Abstract
Objective
Standard competitive repopulation assays have proven valuable in evaluating engraftment potential in ablated hosts, permitting comparisons between various test cell populations. However, no similar method exists to compare engraftment of test cells in submyeloablated hosts, which would be helpful given the applications of reduced-intensity conditioning for hematopoietic gene-replacement therapy and other cellular therapies. Here, we developed a novel assay to quantitate engraftment of hematopoietic stem cells in submyeloablated hosts.
Methods
Engraftment of murine marrow cells transduced with retroviral vectors using two separate protocols was compared to engraftment of fresh untreated competitor cells within low-dose radiation conditioned hosts using a “three-way” marking system, so that test, competitor and host cell chimerism could be reliably determined post-transplant.
Results
We demonstrate that the repopulating ability of marrow cells transduced using two distinct protocols was reduced ~10-fold compared to fresh competitor cells in submyeloablated hosts utilizing the novel “three-way” transplant assay.
Conclusions
Murine marrow cells transduced using a clinically-applicable protocol acquire an engraftment defect in submyeloablated hosts, similar to cells transduced using a research protocol. We conclude that the submyeloablative competitive repopulation assay described here will be of benefit to comparatively assess the engraftment ability of manipulated hematopoietic stem cells using various culture protocols, such as to test the impact of modifications in transduction protocols needed to attain therapeutic levels of gene-corrected blood cells, or the effect of ex vivo expansion protocols on engraftment potential.
Keywords: Engraftment, conditioning, submyeloablative, transduction, hematopoietic stem cells
INTRODUCTION
Submyeloablative, or reduced-intensity, conditioning for hematopoietic stem cell (HSC) transplantation has received considerable recent attention. For allogeneic HSC transplantation, submyeloablative conditioning permits the transplantation of older adults or patients with end-organ impairments who otherwise would not tolerative the toxicity of a fully ablative transplant. For autologous HSC transplantation, submyeloablative conditioning shows promise for the treatment of autoimmune diseases, such as type I diabetes [1] and lupus [2] and for cellular therapy for regenerative medicine applications [3]. The use of submyeloablative conditioning in experimental transplantation models has also assisted in the elucidation of mechanisms of HSC engraftment [4–6]. Furthermore, gene transfer using recombinant retroviral (and other viral) vectors, followed by autologous transplantation of the transduced cells has been advocated for the potential correction of genetic blood cell diseases [7–9]. Because short- and long-term toxicities preclude the use of ablative conditioning in patients with chronic, non-malignant blood disorders, the development of reduced-toxicity conditioning regimens that permit engraftment of clinically beneficial numbers of retrovirus-transduced autologous cells is highly desirable.
Reduced-toxicity conditioning, though beneficial for reducing regimen-related toxicity, may negatively impact engraftment of HSC. This has been most clearly shown in transplantation studies using retrovirus-transduced HSC. Initial engraftment studies of gene-marked cells in minimally-conditioned hosts were generally disappointing, with very low or transient levels of gene-marked cells reported in numerous murine [10–12] and large animal studies [13–16]. We previously reported that transplantation of marrow cells transduced using a protocol in which donor mice were treated 5-fluorouracil (5-FU) and marrow cells were cultured in serum-containing medium produced three-fold lower engraftment in 160 cGy-conditioned hosts than did similar numbers of fresh marrow cells, despite the transduced cell population being enriched for primitive-phenotype marrow cells [17]. Li et al. [18] described a clinically-relevant murine transduction protocol designed to simulate human gene transfer protocols. These authors demonstrated that transduced cells displayed high levels of gene marking, and efficiently repopulated lethally irradiated hosts.
Our objective in this study was to determine how well marrow cells transduced using a clinically-relevant protocol (i.e., without 5-FU and bovine serum, and a shorter culture period) engraft in submyeloablated hosts, compared to cells transduced using a 5-FU- and serum-containing protocol. Because the means to quantitate and definitively compare engraftment in submyeloablated hosts has not been available, we developed a novel competitive repopulation assay in submyeloablated hosts for the purpose of comparing the influence of different culture/transduction conditions on HSC engraftment in submyeloablated hosts.
MATERIALS AND METHODS
Mice
Wild-type C57Bl/6J (Bl/6; CD45.2+) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). B6.SJL-PtrcaPep3b/BoyJ (Boy J; CD45.1+) and Bl/6xBoy J F1 (F1; CD45.1+/CD45.2+) mice were obtained from an on-site breeding colony. All mice were maintained under pathogen-free conditions, and were fed autoclaved food and acidified water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine.
