cells as the primary target.
Materials and Methods
Colon Cancer Cell Line and Culture Conditions
SW620 human colon cancer cells obtained from Dr. Gary Gallick, M. D. Anderson Cancer Center [29] were maintained in minimal essential medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, l-glutamine, a two-fold vitamin solution (Life Technologies, Grand Island, NY), and a penicillin/streptomycin mixture (Flow Laboratories, Rockville, MD). Adherent monolayer cultures were maintained on plastic and incubated at 37°C in a mixture of 5% CO2 and 95% air. The cultures were free of Mycoplasma and pathogenic murine viruses (assayed by Science Applications International Co., Frederick, MD) and were maintained for no longer than 12 weeks after recovery from frozen stocks.
In Vivo Selection of Highly Tumorigenic Variants from the SW620 Human Colon Cancer Cell Line
SW620 cells were injected into the cecal wall of nude mice. Three months after the injection, cecal tumors were harvested and treated with DNase and collagenase as described previously [30]. Cells were established in culture. Primary cultures were passaged in vitro two or three times, and then cells were harvested by trypsinization and were injected into the cecum of another set of nude mice. The selection cycle was repeated twice to yield the cell line designated as SW620CE2.
Small Hairpin RNA of TGF-α and Lentivirus Production
Sense and antisense oligonucleotides from the TGF-α mRNA (Accession No. NM-003236) was designed with a hairpin and sticky ends (ClaI and MluI) for use with the lentiviral system developed and kindly provided by Didier Trono, University of Geneva, Switzerland [31]. The target sequences for TGF-α shRNA were 5′-GCATGTGTCTGCCATTCTG-3′ and 5′-CAGAATGGCAGACACATGC-3′. The target sequences for nonspecific control shRNA (nontargeting shRNA) were 5′-TAAGGCTATGAAGAGATAC-3′ and 5′-GTATCTCTTCATAGCCTTA-3′. We then ligated these oligos and annealed them into the lentiviral gene transfer vector, pLVTHM, that drives the expression of the green fluorescent protein for independent monitoring of transfection/infection efficiencies, using the ClaI and MluI restriction enzyme sites. The lentivirus was then produced by transfecting human embryonic kidney cells (293FT) with the sequenced-verified pLVTHM vector, the packaging plasmid (MD2G), and envelope plasmid (PAX2) required for viral production. Three days later, the viral supernatant was collected and filtered to remove cellular debris. SW620CE2 cells were transduced with the lentivirus and green fluorescent protein-positive populations were enriched to 100% by fluorescence-activated cell sorting.
Reverse Transcription-Polymerase Chain Reaction
Total RNAs were isolated with RNeasy kit (Qiagen, Hilden, Germany), according to the manufacturer's recommended instructions, and cDNAs were synthesized from 1 µg of each total RNA preparation by use of oligo(dT) primers and reverse transcriptase (Reverse Transcription System; Promega, San Luis Obispo, CA). We prepared appropriate dilutions of each single-stranded cDNA for subsequent polymerase chain reaction (PCR) amplification and monitored the reactions by using β-actin (ACTB) as a quantitative control. The primer sequences were 5′-CATCCACGAAACTACCTTCAACT-3′ and 5′-TCTCCTTAGAGAGAAGTGGGGTG-3′, for ACTB; 5′-CTGGCTGTCCTTATCATCAC-3′ and 5′GACGGAGTTCTTGACAGAGT-3′ for TGF-α; 5′-TTTCGATACCCAGGACCAAGCCACAGCAGG-3′ and 5′AATATTCTTGCTGGATGCGTTTCTGTA-3′ for EGFR, and 5′-ACATCTTCCAGGAGTACCCTGATGAG-3′ and 5′GCATTCACATTTGTTGTGCTGT-3′ for vascular endothelial growth factor A (VEGFA); 5′-CATCACATCCACTGGTATT-3′ and 5′-GCCAAGCTTGTACCATGTG-3′ for vascular endothelial growth factor receptor 2 (VEGFR2). All reactions had an initial denaturation step at 94°C for 4 minutes, followed by 19 cycles (for ACTB), 28 cycles (for TGF-α), 33 cycles (for EGFR or VEGFA), or 35 cycles (for VEGFR2) at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. All reactions took place in a Mastercycler gradient 5331 PCR machine (Eppendorf AG, Cologne, Germany). The PCR products were separated by electrophoresis on 1.5%agarose gels and stained with ethidium bromide. The experiment was performed three times.
In Vitro Production of TGF-α
The production and secretion of TGF-α by human colon cancer cell lines (SW620CE2, SW620CE2 nontargeting shRNA, and SW620CE2 TGF-α shRNA) were determined 48 hours after plating 3 x 105 cells in 0.8 ml of serum-free medium (minimal essential medium) into six-well tissue culture plates. The supernatants of wells from each plate were collected and analyzed for the level of TGF-α using enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN). The concentration of TGF-α was standardized by cell number.
Western Blot Analysis
Adherent cells were washed with phosphate-buffered saline (PBS) containing 5 mM EDTA and 1 mM sodium orthovanadate and then scraped into lysis buffer (20 mM Tris-HCl [pH 8.0], 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 20 µM leupeptin, and aprotinin at 0.15 U/ml), and the mixture was incubated for 20 minutes on ice. The lysed cells were centrifuged at 16,000g for 15 minutes at 4°C, and the supernatant was collected. Proteins in the supernatant were quantified by spectrophotometry, and a constant amount of protein was loaded per lane, resolved by sodium dodecyl sulfate, 7.5% polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (pore size, 0.45 µm). The membranes were incubated with 5% milk in Tris-buffered saline (TBS, 20 mM Tris-HCl [pH 7.5] and 150 mM NaCl) to block nonspecific binding and were then probed with either a rabbit anti-human EGFR polyclonal antibody (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) or a rabbit anti-human β-actin (1:2000 dilution; Sigma, St. Louis, MO) in Tween-TBS (TTBS, 0.1% Tween 20 in TBS). Blots were then incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:2000 dilution; Sigma) in TTBS. Antibody-reactive protein bands were visualized with an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ).
Reagents
PKI166 (4-[R]-phenethylamino-6-[hydroxyl] phenyl-7H-pyrrolo [2,3-d]-pyrimidine), a novel and specific EGFR-TKI, was synthesized and provided by Novartis Pharma (Basel, Switzerland) [32]. For in vivo administration, PKI166 was dissolved in DMSO/0.5% Tween 80 and was then diluted 1:20 in water. Irinotecan (Camptozar; Pharmacia, Kalamazoo, MI) was kept at room temperature and dissolved in 0.9% NaCl on the day of intraperitoneal (i.p.) injection.
