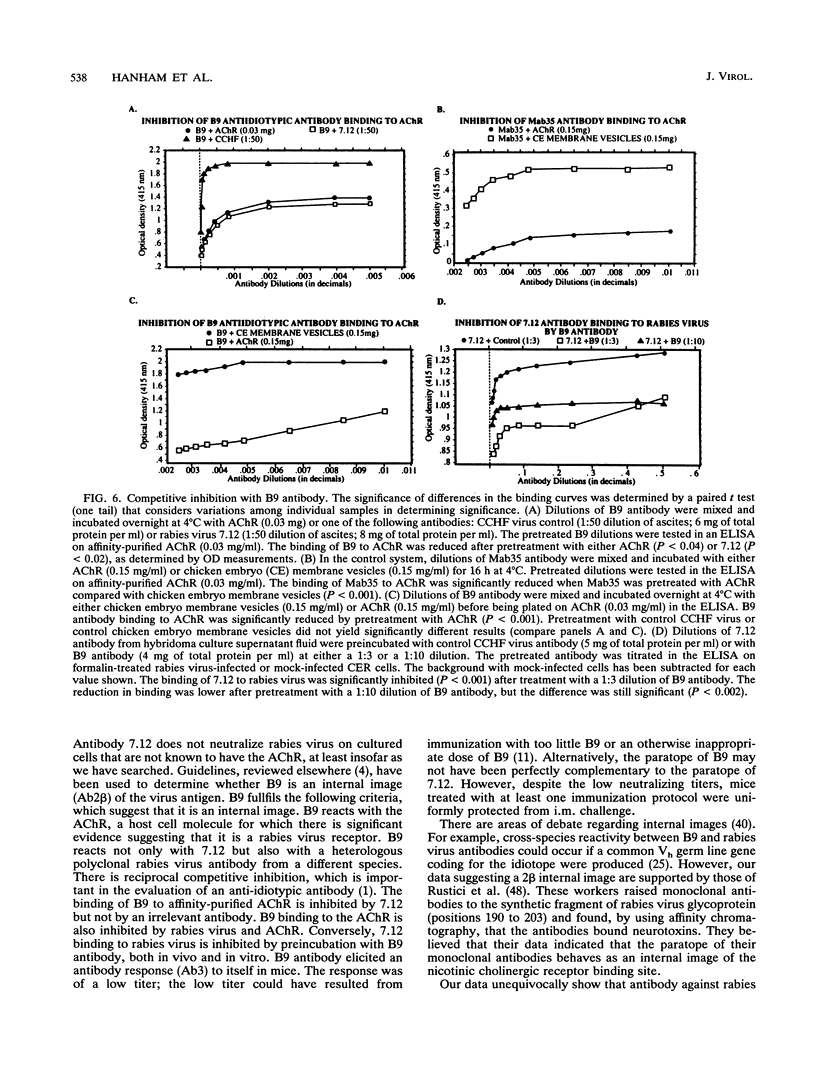
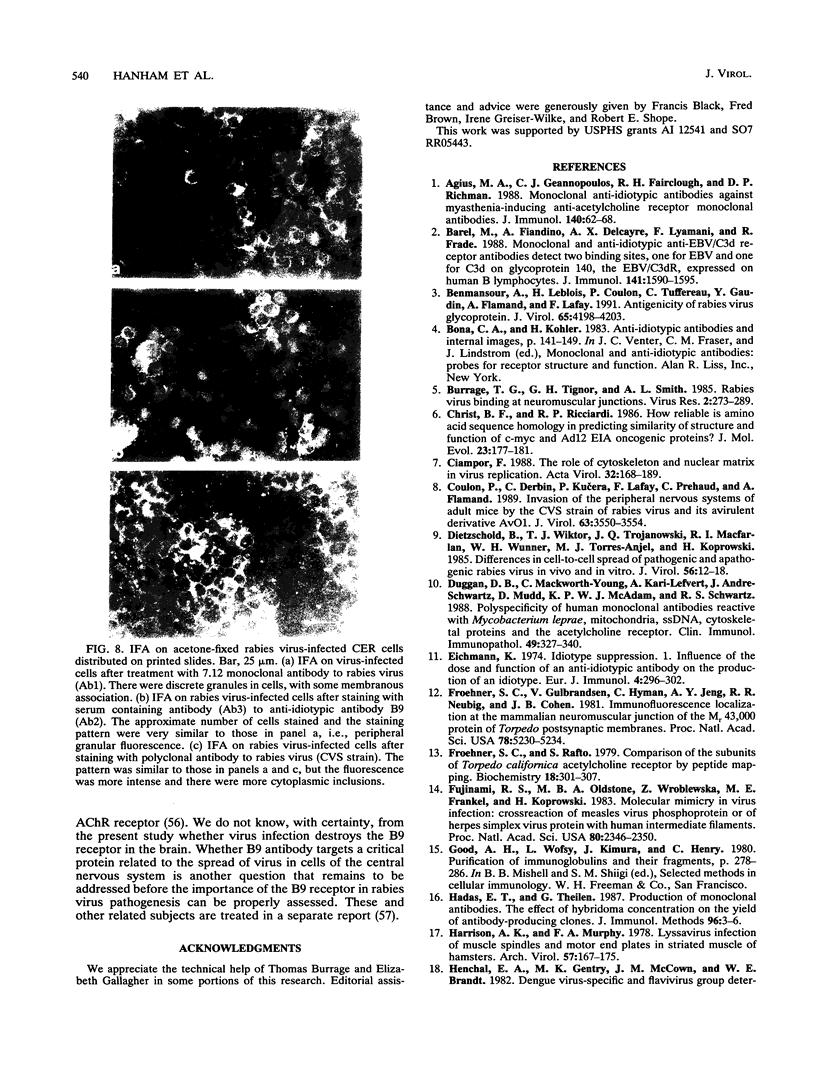
Abstract
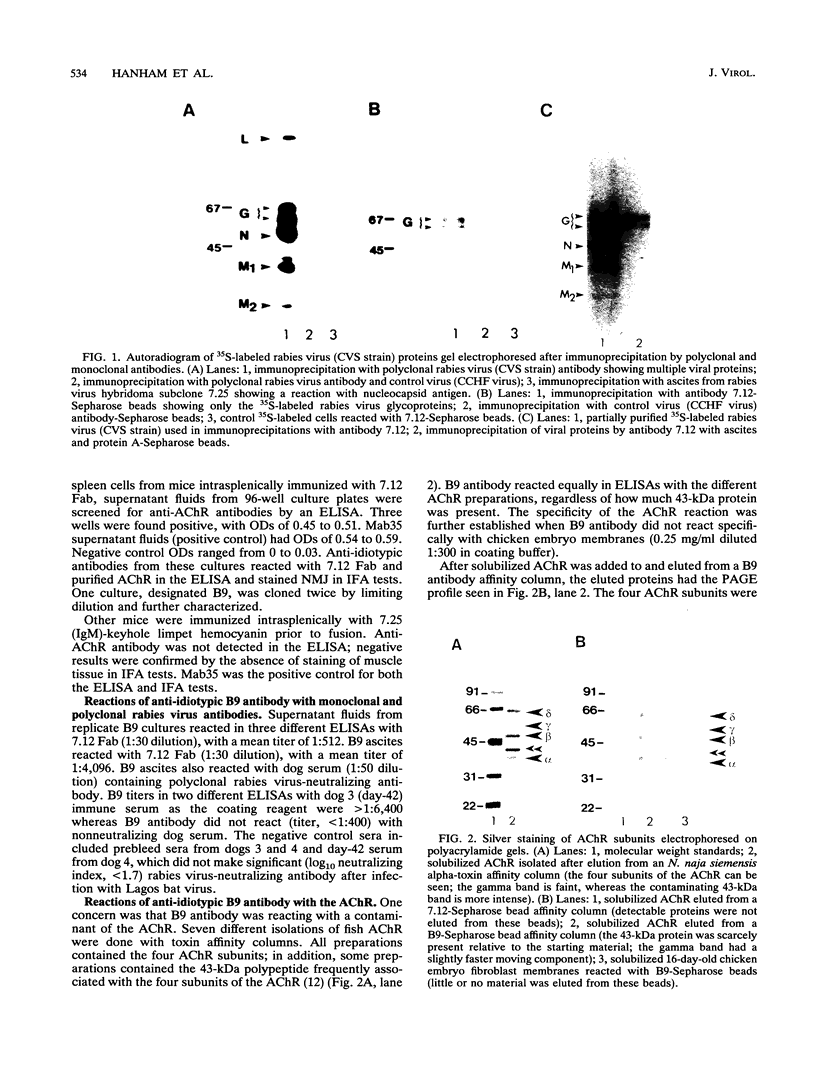
We have developed idiotype-anti-idiotype monoclonal antibodies that provide evidence for rabies virus binding to the acetylcholine receptor (AChR). Hybridoma cell lines 7.12 and 7.25 resulted after fusion of NS-1 myeloma cells with spleen cells from a BALB/c mouse immunized with rabies virus strain CVS. Antibody 7.12 reacted with viral glycoprotein and neutralized virus infectivity in vivo. It also neutralized infectivity in vitro when PC12 cells, which express neuronal AChR, but not CER cells or neuroblastoma cells (clone N18), which have no AChR, were used. Antibody 7.25 reacted with nucleocapsid protein. Anti-idiotypic monoclonal antibody B9 was produced from fusion of NS-1 cells with spleen cells from a mouse immunized with 7.12 Fab. In an enzyme-linked immunosorbent assay and immunoprecipitation, B9 reacted with 7.12, polyclonal rabies virus immune dog serum, and purified AChR. The binding of B9 to 7.12 and immune dog serum was inhibited by AChR. B9 also inhibited the binding of 7.12 to rabies virus both in vitro and in vivo. Indirect immunofluorescence revealed that B9 reacted at neuromuscular junctions of mouse tissue. B9 also reacted in indirect immunofluorescence with distinct neurons in mouse and monkey brain tissue as well as with PC12 cells. B9 staining of neuronal elements in brain tissue of rabies virus-infected mice was greatly reduced. Rabies virus inhibited the binding of B9 to PC12 cells. Mice immunized with B9 developed low-titer rabies virus-neutralizing antibody. These mice were protected from lethal intramuscular rabies virus challenge. In contrast, anti-idiotypic antibody raised against nucleocapsid antibody 7.25 did not react with AChR.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius M. A., Geannopoulos C. J., Fairclough R. H., Richman D. P. Monoclonal anti-idiotopic antibodies against myasthenia-inducing anti-acetylcholine receptor monoclonal antibodies. Preponderance of nonparatope-directed antibodies affecting antigen binding. J Immunol. 1988 Jan 1;140(1):62–68. [PubMed] [Google Scholar]
- Barel M., Fiandino A., Delcayre A. X., Lyamani F., Frade R. Monoclonal and anti-idiotypic anti-EBV/C3d receptor antibodies detect two binding sites, one for EBV and one for C3d on glycoprotein 140, the EBV/C3dR, expressed on human B lymphocytes. J Immunol. 1988 Sep 1;141(5):1590–1595. [PubMed] [Google Scholar]