Retroviral vectors and supernatant production
The construction of retroviral vectors MSCV-m91Neo [19, 20] encoding murine gp91phox and neomycin-resistance genes; MFG-EGFP [21] encoding the enhanced green fluorescent protein (GFP); SFFV-91-LNGFR [22] encoding a truncated human low-affinity nerve growth factor receptor (LNGFR); and SFalltCD34 [18] (a kind gift from Dr. Christopher Baum, encoding a truncated human CD34 (tCD34)) used in this study have been described previously. For MFG-EGFP and SFalltCD34, supernatants were generated prior to each transduction experiment by transient plasmid transfection of ecotropic Phoenix packaging cells [23]. For MSCV-m91Neo and SFFV-91-LNGFR, supernatant was collected from stable producer cell lines. Experiments utilizing ecotropic supernatant were carried out in a biological safety cabinet in a BL-2 laboratory, and transplanted animals were handled under BL-1 guidelines as per approved institutional protocols.
Marrow cell isolation and retroviral transduction - “5-FU protocol”
Marrow cell isolation and retroviral transduction was performed as detailed previously [17]. Briefly, whole bone marrow (WBM) from Bl/6 donor mice injected with 5-FU (5-FU; Adrucil, Pharmacia, Kalamazoo, MI, USA) three days prior to marrow harvest was isolated by crushing the hind limb bones in α-minimal essential medium (α-MEM). Donor marrow was pooled and prestimulated in α-MEM supplemented with 30% fetal calf serum, 100 ng/mL stem cell factor (SCF) and 100 units/mL interleukin-6 (IL-6; both from PeproTech, Rocky Hill, NJ, USA) for 48 hours. Prestimulation was followed by co-culture for 48 hours on mitomycin-C (Bedford Laboratories, Bedford, OH, USA) treated packaging cells in fresh medium supplemented with cytokines as described above plus 8 µg/ml Polybrene (Sigma, St. Louis, MO, USA).
Marrow cell isolation and retroviral transduction - “lineage-depletion protocol”
Retroviral transduction was performed as adapted from Li and colleagues [18]. Briefly, WBM from unmanipulated Bl/6 donor mice was isolated as noted above and subjected to immunomagnetic lineage depletion using a mouse Lineage Cell Depletion kit and VarioMACS apparatus (Miltenyi Biotec, Auburn, CA, USA). The lineage-depleted (lin−) cells were prestimulated in serum-free medium (SFM; STEMSPAN SF, StemCell Technologies, Vancouver, Canada) supplemented with 100 ng/mL SCF and 100 units/mL IL-6 for 48 hours, followed by one overnight transduction on retroviral supernatant-preloaded, RetroNectin (Takara Shuzo, Otsu, Japan)-coated plates, prepared as described [18].
Analysis of transduced cells
Bulk transduction efficiency was determined immediately prior to transplant by GFP fluorescence or by staining with anti-LNGFR or anti-CD34 (Becton-Dickinson (BD) Pharmingen, San Diego, CA, USA). For marrow transduced with MSCV-m91Neo, progenitor colonies were plated in the presence and absence of G418 (Sigma) and the percentage of G418-resistant colonies was determined [19]. In some experiments, transduced lin− cells were analyzed for the percentage of lin− and/or Sca-1+lin−c-kit+ (SLK) cells by staining with fluorescein isothiocyanate-conjugated Sca-1, phycoerythrin-conjugated lineage markers Gr-1, B220 and CD3, and allophycocyanin-conjugated c-kit antibodies (BD-Pharmingen). Cells were analyzed using a FACSCalibur instrument and CELLQuest software (BD) as previously described [24].
Transplantation into submyeloablated hosts
3 × 105–106 fresh or transduced lin− cells from Bl/6 donors were transplanted into 300 cGy- or 550 cGy-conditioned (137Cs source, single dose) Boy J hosts by tail vein injection as previously described [17, 24]. Peripheral blood total donor chimerism was determined monthly for 4–6 months post-transplant using antibodies to CD45.1 and CD45.2 (BD-Pharmingen) [17, 24]. The fraction of donor-derived cells expressing the transgene was determined as described above.
Competitive repopulation assays
Estimation of stem cell function in lethally irradiated hosts was performed using a competitive repopulation assay (CRA) as adapted from Harrison and colleagues [25, 26]. In CRAs performed in ablated hosts, varying ratios (1:1; 1:3) of freshly-isolated or transduced Bl/6 lin− “test” cells were mixed with freshly-isolated Boy J lin− “competitor” cells, and transplanted into 1100 cGy-conditioned (split dose) Boy J hosts. In CRAs performed in submyeloablated hosts (see Figure 3 and Results for a more detailed description), 106 freshly-isolated or transduced test cells were mixed with 106 freshly-isolated competitor cells and transplanted into 550 cGy-conditioned hosts. Test, competitor and host peripheral blood chimerism was determined as described above.