Primary antibodies used were as follows: rabbit anti-phosphorylated EGFR (pEGFR; Tyr1173; Biosource, Camarillo, CA); mouse anti-EGFR (Zymed, San Francisco, CA); mouse anti-TGF-α (Oncogene, Boston, MA) rabbit anti-EGF (Santa Cruz Biotechnology); rat anti-mouse CD31 (BD PharMingen, San Diego, CA); and rabbit anti-Ki-67 antigen (Vector Laboratories, Burlingame, CA) for immunohistochemistry, and rabbit anti-EGFR (SC03; Santa Cruz Biotechnology) for Western blot analysis. The following secondary antibodies were used for colorimetric immunohistochemistry: peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA); peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories); and peroxidase-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories). The following fluorescent secondary antibodies were used: Cy3-conjugated goat anti-rabbit IgG; Cy3-conjugated goat anti-mouse IgG; Cy3-conjugated goat anti-rat IgG; and Cy5-conjugated goat anti-rat IgG (all obtained from Jackson ImmunoResearch Laboratories). The following secondary antibodies were used for Western blot analysis: peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories). Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining was done using a commercial apoptosis detection kit (Promega, Madison, WI) with modifications.
Animals and Orthotopic Implantation of Tumor Cells
Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the US Department of Agriculture, the US Department of Health and Human Services, and the National Institutes of Health. The mice were used in accordance with institutional guidelines when they were 8 to 12 weeks old.
To produce cecal tumors, SW620CE2 WT, SW620CE2 nontargeting shRNA, and SW620CE2 TGF-α shRNA cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Trypsinization was stopped with medium containing 10% fetal bovine serum, and the cells were then washed once in serum-free medium and resuspended in Hanks' balanced salt solution. Only suspensions consisting of single cells with >90% viability were used. A total of 5 x 105 cells in 50 µl of Hanks' balanced salt solution were injected into the cecal wall of nude mice under a dissecting microscope as described previously [27].
Treatment of Established Human Colon Carcinomas Growing in the Cecum of Athymic Nude Mice
Fourteen days after injection of tumor cells when cecal tumors reached the size of 4 to 5 mm in diameter, groups of 10 mice each were randomly assigned to receive one of the following four treatments: 1) oral administration of water diluted at 1:20 with DMSO-0.5% Tween 80 (diluent) three times per week and i.p. injection of PBS once a week (control group); 2) oral administration of PKI166 (100 mg/kg) three times per week and i.p. injection of PBS once a week; 3) oral administration of diluent by three times per week and i.p. injection of irinotecan (10 mg/kg) once a week; and 4) combination of oral PKI166 (100 mg/kg) three times per week and i.p. injection of irinotecan (10 mg/kg) once a week. All treatments were carried out for 5 weeks.
Necropsy Procedures and Histologic Studies
The mice were euthanized by methoxyflurane, and their body weight was recorded. On necropsy, tumors growing in the cecum and peritoneum were excised and weighed. For immunohistochemical and hematoxylin and eosin staining procedures, one part of the tumor tissue was fixed in formalin and embedded in paraffin and another was embedded in optimal cutting temperature compound (Miles, Elkhart, IN), rapidly frozen in liquid nitrogen, and stored at -80°C. All macroscopically enlarged mesenteric lymph nodes were harvested, and the presence of metastatic disease was confirmed by histologic examination.
Immunohistochemical Staining for TGF-α and EGF
Paraffin-embedded tissues were used for immunohistochemical analyses of TGF-α and EGF. The sections were deparaffinized in xylene, dehydrated with alcohol, and rehydrated in PBS. Endogenous peroxidase was blocked with 3% hydrogen peroxide in PBS. The slides were placed in a humidified chamber and incubated with protein blocking solution (5% normal horse serum and 1% normal goat serum in PBS) for 20 minutes at room temperature and incubated overnight at 4°C with primary antibody against TGF-α (1:100) and EGF (1:100). For TGF-α staining, the slides were incubated overnight at 4°C with goat anti-mouse IgG, Fab fragment (Jackson ImmunoResearch Laboratories) to block endogenous immunoglobulins, followed by incubation with the primary antibody. Slides were washed with PBS three times, incubated with peroxidase-conjugated secondary antibody (1:500) for 1 hour, and then positive reaction was detected by exposure to stable 3,3′-diaminobenzidine (Phoenix Biotechnologies, Huntsville, AL). The slides were counterstained with Gill's no. 3 hematoxylin. Sections stained for immunoperoxidase or hematoxylin and eosin were examined in a fluorescence microscope (Microphot-FX; Nikon Instruments, Inc., Melville, NY) equipped with a three-chip charged coupled device color video camera (Model DXC990; Sony Corp., Tokyo, Japan). Digital images were captured using Optimas Image Analysis software (Media Cybernetics, Silver Spring, MD).
Double Immunofluorescence Staining for EGFR or pEGFR and CD31 in Tumor Tissues
Frozen sections of cecal tumors from nude mice were cut into 4-µm sections, mounted on positively charged slides, and stored at -80°C. Slides were fixed in cold acetone for 10 minutes, placed in a light-shielded humidified chamber, incubated with protein blocking solution (5% normal horse serum and 1% normal goat serum in PBS) for 20 minutes at room temperature, and incubated overnight at 4°C with primary antibody against EGFR (1:200) or pEGFR (1:100). For EGFR staining, the slides were incubated overnight at 4°C with goat anti-mouse IgG, Fab fragment (Jackson ImmunoResearch Laboratories) to block endogenous immunoglobulins, followed by incubation with the primary antibody. The slides were washed with PBS three times and then incubated for 1 hour at room temperature with goat anti-mouse or -rabbit Cy3 secondary antibody (1:500). Then, the slides were incubated overnight at 4°C with an antibody against CD31 (1:800). The slides were washed with PBS three times and then incubated for 1 hour at room temperature with goat anti-rat Cy5 secondary antibody (1:500). Nuclear counterstain with Sytox green was applied for 10 minutes, and a mounting medium (90% glycerol, 10% PBS, and 0.1 M propyl gallate) was placed on each sample, which were then covered with a glass coverslip (Fischer Scientific, Pittsburgh, PA). Endothelial cells (CD31- positive cells) were identified by green fluorescence, whereas EGFR- or pEGFR-positive cells were identified by red fluorescence. The presence of EGFR or pEGFR on endothelial cells was detected by colocalization of red and green fluorescence, which appeared yellow.
