Abstract
Mechanisms to compensate for dosage differences of genes on sex chromosomes are widespread in animals and have been thought to be critical for viability. However, in birds, compensation is inefficient, implying that for many genes dosage compensation is not critical, and for some genes, dosage differences have even been selected for.
In many species of animals, and even a few plants, males and females differ in their sex chromosomes. For example, male mammals have one X chromosome and a much smaller Y chromosome, while females have two X chromosomes. Birds have a different system: females have one Z chromsome and a much smaller W chromosome, and males have two Z chromosomes. Such heteromorphic sex chromosomes are a great embarrassment to proper gene regulation because they leave many genes present in a single dose in the heterogametic sex (XY male mammals and ZW female birds). This causes problems in maintaining a balance of expression between genes on the autosomes and those on the X or Z chromosome. Another problem that arises is that the dosage of genes on the sex chromosomes is different between the sexes. Mechanisms that compensate for these differences, so-called dosage-compensation mechanisms, evolved to balance gene expression between sex chromosomes and autosomes, and between males and females.
In organisms with XY male heterogamety, gene-dosage differences between the sexes are compensated by a variety of mechanisms. In Drosophila, the single X is upregulated in males but not females. In Caenorhabditis elegans, the X chromosome is upregulated in XO males and XX hermaphrodites, and then both X chromosomes are downregulated in hermaphrodites. In mammals, one of the two X chromosomes in females is rendered transcriptionally inactive, and expression from the single active X is up-regulated in both males and females to match expression from the autosomes [1]. Given the severe effects of monosomy (the presence of only one copy of a chromosome) of even the smallest human autosome, it has been thought that dosage compensation of the genes on the X chromosome is essential for survival.
Genes borne on the Z chromosome in ZW female:ZZ male systems of female heterogamety such as birds must, therefore, surely also be dosage compensated to avoid severe effects of monosomy for Z-borne genes in females, and a disruptive 2:1 dosage difference between the sexes. It was therefore puzzling to discover, in the 1970s, that the products of three genes on the bird Z chromsome are present in double the concentration in males than in females [2]. We expected that further studies at the transcriptional level would show that these genes were exceptional, and that most or all of the 841 protein-coding genes on the 74.6 Mb chicken Z chromosome (NCBI chicken build 2.1, November 2006) are dosage compensated. Indeed, a study using real-time PCR showed that six out of the nine chicken Z genes studied had male:female transcript ratios around 1, although both alleles were transcribed in males, presumably each at a lower level [3].
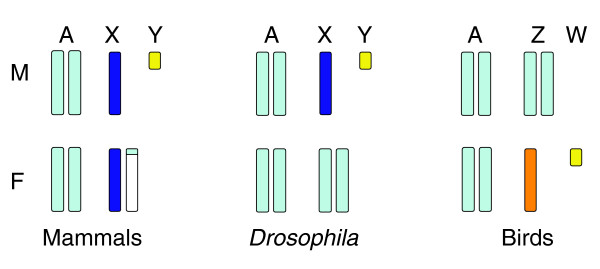
Nevertheless, a comprehensive study in two bird species of transcript ratios between Z chromosomes and autosomes, and males and females, now shows that most genes are not completely dosage compensated, at least at the transcriptional level. In this issue of Journal of Biology, a team from Art Arnold's laboratory [4] have investigated male:female expression ratios for more than 1,000 genes on chicken microarrays, backed up by custom arrays of more than 100 zebra finch genes. Using this approach, Itoh and Melamed et al. [4] evaluated global expression of genes on the Z chromosome versus autosomal genes in both sexes. Overall, they calculated male-to-female gene-expression ratios to be between 1 and 2, often showing a distribution that looks suspiciously bimodal, as would be expected if some genes were more-or-less compensated and others not. This contrasts with an average male-to-female expression ratio of 1 for human and mouse, determined on microarrays using the same techniques. Z chromosome:autosome ratios were balanced in male chickens, but were somewhat lower (around 80%) in females, consistent with partial dosage compensation by upregulation in the heterogametic sex. In the homogametic sex the data can be interpreted as complete absence of dosage compensation, or possibly the partial repression of each allele. The striking differences between mammals, Drosophila and birds in male:female expression ratios are illustrated in Figure 1.
Figure 1.
Regulation of gene expression on sex chromosomes relative to autosomes in males (M) and females (F) of mammals and Drosophila (XX female:XY male), and birds (ZW female:ZZ male). Basal autosome-equivalent expression level is in light blue; upregulated regions are represented in dark blue and inactivated regions in white. Genes on the two Z chromosomes of the male bird are presumed to have equal expression to the autosomes, but the single Z of female birds is only partially dosage compensated (orange shading represents a mixture of genes compensated to various degrees). The heterochromatic and largely inactive or specialized Y and W chromosomes are represented in yellow.
So it looks as if most genes on the bird Z chromosome are not fully dosage compensated, at least at the transcriptional level. How can this be? It is difficult to imagine that all these genes are independently regulated at some posttranscriptional level, but equally hard to imagine any global posttranscriptional control that affects Z-borne genes specifically. Besides, the tiny scrap of old isozyme data in birds shows that there is no dosage compensation at the protein level for the three genes studied [2].