- Benmansour A., Leblois H., Coulon P., Tuffereau C., Gaudin Y., Flamand A., Lafay F. Antigenicity of rabies virus glycoprotein. J Virol. 1991 Aug;65(8):4198–4203. doi: 10.1128/jvi.65.8.4198-4203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage T. G., Tignor G. H., Smith A. L. Rabies virus binding at neuromuscular junctions. Virus Res. 1985 Apr;2(3):273–289. doi: 10.1016/0168-1702(85)90014-0. [DOI] [PubMed] [Google Scholar]
- Ciampor F. The role of cytoskeleton and nuclear matrix in virus replication. Acta Virol. 1988 Mar;32(2):168–189. [PubMed] [Google Scholar]
- Coulon P., Derbin C., Kucera P., Lafay F., Prehaud C., Flamand A. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivative AvO1. J Virol. 1989 Aug;63(8):3550–3554. doi: 10.1128/jvi.63.8.3550-3554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Trojanowski J. Q., Macfarlan R. I., Wunner W. H., Torres-Anjel M. J., Koprowski H. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J Virol. 1985 Oct;56(1):12–18. doi: 10.1128/jvi.56.1.12-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts D. L., Lentz T. L. Synthetic peptides of neurotoxins and rabies virus glycoprotein behave as antagonists in a functional assay for the acetylcholine receptor. Pept Res. 1989 May-Jun;2(3):221–226. [PubMed] [Google Scholar]
- Duggan D. B., Mackworth-Young C., Kari-Lefvert A., Andre-Schwartz J., Mudd D., McAdam K. P., Schwartz R. S. Polyspecificity of human monoclonal antibodies reactive with Mycobacterium leprae, mitochondria, ssDNA, cytoskeletal proteins, and the acetylcholine receptor. Clin Immunol Immunopathol. 1988 Dec;49(3):327–340. doi: 10.1016/0090-1229(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Gulbrandsen V., Hyman C., Jeng A. Y., Neubig R. R., Cohen J. B. Immunofluorescence localization at the mammalian neuromuscular junction of the Mr 43,000 protein of Torpedo postsynaptic membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5230–5234. doi: 10.1073/pnas.78.8.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Rafto S. Comparison of the subunits of Torpedo californica acetylcholine receptor by peptide mapping. Biochemistry. 1979 Jan 23;18(2):301–307. doi: 10.1021/bi00569a011. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrist B. F., Ricciardi R. P. How reliable is amino acid sequence homology in predicting similarity of structure and function of c-myc and Ad12 E1A oncogenic proteins? J Mol Evol. 1986;23(2):177–181. doi: 10.1007/BF02099912. [DOI] [PubMed] [Google Scholar]