Immunohistochemical Determination of Ki-67 Antigen, CD31, and TUNEL
Paraffin-embedded tissues were used for immunohistochemical staining for Ki-67 as previously described [33]. Ki-67 labeling index (LI) was determined by light microscopy at the site of the greatest number of Ki-67-positive cells. The representative areas were determined by scanning tumor sections using low power (magnification, x40). For Ki-67 LI, the number of positive cells among approximately 1000 tumor cells was calculated as a percentage. Frozen tissues were used for quantifying mean vessel density (MVD). Frozen sections were fixed in cold acetone (10 minutes), and immunohistochemical procedures were done as described previously [13]. For the quantification of MVD, 10 random 0.159-mm2 fields at a magnification of x100 were captured, and CD31-positive cells were quantified according to a method described previously [12]. Analysis of apoptotic cells was done by using a commercially available TUNEL kit (Promega, Madison, WI). To quantify the apoptotic index, the TUNEL-positive cells were counted in 10 random 0.159-mm2 fields at a magnification of x100.
Double Immunofluorescence Staining for CD31 and TUNEL
Frozen sections of cecal tumors were used for assay. Specimens were cut into 4-µm sections, mounted on positively charged slides, and stored at -80°C. Slides were fixed in cold acetone for 10 minutes, placed in a light-shielded humidified chamber, incubated with protein blocking solution (5% normal horse serum and 1% normal goat serum in PBS) for 20 minutes at room temperature, and incubated overnight at 4°C with primary antibody against CD31 (1:800). The slides were washed with PBS three times and then incubated for 1 hour at room temperature with goat anti-rat Cy3 secondary antibody (1:500). Then, TUNEL assay was done by using a commercially available TUNEL kit. Nuclear counterstain with Sytox green was applied for 10 minutes, and slides were covered with a glass coverslip (Fischer Scientific) as described in the above paragraphs. TUNEL-positive apoptotic cells were detected by localized green fluorescence within the cell nuclei, and endothelial cells (CD31-positive cells) were identified by red fluorescence. Apoptotic endothelial cells were detected by colocalization of red and green fluorescence, which appeared yellow, within the nuclei. The total number of apoptotic cells was quantified in 10 randomly selected microscopic fields and expressed as the ratio of apoptotic endothelial cells to the total number of endothelial (magnification, x400).
Confocal Microscopy
Confocal fluorescence images were obtained by using x20 or x40 objectives on a laser scanning microscope (LSM 510; Carl Zeiss Inc., Thornwood, NY) equipped with a motorized Axioplan microscope, argon laser (458/477/488/514 nm, 30 mW), HeNe laser (543 nm, 1 mW and 633 nm, 5 mW), LSM 510 control and image acquisition software, and appropriate filters (Chroma Technology Corp., Brattleboro, VT). Confocal images were exported to Adobe Photoshop software, and montages were prepared.
Statistical Analysis
We used the Mann-Whitney U test to compare the body weight of mice, tumor weight, the number of Ki-67-positive cells, the MVD (CD31/PECAM-1), and the number of TUNEL-positive cells.
Results
Expression of TGF-α and EGFR in SW620CE2 Parent, SW620CE2 Nontargeting shRNA, and SW620CE2 TGF-α shRNA Human Colon Carcinoma Cells In Vitro
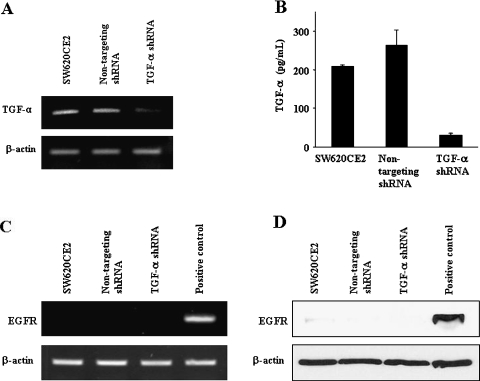
In the first set of experiments, we examined the expression of TGF-α in SW620CE2 parent, SW620CE2 nontargeting shRNA, and SW620CE2 TGF-α shRNA cells growing in culture by RTPCR and ELISA (Figure 1, A and B). SW620CE2 parent cells and SW620CE2 nontargeting shRNA cells expressed high levels of TGF-α. The expression of TGF-α by SW620CE2 TGF-α shRNA cells was reduced by more than 80%. Because immunohistochemistry as a single parameter may not determine absolute presence or absence of the EGFR on colon cancer cells [34], we also examined the in vitro expression of EGFR by RT-PCR and Western blot analysis (Figure 1, C and D). SW620CE2 parent, SW620CE2 nontargeting shRNA, and SW620CE2 TGF-α shRNA cells expressed minimal levels of EGFR protein or mRNA. HT29 human colon carcinoma cells [26,27] used as a positive control expressed high levels of EGFR. The SW620CE2 cells do not express the VEGFR2 but do express VEGFA. Transduction with nontargeting shRNA or TGF-α shRNA did not change these properties (Figure 1).
Figure 1.
The expression of TGF-α and EGFR by SW620CE2, SW620CE2 nontargeting shRNA, and SW620CE2 TGF-α shRNA cells growing in culture. RT-PCR (A), ELISA (B), and Western blot analysis (D) reveal that using the lentiviral system, the expression of TGF-α was decreased in the SW620CE2 TGF-α shRNA cells compared to the other cells, SW620CE2 WT and SW620CE2 nontargeting shRNA cells that expressed minimal levels of EGFR mRNA (C) and protein (D). HT29 human colon carcinoma cells [27] were used as the positive control for EGFR expression.
Treatment of SW620CE2 WT, SW620CE2 Nontargeting shRNA, or SW620CE2 TGF-α shRNA Human Colon Cancer Cells Growing in the Cecum of Nude Mice
In the next set of experiments, we determined the therapeutic effects of PKI166, irinotecan, or the combination of PKI166 and irinotecan, and the growth and metastasis of SW620CE2 WT, SW620CE2 nontargeting shRNA, or SW620CE2 TGF-α shRNA human colon cancer cells growing in the cecum of nude mice (orthotopic animal model). Tumor cells were injected into the cecal wall of nude mice. Treatment began 2 weeks later when the tumors were established. After 5 weeks of treatment, all mice were euthanized and necropsied. All three cell lines produced cecal tumors in all injected mice (Table 1), suggesting that autocrine-paracrine loops of TGF-α/EGFR are not required for tumor growth. None of the treatments significantly affected body weight.
Table 1.