Figure 3.

Schematic of submyeloablative CRA. In this assay, test cells (in this illustration, transduced Bl/6 marrow cells) were mixed with fresh Boy J competitor cells and transplanted into 550 cGy-conditioned Bl/6 × Boy J F1 hosts. Note that because the three mouse strains have similar HSC function (see Results for details), any of the strains can be used as the source of test cells, competitor cells or hosts, as long as the strains remain consistent within each independent experiment. Thus, if submyeloablative CRAs are performed in parallel using untreated and transduced test cells, the engraftment of transduced test cells (relative to untreated control cells) can be deduced.
Statistics
All data are represented as the mean ± standard error of the mean (SEM). The data were compared using the unpaired Student’s t test, or Welch’s unpaired t test if the SEMs were significantly different, using InStat Version 3.05 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com).
RESULTS
Characterization of cells transduced using the lineage-depletion protocol
We previously reported that the repopulating ability of marrow transduced using the 5-FU protocol is markedly impaired relative to that of fresh marrow [17]. Because the lineage-depletion transduction protocol [18] outlined above avoids the use of 5-FU and serum, we hypothesized that cells transduced using this protocol would exhibit greater engraftment in submyeloablated hosts than marrow transduced using the 5-FU protocol. To test this hypothesis, we first characterized the phenotype and transduction efficiency of cells transduced using this protocol. We obtained lin− cell purities and bulk (total mononuclear cell) transduction efficiencies similar to that previously reported [18]. We found that the stem/progenitor cell population (SLK cells) was enriched 15 ± 2.7-fold (N = 4 experiments) after lineage depletion. The percentage of SLK cells in transduced lin− populations was very similar to that of freshly-isolated lin− marrow (5.7 ± 0.7% vs. 6 ± 1.2%, N = 4 experiments; P = 0.83), indicating that the cell purity and phenotype of the lin− cells is not substantially altered by this transduction protocol; furthermore, this means that the engraftment potential of fresh and transduced lin− marrow can be directly compared without accounting for differences in HSC content as with the 5-FU protocol (see below). High levels of transduction were achieved with the lineage-depletion protocol: 45.3 ± 10% of lin− cells expressed transgene from several retroviral constructs after a single overnight transduction (N = 8 experiments). We confirmed that lin− cells transduced in this manner repopulated lethally-irradiated hosts; in one experiment, in which 4 × 105 cells were transplanted into each of six ablated hosts, all hosts achieved hematopoietic reconstitution, and leukocyte GFP expression was 18.5 ± 3% six months post-transplant.
Engraftment of transduced lin− cells
Next, engraftment of transduced lin− cells in submyeloablated hosts was compared to that of fresh lin− cells. As shown in Figure 1A, very low donor chimerism (“Td CD45.2+”; 3 ± 0.5%, N = 13 hosts) was observed 4–5 months after transplantation of 106 transduced Bl/6 lin− cells into 300 cGy-conditioned Boy J hosts, significantly less than that achieved with fresh lin− marrow (29 ± 5.9%; P = 0.0012). Of the engrafted donor cells, however, 43.1 ± 6.8% expressed the transgene (either GFP or tCD34; “Td CD45.2+/Tg+”), indicating that although transduced HSC engrafted poorly, efficient marking was achieved. To potentially improve engraftment of transduced cells, the conditioning radiation dose was increased to 550 cGy. Transplantation of 106 fresh lin− cells resulted in significantly higher engraftment in 550 cGy-, compared to 300 cGy-, conditioned hosts (90.2 ± 0.6% (Fig. 1B) vs. 29 ± 5.9% (Fig. 1A); P < 0.0001). When transduced lin− cells were transplanted into 550 cGy-conditioned hosts (Fig. 1B), significantly improved donor chimerism was detected (35 ± 9.2% (N = 4); P = 0.036 vs. chimerism in 300 cGy-conditioned hosts (3 ± 0.5%; Fig. 1A), respectively). The fraction of long-term engrafted cells expressing the transgene was at least equivalent to that of the bulk transduced population prior to transplant. These data demonstrate that lin− cells transduced in this manner engraft less well in submyeloablated hosts than do fresh lin− cells. Furthermore, since a modest increase in conditioning radiation led to a ≥11- fold increase in donor chimerism, these data confirm that engraftment is determined in part by the degree of host HSC impairment [17, 24, 27].
Figure 1.