- Hadas E., Theilen G. Production of monoclonal antibodies. The effect of hybridoma concentration on the yield of antibody-producing clones. J Immunol Methods. 1987 Jan 26;96(1):3–6. doi: 10.1016/0022-1759(87)90359-0. [DOI] [PubMed] [Google Scholar]
- Harrison A. K., Murphy F. A. Lyssavirus infection of muscle spindles and motor end plates in striated muscle of hamsters. Arch Virol. 1978;57(2):167–175. doi: 10.1007/BF01315678. [DOI] [PubMed] [Google Scholar]
- Henchal E. A., Gentry M. K., McCown J. M., Brandt W. E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982 Jul;31(4):830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Liu D. S., Yamamoto T., Konno H. On the replication and spread of rabies virus in the human central nervous system. J Neuropathol Exp Neurol. 1985 Mar;44(2):185–195. doi: 10.1097/00005072-198503000-00007. [DOI] [PubMed] [Google Scholar]
- Jackson A. C. Biological basis of rabies virus neurovirulence in mice: comparative pathogenesis study using the immunoperoxidase technique. J Virol. 1991 Jan;65(1):537–540. doi: 10.1128/jvi.65.1.537-540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. C., Reimer D. L. Pathogenesis of experimental rabies in mice: an immunohistochemical study. Acta Neuropathol. 1989;78(2):159–165. doi: 10.1007/BF00688204. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kauffman R. S., Noseworthy J. H., Nepom J. T., Finberg R., Fields B. N., Greene M. I. Cell receptors for the mammalian reovirus. II. Monoclonal anti-idiotypic antibody blocks viral binding to cells. J Immunol. 1983 Nov;131(5):2539–2541. [PubMed] [Google Scholar]
- Keay S., Rasmussen L., Merigan T. C. Syngeneic monoclonal anti-idiotype antibodies that bear the internal image of a human cytomegalovirus neutralization epitope. J Immunol. 1988 Feb 1;140(3):944–948. [PubMed] [Google Scholar]