Therapy for SW620CE2, SW620CE2 Nontargeting shRNA, and SW620CE2 TGF-α shRNA Tumors Growing in the Cecal Wall of Nude Mice.
| Body Weight (g), Median (Range) | Tumor Incidence | Tumor Weight (g), Median (Range) | Incidence of Lymph Node Metastasis | ||
| WT | Control | 30.7 (27.5–33.6) | 10/10 | 0.31 (0.14–0.46) | 7/10 |
| Irinotecan | 31.5 (28.2–35.2) | 10/10 | 0.20 (0.14–0.46)* | 4/10 | |
| PKI166 | 30.2 (28.2–35.2) | 10/10 | 0.15 (0.09–0.31)* | 3/10 | |
| PKI166/Irinotecan | 30.4 (27.5–33.9) | 10/10 | 0.10 (0.04–0.20)†,‡ | 0/10 | |
| Nontargeting shRNA | Control | 31.3 (27.1–35.3) | 9/9 | 0.29 (0.17–0.42) | 6/9 |
| Irinotecan | 32.0 (27.6–33.9) | 10/10 | 0.19 (0.13–0.25)* | 5/10 | |
| PKI166 | 31.5 (28.0–33.6 | 9/9 | 0.16 (0.08–0.28)* | 2/9 | |
| PKI166/Irinotecan | 30.4 (27.3–33.5) | 10/10 | 0.09 (0.04–0.19)†,‡ | 0/10 | |
| TGF-α | Control | 31.7 (27.8–33.4) | 9/9 | 0.24 (0.15–0.43) | 3/9 |
| Irinotecan | 31.5 (27.8–33.4) | 10/10 | 0.15 (0.07–0.28)* | 2/10 | |
| PKI166 | 30.4 (27.5–31.7) | 10/10 | 0.22 (0.13–0.35) | 3/10 | |
| PKI166/Irinotecan | 30.9 (27.5–32.7) | 10/10 | 0.16 (0.05–0.24)* | 2/10 |
P < .05, compared with control group.
P < .001, compared with control group.
P < .05, compared with irinotecan group.
In mice injected with SW620CE2 WT tumors, control mice had the largest tumors (median, 0.31 g; range, 0.14–0.46 g) (Table 1). Mesenteric lymph node metastasis was found in 7 of 10 mice. Treatment with only PKI166 significantly reduced the weight of cecal tumors (median, 0.15 g; range, 0.09–0.31 g; P < .05, compared with control). Three of 10 mice had lymph node metastasis. Treatment with only irinotecan also inhibited tumor growth (median, 0.20 g; range, 0.13–0.34 g; P < .05, compared with control). Lymph node metastasis was found in 4 of 10 mice. Treatment with oral administration of PKI166 and i.p. injection of irinotecan produced the most significant inhibition of cecal tumor (median, 0.10 g; range, 0.04–0.20 g; P < .001, compared with control and P < .05, compared with irinotecan alone) and completely inhibited metastasis to regional lymph nodes (P < .05, compared with control).
In mice injected with SW620CE2 nontargeting shRNA tumor cells (Table 1), control mice had the largest tumors (median, 0.29 g; range, 0.17–0.42 g), and 6 of 9 mice had metastasis in the regional lymph nodes. Oral administration of PKI166 significantly reduced the weight of the cecal tumors (median, 0.16 g; range, 0.08–0.28 g; P < .05, compared with control) and decreased the incidence of lymph node metastasis to 2 of 9 mice. Intraperitoneal injection of irinotecan also inhibited cecal tumor growth (median, 0.19 g; range, 0.13–0.25 g; P < .05, compared with control). Oral administration of PKI166 and i.p. injection of irinotecan produced the most significant inhibition of cecal tumor growth (median, 0.09 g; range, 0.04–0.19 g; P < .001, compared with control and P < .05, compared with irinotecan alone) and completely inhibited lymph node metastasis (P < .05, compared with control). The results obtained with the SW620CE nontargeting shRNA were therefore similar to that obtained with the SW620CE2 WT tumors.
In mice injected with SW620CE2 TGF-α shRNA tumor cells, the control group had the largest cecal tumors (median, 0.24 g; range, 0.15–0.43 g), and 3 of 9 mice had lymph node metastasis (Table 1). Oral administration of PKI166 did not produce significant changes in tumor weight (median, 0.22 g; range, 0.13–0.35 g). Treatment with irinotecan alone inhibited tumor growth (median, 0.15 g; range, 0.07–0.28 g; P < .05, compared with control). The weight of cecal tumors in mice treated with the combination of oral PKI166 and i.p. irinotecan was comparable to mice treated with only irinotecan (median, 0.16 g; range, 0.05–0.24 g; P < .001). The incidence of lymph node metastasis was higher in mice with SW620CE2 WT and SW620CE nontargeting shRNA than in mice with SW620CE2 TGF-α shRNA cecal tumors.
Immunohistochemical Analysis
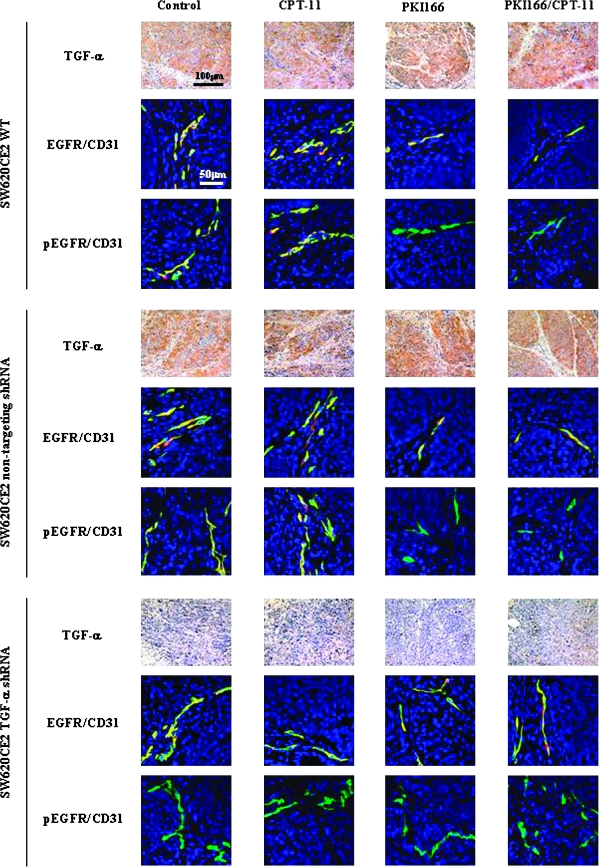
Next, we determined the expression of TGF-α, EGF, EGFR, and phosphorylated EGFR (pEGFR) in tumors by immunohistochemical analysis. SW620CE2 WT and SW620CE nontargeting shRNA tumors expressed high levels of TGF-α, whereas the SW620CE2 TGF-α shRNA tumor did not (Figure 2). Because the immunohistochemistry was carried out on cells transfected with the TGF-α shRNA at least 12 weeks before the assay, the absence of TGF-α expression verified the stability of the transfection. None of the tumors expressed EGF (data not shown).