Are dosage differences better tolerated in birds than mammals? There are a few clues that sex-chromosome dosage is handled differently. For instance, triploid human fetuses usually do not survive until birth, whereas triploid chickens can be obtained with ZZZ or ZZW (but never ZWW) sex-chromosome constitutions [5]. No diploid chickens with a sex chromosome complement ZZZ or Z0 have been observed, however, and it has been proposed that a locus on the W chromosome elicits at least partial up-regulation of the Z chromosome in ZW females [6].
There are two broad alternative explanations for the less efficient dosage compensation in birds. One is that dosage differences for many genes really are not as important as we have believed. Another is that differences in expression of Z-borne genes between males and females have been selected for in birds to control sex-specific characters.
Perhaps we should not be surprised to find that we have overrated the importance of dosage compensation. After all, some quite large deletions of human chromosomes have a phenotype equivalent to single gene defects. We have known since the 1970s that X-chromosome inactivation in marsupials is incomplete, at least in some tissues [7], and we now know that approximately 150 of the 1,000 genes on the human X are not completely silenced by X inactivation [8]. These escaping genes, expressed from both alleles (albeit at a reduced level from the inactive X) lie mostly on the part of the X that was added only during the last 100 million years [9], so may merely represent relics of the ancient autosomal region that was recently added to the X and Y. They are being slowly recruited into the X-chromosome inactivation system, but there appears to be no particular hurry, suggesting that their dosage inequality is not a huge problem that needs to be solved immediately.
Against this laissez faire argument, however, are arguments that the expression of escaper genes on the inactive X chromosome are maintained by specific mechanisms such as insulator elements [10]. This suggests that mechanisms have evolved to exploit differences in the expression of some genes between the sexes. There are no genes on the human X whose dosage-sensitive function is known to be required for sex determination or differentiation, but at least one gene on the marsupial X seems to have a dosage-sensitive role in mammary and scrotal differentiation [7].
Perhaps, then, the mammalian X chromosome is a mosaic of genes that must be dosage compensated, genes that must be differentially expressed in males and females, and genes for which dosage really does not matter. Genes that must be dosage compensated, because they lie in dosage-sensitive pathways, seeded domains that became subject to X inactivation, and recruited adjacent genes for which dosage differences were also deleterious or not critical, or were only weakly selected against. In contrast, genes for which a sex-differential role was selected, and thus which needed to avoid dosage compensation, established domains that are protected from inactivation. This process may have shaped the X chromosomes of different mammalian lineages independently; for instance, genes that escape X inactivation in humans do not necessarily escape in the mouse.
Is a similar process shaping the transcriptional activity profile of the bird Z chromosome? Is this chromosome, too, a mosaic of genes or regions showing equal expression in males and females (and equivalent expression to autosomes), and genes or regions that have assumed sex-specific roles? This would be consistent with data hinting that patterns of dosage compensation are different in different tissues (in human and mouse, as well as chicken and zebra finch). At least one gene on the bird Z chromosome, DMRT1, has a dosage-sensitive role in sex determination in humans, and its differential dosage in birds might be critical for sex determination.
Although the mammalian X and the bird Z chromosomes are genetically non-homologous, and their representation in the two sexes is reversed in these systems of male heterogamety and female heterogamety, respectively, and although the molecular mechanisms of dosage compensation may be different, the regulation of activity of the X and Z chromosomes seems to have been shaped by similar evolutionary forces.
References
- Cheng MK, Disteche CM. A balancing act between the X chromosome and the autosomes. J Biol. 2006;5:2. doi: 10.1186/jbiol32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baverstock PR, Adams M, Polkinghorne RW, Gelder M. A sex-linked enzyme in birds – Z-chromosome conservation but no dosage compensation. Nature. 1982;296:763–766. doi: 10.1038/296763a0. [DOI] [PubMed] [Google Scholar]
- McQueen HA, McBride D, Miele G, Bird AP, Clinton M. Dosage compensation in birds. Curr Biol. 2001;11:253–257. doi: 10.1016/S0960-9822(01)00070-7. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF, Schadt EE, Lusis AJ, Arnold AP. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6:3. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne MH, Sheldon BL. Triploid intersex and chimeric chickens: useful models for studies of avian sex determination. In: Reed K, Graves JAM, editor. Sex Chromosomes and Sex Determination Genes. Switzerland: Harwood Academic Publishers; 1993. pp. 201–208. [Google Scholar]
- Graves JA. Sex and death in birds: a model of dosage compensation that predicts lethality of sex chromosome aneuploids. Cytogenet Genome Res. 2003;101:278–282. doi: 10.1159/000074349. [DOI] [PubMed] [Google Scholar]
- Cooper DW, Johnston PG, Watson JM, Graves JAM. X-inactivation in marsupials and monotremes. Semin Dev Biol. 1993;4:117–128. doi: 10.1006/sedb.1993.1014. [DOI] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Graves JAM. The origin and function of the mammalian Y chromosome and Y-borne genes – an evolving understanding. BioEssays. 1995;17:311–320. doi: 10.1002/bies.950170407. [DOI] [PubMed] [Google Scholar]
- Filippova GN, Cheng MK, Moore JM, Truong JP, Hu YJ, Nguyen DK, Tsuchiya KD, Disteche CM. Boundaries between chromosomal domains of X inactivation and escape bind CTCF and lack CpG methylation during early development. Dev Cell. 2005;8:31–42. doi: 10.1016/j.devcel.2004.10.018. [DOI] [PubMed] [Google Scholar]