- Krah D. L., Choppin P. W. Mice immunized with measles virus develop antibodies to a cell surface receptor for binding virus. J Virol. 1988 May;62(5):1565–1572. doi: 10.1128/jvi.62.5.1565-1572.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera P., Dolivo M., Coulon P., Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985 Jul;55(1):158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F., Coulon P., Astic L., Saucier D., Riche D., Holley A., Flamand A. Spread of the CVS strain of rabies virus and of the avirulent mutant AvO1 along the olfactory pathways of the mouse after intranasal inoculation. Virology. 1991 Jul;183(1):320–330. doi: 10.1016/0042-6822(91)90145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L., Burrage T. G., Smith A. L., Crick J., Tignor G. H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982 Jan 8;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Chester J., Benson R. J., Hawrot E., Tignor G. H., Smith A. L. Rabies virus binding to cellular membranes measured by enzyme immunoassay. Muscle Nerve. 1985 May;8(4):336–345. doi: 10.1002/mus.880080411. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Hawrot E., Wilson P. T. Synthetic peptides corresponding to sequences of snake venom neurotoxins and rabies virus glycoprotein bind to the nicotinic acetylcholine receptor. Proteins. 1987;2(4):298–307. doi: 10.1002/prot.340020406. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Wilson P. T., Hawrot E., Speicher D. W. Amino acid sequence similarity between rabies virus glycoprotein and snake venom curaremimetic neurotoxins. Science. 1984 Nov 16;226(4676):847–848. doi: 10.1126/science.6494916. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Merlie J. Immunization of rats with polypeptide chains from torpedo acetylcholine receptor causes an autoimmune response to receptors in rat muscle. Proc Natl Acad Sci U S A. 1978 Feb;75(2):769–773. doi: 10.1073/pnas.75.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Roeder D. J., Consigli R. A. Anti-idiotypic antibodies to a polyomavirus monoclonal antibody recognize cell surface components of mouse kidney cells and prevent polyomavirus infection. J Virol. 1987 Sep;61(9):2747–2753. doi: 10.1128/jvi.61.9.2747-2753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology. 1989 Apr;169(2):354–364. doi: 10.1016/0042-6822(89)90161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I. Receptor anti-idiotypes. Mimics--or gimmicks? Nature. 1990 Oct 4;347(6292):424–425. doi: 10.1038/347424a0. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Ni Y. J., Kawai A. Syncytium formation is induced in the murine neuroblastoma cell cultures which produce pathogenic type G proteins of the rabies virus. Virology. 