Figure 2.
Immunohistochemical analysis of the expression of SW620CE2 WT, SW620CE2 nontargeting shRNA and SW620CE2 TGF-α shRNA cells growing in the cecum of nude mice. Human colon cancer cells were implanted into the cecum of nude mice. Two weeks later, treatment with saline (control), irinotecan (CPT-11; 10 mg/kg, i.p. injection, once per week), PKI166 (100 mg/kg, p.o., three times per week), or PKI166 and irinotecan began. Five weeks later, the mice were euthanized and autopsied. Cecal tumors were resected and fixed in buffered formalin or embedded in optimal cutting temperature compound for frozen sections. Paraffin-embedded tissues were processed for immunohistochemical analysis of TGF-α. SW620CE2 WT and SW620CE2 nontargeting shRNA tumor cells expressed TGF-α, whereas SW620CE2 TGF-α shRNA tumor cells did not. Treatment with irinotecan alone, PKI166 alone, or the combination of PKI166 and irinotecan did not alter the expression level of TGF-α. Frozen sections were used for double immunofluorescence staining for CD31 (green) and EGFR or pEGFR (red). Colocalization of CD31 and EGFR or pEGFR produces yellow fluorescence. EGFR and pEGFR were not expressed in any of the tumor cells. In contrast, most tumor-associated endothelial cells from all the groups expressed high level of EGFR. The phosphorylation of EGFR was diminished on tumor-associated endothelial cells from SW620CE2 WT and SW620CE2 nontargeting shRNA tumors from mice treated with PKI166 alone or PKI166 and irinotecan. In contrast, the EGFR on tumor-associated endothelial cells from any of the SW620CE2 TGF-α shRNA tumors were not phosphorylated.
Dual localization of CD31 (green) and EGFR (red) or pEGFR (red) confirmed that tumor cells in all three colon carcinoma groups did not express the EGFR (and hence pEGFR). In all groups, tumor-associated endothelial cells expressed the EGFR (yellow). In the SW620CE WT tumors and SW620CE nontargeting shRNA treated with PKI166 or PKI166 plus irinotecan, the EGFR was not phosphorylated. In the SW620CE2 TGF-α shRNA tumor, tumor-associated endothelial cells expressed EGFR (yellow) that was not phosphorylated (Figure 2).
Cell Proliferation (Ki-67), Apoptosis (TUNEL), and MVD in Cecal Tumors
Cell proliferation was evaluated by staining for Ki-67 (Table 2). In SW620CE2 WT tumors, the median number of Ki-67 LI of control group was 17 (range, 8–28). Treatment with irinotecan alone or PKI166 alone significantly decreased the number of Ki-67 LI (median, 11; range, 4–19 and median, 10; range, 4–20, respectively; P < .05, compared with control). Treatment with both PKI166 and irinotecan produced the most significant decrease in cell proliferation (median, 6; range, 3–15; P < .01, compared with control). In SW620CE2 nontargeting shRNA tumors, the median number of Ki-67 LI of control group was 19 (range, 10–32). Treatment with irinotecan alone or PKI166 alone significantly decreased the number of Ki-67 LI (median, 10; range, 7–21 and median, 11; range, 6–22, respectively; P < .05, compared with control). Treatment with both PKI166 and irinotecan produced the most significant decrease in cell proliferation (median, 7; range, 3–18; P < .01, compared with control). In SW620CE2 TGF-α shRNA tumors, the median number of Ki-67 LI of control group was 14 (range, 9–24). The treatment with irinotecan alone significantly decreased Ki-67 LI (median, 9; range, 4–18; P < .05, compared with control), whereas treatment with PKI166 alone did not. Treatment of mice with both PKI166 and irinotecan produced the same results as irinotecan administered alone (median, 8; range, 4–16; P < .05, compared with control).
Table 2.
Immunohistochemical Analysis of SW620CE2 Human Colon Cancer Cells Treated with TGF-α shRNA or Nontargeting shRNA Growing in the Cecal Wall of Nude Mice.
| Tumor Cells |
Endothelial Cells |
||||
| Ki-67 LI | TUNEL | MVD | TUNEL | ||
| WT | Control | 17 (8–28) | 1 (0–4) | 48 (28–73) | 0 (0–3) |
| Irinotecan | 11 (4–19)* | 6 (1–18)* | 43 (27–60) | 1 (0–6) | |
| PKI166 | 10 (4–20)* | 9 (1–22)* | 16 (7–25)† | 6 (0–11)* | |
| PKI166/Irinotecan | 6 (3–15)*,‡ | 15 (4–28)†,‡ | 11 (5–28)†,‡ | 8 (0–19)*,‡ | |
| Nontargeting shRNA | Control | 19 (10–32) | 1 (0–4) | 43 (28–70) | 0 (0–4) |
| Irinotecan 10 (7–21)* | 5 (1–16)* | 40 (22–72) | 1 (0–55) | ||
| PKI166 | 11 (6–22)* | 7 (2–19)* | 15 (8–32)† | 7 (0–18)* | |
| PKI166/Irinotecan | 7 (3–18)*,‡ | 17 (3–26)†,‡ | 12 (5–22)†,‡ | 8 (0–14)*,‡ | |
| TGF-α shRNA | Control | 14 (9–24) | 1 (0–3) | 39 (22–62) | 0 (0–4) |
| Irinotecan | 9 (4–18)* | 5 (1–14)* | 36 (21–59)* | 1 (0–5) | |
| PKI166 | 12 (7–25) | 1 (0–4) | 35 (18–49) | 1 (0–5) | |
| PKI166/Irinotecan | 8 (4–16)* | 6 (1–19)* | 33 (20–52)* | 2 (0–6) | |
P < .05, compared with control group.
P < .001, compared with control group.
P < .05, compared with irinotecan group.
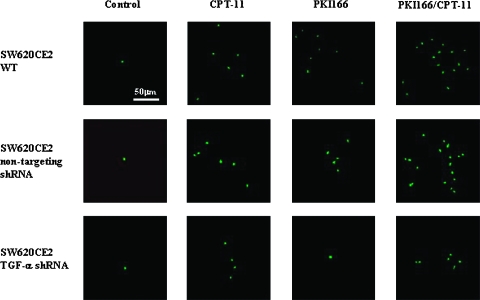
The induction of apoptosis in the tumors at the end of 5 weeks of treatment was determined by the TUNEL assay (Figure 3). In control mice injected with SW620CE2 WT cells, the median number of apoptotic tumor cells was 1 (range, 0–4). Treatment with irinotecan alone or PKI166 alone significantly increased the number of apoptotic tumor cells (median, 6; range, 1–18 and median, 9; range, 1–22, respectively; P < .05, compared with control). The most significant induction of apoptosis was observed in tumors in from mice treated with both PKI166 and irinotecan (median, 15; range, 4–28; P < .001, compared with control) (Table 2). In the SW620CE2 nontargeting shRNA tumors, the median number of apoptotic tumor cells in control treatment group was 1 (range, 0–4). Treatment with irinotecan alone or PKI166 alone increased the number of apoptotic tumor cells (median, 5; range, 1–16 and median, 7; range, 2–19; respectively; P < .05, compared with control). Similar to the SW620CE2 WT tumors, the most significant induction of apoptosis was produced by the combination treatment of PKI166 and irinotecan (median, 17; range, 3–26; P < .001, compared with control) (Table 2).
Figure 3.
Analysis of apoptosis of tumor cells and tumor-associated endothelial cells. Cecal tumors from different treatment groups were analyzed by immunohistochemistry for apoptosis (TUNEL). In control mice injected with SW620CE TGF-α shRNA cells, treatment with irinotecan (CPT-11) significantly increased the number of apoptotic cells. Note that in SW620CE and SW620CE nontargeting shRNA tumors, treatment with irinotecan or PKI166 increased the number of apoptotic cells. Treatment with both irinotecan and PKI166 produced additive effects. In the SW620CE TGF-α shRNA neither the CPT-11 nor the PKI166 produced significant apoptosis.
In control mice injected with SW620CE TGF-α shRNA cells and treated with saline, the median number of apoptotic tumor cells was 1 (range, 0–3). Treatment with irinotecan significantly increased the number of apoptotic tumor cells (median, 5; range, 1–14; P < .05, compared with control), whereas treatment with only PKI166 did not (Figure 3). Treatment with both PKI166 and irinotecan produced the same induction of apoptosis as irinotecan (median, 6; range, 1–19; P < .05, compared with control).
Microvessel Number and Apoptosis of Endothelial Cells in Cecal Tumors
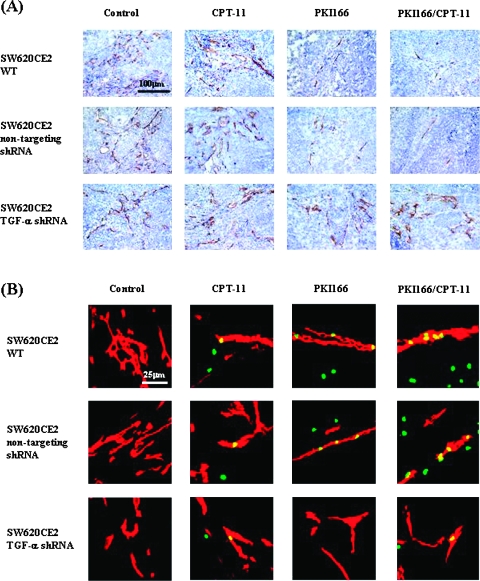
MVD was determined by staining for CD31 (Figure 4A; Table 2). In SW620CE2 WT tumors from mice treated with saline, the median number of MVD was 48 (range, 28–73). Treatment with irinotecan did not change the MVD. Treatment with PKI166 alone significantly decreased the number of microvessels (median, 16; range, 7–25; P < .001, compared with control). Treatment with both PKI166 and irinotecan also produced a significant decrease of vessels (median, 11; range, 5–28; P < .001, compared with control). In SW620CE2 nontargeting shRNA tumors from mice treated with saline (control), the median number of microvessels was 43 (range, 28–70). Treatment with irinotecan did not decrease the MVD. Treatment with PKI166 alone significantly decreased the number of MVD (median, 15; range, 8–32; P < .001, compared with control). Treatment with PKI166 and irinotecan also produced significant decrease in the MVD (median, 12; range, 5–22; P < .001, compared with control). In SW620CE2 TGF-α shRNA tumors from mice treated with saline, the median number of microvessels was 39 (range, 22–62). Treatment with irinotecan alone, PKI166 alone, or combination of PKI166 and irinotecan did not produce a significant decrease in the MVD (median, 36; range, 21–59; median, 35; range, 18–49; and median, 33; range, 20–52, respectively; P > .05, compared with control).
Figure 4.
Analysis for MVD. (A) MVD was determined by staining for CD31. In SW620CE2 WT and SW620CE2 nontargeting shRNA tumors, treatment with PKI166 alone and with PKI166 and irinotecan (CPT-11) significantly decreased the number of MVD. In SW620CE2 TGF-α shRNA tumors, treatment with PKI166 alone, irinotecan alone, or the combination of PKI166 and irinotecan did not significantly decreased the number of MVD. (B) Apoptosis of endothelial cells was determined by double staining for CD31 (red) and TUNEL (green). In cecal tumors produced by SW620CE2 WT and SW620CE2 nontargeting shRNA cells, treatment with PKI166 and irinotecan produced apoptosis in tumor-associated endothelial cells. None of the treatments induced apoptosis in tumor-associated endothelial cells of the SW620CE TGF-α tumors.
Apoptosis of endothelial cells was determined by double staining for CD31 (red) and TUNEL (green) (Figure 4B; Table 2). In SW620CE2 WT tumors from control mice, the median number of apoptotic endothelial cells was 0 (range, 0–3). Treatment with irinotecan did not produce apoptosis in endothelial cells. Treatment with PKI166 alone significantly increased the number of apoptotic endothelial cells (median, 6; range, 0–11; P < .05, compared with control).
Treatment with both PKI166 and irinotecan (median, 8; range, 0–19; P < .05, compared with control) also produced significant increase in apoptosis of tumor-associated endothelial cells. In SW620CE2 nontargeting shRNA tumors, the median number of apoptotic endothelial cells in control tumors was 0 (range, 0–4). Treatment with PKI166 alone significantly increased the number of apoptotic endothelial cells (median, 7; range, 0–18; P < .05, compared with control) also did the combination of PKI166 and irinotecan (median, 8; range, 0–14; P < .05, compared with control). In SW620CE2 TGF-α shRNA tumors from mice treated with saline (control), the median number of apoptotic endothelial cells was 0 (range, 0–4). Treatment with irinotecan alone, PKI166 alone, or the combination of PKI166 and irinotecan did not produce a significant increase in apoptosis of tumor-associated endothelial cells (median, 1; range, 0–5; median, 1; range, 0–5; and median, 2; range, 0–6, respectively; P > .05, compared with control).