1992 Jul;189(1):203–216. doi: 10.1016/0042-6822(92)90696-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosckovitz R., Gershoni J. M. Three possible disulfides in the acetylcholine receptor alpha-subunit. J Biol Chem. 1988 Jan 15;263(2):1017–1022. [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P. Early street rabies virus infection in striated muscle and later progression to the central nervous system. Intervirology. 1974;3(4):256–268. doi: 10.1159/000149762. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Pachner A. R., Sourojon M., Fuchs S. Anti-idiotypic antibodies to anti-acetylcholine receptor antibody: characterization by ELISA and immunoprecipitation assays. J Neuroimmunol. 1986 Sep;12(3):205–214. doi: 10.1016/s0165-5728(86)80004-2. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Wunner W. H. Rabies virus interaction with various cell lines is independent of the acetylcholine receptor. Arch Virol. 1985;84(3-4):277–282. doi: 10.1007/BF01378980. [DOI] [PubMed] [Google Scholar]
- Rustici M., Santucci A., Lozzi L., Petreni S., Spreafico A., Neri P., Bracci L., Soldani P. A monoclonal antibody to a synthetic fragment of rabies virus glycoprotein binds ligands of the nicotinic cholinergic receptor. J Mol Recognit. 1989 Sep;2(2):51–55. doi: 10.1002/jmr.300020202. [DOI] [PubMed] [Google Scholar]
- Schumacher C. L., Dietzschold B., Ertl H. C., Niu H. S., Rupprecht C. E., Koprowski H. Use of mouse anti-rabies monoclonal antibodies in postexposure treatment of rabies. J Clin Invest. 1989 Sep;84(3):971–975. doi: 10.1172/JCI114260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H. Host cell receptors for two strains of Sindbis virus. Arch Virol. 1980;66(1):11–26. doi: 10.1007/BF01315041. [DOI] [PubMed] [Google Scholar]
- Spitz M., Spitz L., Thorpe R., Eugui E. Intrasplenic primary immunization for the production of monoclonal antibodies. J Immunol Methods. 1984 May 11;70(1):39–43. doi: 10.1016/0022-1759(84)90387-9. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R. Rabies pathogenesis: fast times at the neuromuscular junction. J Infect Dis. 1985 Dec;152(6):1362–1363. doi: 10.1093/infdis/152.6.1362. [DOI] [PubMed] [Google Scholar]
- Superti F., Seganti L., Tsiang H., Orsi N. Role of phospholipids in rhabdovirus attachment to CER cells. Brief report. Arch Virol. 1984;81(3-4):321–328. doi: 10.1007/BF01310002. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M., Whiting P. J., Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987 Oct;7(10):3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tignor G. H., Shope R. E., Bhatt P. N., Percy D. H. Experimental infection of dogs and monkeys with two rabies serogroup viruses, Lagos bat and Mokola (IbAn 27377): clinical, serologic, virologic, and fluorescent-antibody studies. J Infect Dis. 1973 Oct;128(4):471–478. doi: 10.1093/infdis/128.4.471. [DOI] [PubMed] [Google Scholar]
- Tignor G. H., Smith A. L., Casals J., Ezeokoli C. D., Okoli J. Close relationship of Crimean hemorrhagic fever-Congo (CHF-C) virus strains by neutralizing antibody assays. Am J Trop Med Hyg. 1980 Jul;29(4):676–685. doi: 10.4269/ajtmh.1980.29.676. [DOI] [PubMed] [Google Scholar]
- Tsiang H., de la Porte S., Ambroise D. J., Derer M., Koenig J. Infection of cultured rat myotubes and neurons from the spinal cord by rabies virus. J Neuropathol Exp Neurol. 1986 Jan;45(1):28–42. doi: 10.1097/00005072-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Tuffereau C., Leblois H., Bénéjean J., Coulon P., Lafay F., Flamand A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 1989 Sep;172(1):206–212. doi: 10.1016/0042-6822(89)90122-0. [DOI] [PubMed] [Google Scholar]
- UytdeHaag F. G., Bunschoten H., Weijer K., Osterhaus A. D. From Jenner to Jerne: towards idiotype vaccines. Immunol Rev. 1986 Apr;90:93–113. doi: 10.1111/j.1600-065x.1986.tb01479.x. [DOI] [PubMed] [Google Scholar]
- Walker K. Z., Gibson J., Axiak S. M., Prentice R. L. Potentiation of hybridoma production by the use of mouse fibroblast conditioned media. J Immunol Methods. 1986 Apr 3;88(1):75–81. doi: 10.1016/0022-1759(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Watson H. D., Tignor G. H., Smith A. L. Entry of rabies virus into the peripheral nerves of mice. J Gen Virol. 1981 Oct;56(Pt 2):372–382. doi: 10.1099/0022-1317-56-2-371. [DOI] [PubMed] [Google Scholar]
- Whiting P. J., Schoepfer R., Swanson L. W., Simmons D. M., Lindstrom J. M. Functional acetylcholine receptor in PC12 cells reacts with a monoclonal antibody to brain nicotinic receptors. Nature. 1987 Jun 11;327(6122):515–518. doi: 10.1038/327515a0. [DOI] [PubMed] [Google Scholar]
- Whiting P., Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci U S A. 1987 Jan;84(2):595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]