Discussion
We here present compelling evidence to support the important role of paracrine activation of EGFR in tumor-associated endothelial cells in the colon for mediating response to EGFR kinase inhibitors. In the current study, we report that the systemic administration of the EGFR-TKI PKI166 to nude mice bearing the human SW620CE2 colon cancer leads to significant inhibition of cecal tumor growth and lymph node metastasis. The SW620CE2 cells do not express EGFR, HER2, or VEGFR but do express the EGFR ligands TGF-α/EGF. Colon tumors produced by SW620CE2 cells treated with TGF-α shRNA were resistant to PKI166. The expression of activated EGFR by tumor-associated endothelial cells is influenced by the production of TGF-α/EGF by adjacent tumor cells [25–27,35,36] and immunohistochemical analyses of the orthotopic colon tumors revealed that tumor-associated endothelial cells in SW620CE2 tumors (TGF-α+) expressed activated EGFR, whereas tumor-associated endothelial cells in SW620CE2 TGF-α shRNA (TGF-α-) did not. Therapy with PKI166 and irinotecan produced additive apoptosis of tumor-associated endothelial cells in the SW620CE2 cecal tumors (TGF-α+) but not in the SW620CE2 TGF-α shRNA (TGF-α-) cecal tumors. The apoptosis of tumor-associated endothelial cells was associated with a significant inhibition in cecal tumor growth and production of lymph node metastasis. Because neither set of tumors expressed EGFR or HER-2, the data clearly indicate that the susceptibility of the human colon cancer SW620CE2 to therapy by EGFR-TKI is determined by expression of ligand TGF-α/EGF and that the primary target for therapy with the EGFR-TKI is the tumor-associated endothelial cells.
The response of neoplasms to EGFR antagonists has been correlated with EGFR mutations, HER2 expression, Akt activation [21,23,24], and EGFR gene copy number [24,37]. Our present data using colon cancer cells that do not express EGFR, HER2, or VEGFR suggest that the expression of TGF-α/EGF by tumor cells leading to the activation of the EGFR in tumor-associated endothelial cells is a major determinant for response. These data agree with a previous report that human renal cancer that express TGF-α with activated EGFR in tumor-associated endothelial cells respond to treatment by PKI166 [35]. Recent studies report that pancreatic [26], colon [27], prostate [38], ovarian [39], and head and neck [40] neoplasms that express wild-type EGFR and TGF-α/EGF leading to activation of EGFR in tumor-associated endothelial cells respond to treatment with TKI. Moreover, retrospective analysis of a recent clinical trial of cetuximab (Erbitux, a chimeric monoclonal anti-EGFR antibody) showed that colorectal cancer patients with EGFR-negative tumors could respond to therapy [41]. These results have been confirmed in other clinical studies [42] and are also consistent with recent preclinical studies using cetuximab showing that the activity of the agent was unrelated to relative total or activated EGFR expression levels [43]. Collectively, these data recommend that predicting response of individual neoplasms to EGFR-TKI can be best accomplished by careful screening of biopsy specimen for expression of the ligand TGF-α/EGF and phosphorylated EGFR in tumor cells and especially in tumor-associated endothelial cells.
The progressive growth, survival, and metastasis of neoplasms depend on the development and maintenance of an adequate vascular supply [44]. Specifically, the survival of all cells in the body depends on an adequate supply of nutrients and oxygen and removal of waste products, i.e., vascular supply [3,45]. Because the genetic instability of neoplastic cells in general and metastatic cells in particular leads to the generation of biologic heterogeneity in neoplasms, targeting the neovasculature of neoplasms has been explored as an approach to therapy [1,46,47]. Antivascular therapy can destroy tumor cells that require nutrients and oxygen for survival. Endothelial cells in normal tissues rarely divide, whereas up to 2% to 5% of endothelial cells in neoplasms divide daily [48]. These dividing endothelial cells should be sensitive to anticycling drugs, such as irinotecan [3]. However, the major signaling pathways induced by activation of tyrosine kinase receptors are Akt and P13K, which can affect not only cell proliferation but also inhibition of apoptosis [49,50]. In our study, inhibition of EGFR activation on tumor-associated endothelial cells by PKI166 inhibited the dividing endothelial cells' resistance to irinotecan and therefore induced their apoptosis leading to a marked decrease in microvessel density, decreased proliferation of tumor cells, and increased apoptosis of tumor cells. These differences between dividing tumor-associated endothelial cells and quiescent endothelial cells in normal tissues [48] allow selective therapy with EGFR-TKI combined with chemotherapy. Our results may provide an explanation as to why suppression of proliferation of EGFR-positive tumor cells by EGFR targeting drugs—which should render the treated cancer less susceptible to chemotherapy drugs—can lead to increased chemosensitivity. If the primary targets are EGFR-positive tumor-associated endothelial cells, the proliferative status of the tumor cell population may be irrelevant to the effects of the chemotherapy obtained.
Many investigators undertaking clinical trials of EGFR-TKI have ignored the possibility that tumor-associated endothelial cells can be a major target of TKIs of the EGFR. Thus, based on our current findings, we suggest that the clinical use of TKI specific to EGFR will be more effective against neoplasms that express high levels of TGF-α/EGF. In these tumors, destruction of tumor-associated endothelial cells should lead to apoptosis of adjacent tumor cells and stromal cells leading to necrosis of primary neoplasms and metastases.
Acknowledgments
We thank Walter Pagel for critical editorial review and Lola López for expert assistance with the preparation of the manuscript.
Abbreviations
- EGF
epidermal growth factor
- EGFR
EGF receptor
- HER2
human epidermal growth factor receptor 2
- PKI166
4-[R]-phenethylamino-6-[hydroxyl] phenyl-7H-pyrrolo [2,3-d]-pyrimidine
- shRNA
small hairpin RNA
- TGF
transforming growth factor
- TKI
tyrosine kinase inhibitor
Footnotes
This work was supported in part by Cancer Center Support Core grant CA16672 and Specialized Programs of Research Excellence in Prostate Cancer grant CA902701 from the National Cancer Institute, National Institutes of Health.
References
- 1.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. (Review). [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2002;3:453–458. doi: 10.1038/nrc1098. (Timeline). [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 4.De Jong KP, Stelleman R, Karrenbeld A, Koudstaal J, Gouw AS, Sluiter WJ, Peters PM, Slooff MJ, DeVries EG. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology. 1998;28:971–979. doi: 10.1002/hep.510280411. [DOI] [PubMed] [Google Scholar]
- 5.Toschi L, Cappuzzo F. Understanding the new genetics of responsiveness to epidermal growth factor receptor tyrosine kinase inhibitors. Oncologist. 2007;12:211–220. doi: 10.1634/theoncologist.12-2-211. [DOI] [PubMed] [Google Scholar]
- 6.Veale D, Kerr N, Gibson GJ, Kelly PJ, Harris AL. The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long-term survival. Br J Cancer. 1993;68:162–165. doi: 10.1038/bjc.1993.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajput A, Korterba AP, Kreisberg JI, Foster JM, Willson JK, Brattain MG. A novel mechanism of resistance to epidermal growth factor receptor antagonism in vivo. Cancer Res. 2007;67:665–673. doi: 10.1158/0008-5472.CAN-06-2773. [DOI] [PubMed] [Google Scholar]
- 8.Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Romatico G, De Placido S, Bianco AR, Mendelsohn J, Tortora G. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000;6:3739–3747. [PubMed] [Google Scholar]
- 9.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58:903–913. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Ravindranath N, Wion D, Brachet P, Djakiew D. Epidermal growth factor modulates the expression of vascular endothelial growth factor in the human prostate. J Androl. 2001;22:432–443. [PubMed] [Google Scholar]
- 11.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 12.Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9:1200–1210. [PubMed] [Google Scholar]
- 13.Kim SJ, Uehara H, Yazici S, Busby JE, Nakamura T, He J, Maya M, Logothetis C, Mathew P, Kim SJ, et al. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. J Natl Cancer Inst. 2006;98:783–793. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Arteaga CL. Critical update and emerging Rtrends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 15.Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd FA, Rodriguez-Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smiley M, Martins R, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 17.Daniele L, Macri L, Schena M, Dongiovanni D, Bonello L, Armando E, Ciuffreda L, Bertetto O, Bussolati G, Sapino A. Predicting gefitinib responsiveness in lung cancer by fluorescence in situ hybridization/chromogenic in situ hybridization analysis of EGFR and HER2 in biopsy and cytology specimens. Mol Cancer Ther. 2007;6:1223–1229. doi: 10.1158/1535-7163.MCT-06-0719. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli F, Mezzetti A, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, Oh DY, Kim JH, Kim DW, Chung DH, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–2501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]
- 21.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 22.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 23.Tsao MS, Sakurada A, Cutz JC, Zhu C-Q, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Lagarde A, et al. Erlotinib in lung cancer: molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 24.Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Gregorc V, Toschi L, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol. 2005;23:5007–5018. doi: 10.1200/JCO.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 25.Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, Fidler IJ. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161:929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoi K, Kim SJ, Thaker PH, Yazici S, Nam DH, He J, Sasaki T, Chiao PJ, Sclabas GM, Abbruzzese JL, et al. Induction of apoptosis in tumorassociated endothelial cells and therapy of orthotopic human pancreatic carcinoma in nude mice. Neoplasia. 2005;7:696–704. doi: 10.1593/neo.05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoi K, Thaker PH, Yazici S, Rebhun RR, Nam DH, He J, Kim SJ, Abbruszzese JL, Hamilton SR, Fidler IJ. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res. 2005;65:3716–3725. doi: 10.1158/0008-5472.CAN-04-3700. [DOI] [PubMed] [Google Scholar]
- 28.Fidler IJ. Antivascular therapy of cancer metastasis. J Surg Oncol. 2006;94:178–180. doi: 10.1002/jso.20380. (Editorial Commentary). [DOI] [PubMed] [Google Scholar]
- 29.Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, Gallick GE. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinoma implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 31.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traxler P, Allegrini PR, Brandt R, Bruegen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J, et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 33.Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol. 2006;169:2054–2065. doi: 10.2353/ajpath.2006.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saltz L. Epidermal growth factor receptor-negative colorectal cancer: is there truly such an entity? Clin Colorectal Cancer. 2005;5:S98–S100. doi: 10.3816/ccc.2005.s.013. [DOI] [PubMed] [Google Scholar]
- 35.Baker CH, Pino MS, Fidler IJ. Phosphorylated epidermal growth factor receptor on tumor-associated endothelial cells in human renal cell carcinoma is a primary target for therapy by tyrosine kinase inhibitors. Neoplasia. 2006;8:470–476. doi: 10.1593/neo.06172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SJ, Uehara H, Yazici S, Langley RR, He J, Tsan R, Fan D, Killion JJ, Fidler IJ. Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res. 2004;64:4201–4208. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- 37.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59 doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Yazici S, Kim SJ, Busby JE, He J, Thaker P, Yokoi K, Fan D, Fidler IJ. Dual inhibition of the epidermal growth factor and vascular endothelial growth factor phosphorylation for antivascular therapy of human prostate cancer in the prostate of nude mice. Prostate. 2005;65:203–215. doi: 10.1002/pros.20283. [DOI] [PubMed] [Google Scholar]
- 39.Thaker PH, Yazici S, Nilsson MB, Yokoi K, Tsan RZ, He J, Kim SJ, Fidler IJ, Sood AK. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin Cancer Res. 2005;11:4923–4933. doi: 10.1158/1078-0432.CCR-04-2060. [DOI] [PubMed] [Google Scholar]
- 40.Yigitbasi OG, Younes MN, Doan D, Jasser SA, Schiff BA, Bucana CD, Bekele BN, Fidler IJ, Myers JN. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004;64:7977–7984. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- 41.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Shrag D, Schwartz L, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 42.Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP, Pruvot FR. Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer Drugs. 2006;17:855–857. doi: 10.1097/01.cad.0000217425.44584.9f. [DOI] [PubMed] [Google Scholar]
- 43.Wild R, Fager K, Flefleh C, Kan D, Camuso A, McGlinchey K, Rose WC. Cetuximab preclinical antitumor activity (monotherapy and combination based) is not predicted by relative total or activated epidermal growth factor receptor tumor expression levels. Mol Cancer Ther. 2006;5:104–113. doi: 10.1158/1535-7163.MCT-05-0259. [DOI] [PubMed] [Google Scholar]
- 44.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularization and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 45.Folkman J. What is the evidence that tumors are angiogenesis-dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 46.Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, Reinmuth N. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94–104. doi: 10.1016/s0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- 47.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 48.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin GH. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 49.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couple survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 50.Langley RR, Fan D, Tsan RZ, Rebhun R, He J, Kim SJ, Fidler IJ. Activation of the platelet-derived growth factor receptor enhances survival of murine bone endothelial cells. Cancer Res. 2004;64:3727–3730. doi: 10.1158/0008-5472.CAN-03-3863. [DOI] [PubMed] [Google Scholar]