Engraftment of transduced lin− cells in 300 and 550 cGy-conditioned hosts is diminished compared to fresh lin− cells. (A) 106 lin− cells from untreated Bl/6 (CD45.2+) donors were either transplanted directly (fresh) or transduced (Td) with MFG-GFP or SFalltCD34 as described in the Materials and Methods prior to transplantation into 300 cGy-conditioned Boy J (CD45.1+) hosts. Total donor chimerism and the fraction of transgene-marked (Tg+) donor cells were determined by flow cytometry 4–6 months post-transplant. N = 10 hosts from 2 independent experiments for fresh cells; N = 13 hosts from 3 independent experiments for transduced cells. *, P = 0.0012 for transduced lin− cell chimerism compared to that of fresh lin− cells. (B) 106 fresh or SFFV-91-LNGFR-transduced Bl/6 lin− cells were transplanted into 550 cGy-conditioned Boy J hosts. N = 5 hosts for fresh cells; N = 4 hosts for transduced cells. †, P < 0.05 for chimerism of transduced lin− cells in 550 cGy- vs. 300 cGy-conditioned hosts (panel A).
Competitive repopulation of lin− cells in ablated and submyeloablated hosts
To directly determine the stem cell function of transduced lin− cells, CRAs were performed. First, we performed a “classic” CRA in 1100 cGy-conditioned hosts [25, 26]. In the first of two independent assays (Fig. 2A), 3 × 105 Bl/6 lin− cells, either: freshly-isolated (“fresh test cells”) or transduced as described above (“transduced test cells”) were mixed with 3 × 105 freshly-isolated Boy J lin− cells (competitor cells), and the cell mixtures were transplanted into 1100 cGy-conditioned Boy J hosts. Test cell chimerism was determined on at least two occasions ≥4 months post-transplant. The second assay (Fig. 2B) was similar, except that 6 × 105 test cells were mixed with 2 × 105 competitor cells before transplantation. Transgene expression prior to transplant was 37% (LNGFR) and 29% (GFP) in the two experiments, respectively. In both experiments, test cell chimerism in hosts transplanted with transduced test cells was ≥10-fold lower than in hosts transplanted with fresh test cells (P≤0.012). These CRAs indicate that even though lin− cells from non-5-FU-treated mice were used as the target, ex vivo transduction still markedly impaired long-term repopulating ability.
Figure 2.
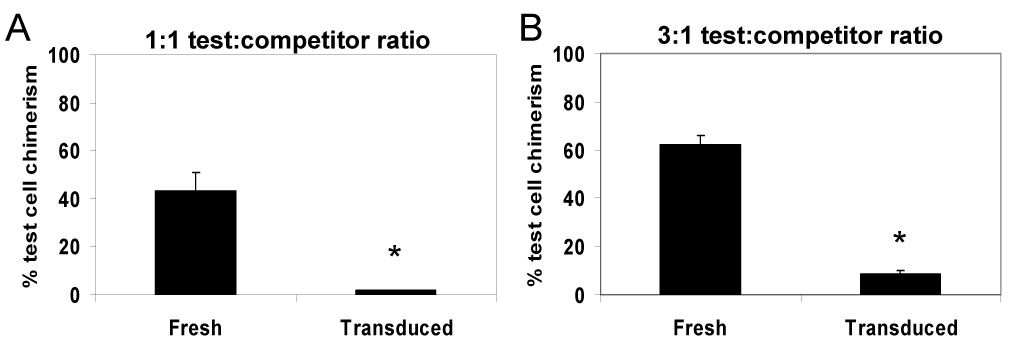
Transduced lin− marrow cells display significantly impaired competitive repopulating ability in 1100 cGy-conditioned hosts. Transduced lin− test cells from Bl/6 donors were mixed with freshly-isolated lin− competitor cells from Boy J donors, and the mixtures were transplanted into 1100 cGy-conditioned Boy J hosts. In the experiment shown in panel (A), 3 × 105 test cells were mixed with 3 × 105 competitor cells prior to transplantation; in the experiment shown in panel (B), 6 × 105 test cells were mixed with 2 × 105 competitor cells. Test cell chimerism is shown 4–5 months post-transplant from two independent experiments. N = 4 hosts for each parameter. *, P < 0.012 comparing fresh test cell chimerism to that of transduced test cells.
Repopulating ability of transduced marrow in submyeloablated hosts
We established that the engraftment of marrow cells transduced using a clinically-relevant lineage-depletion protocol is impaired in submyeloablated hosts (Fig. 1), and long-term repopulating ability of these cells is reduced in CRAs utilizing ablated hosts (Fig. 2). However, the means to quantitate engraftment defects in submyeloablated hosts has not previously been available; such assays would be useful for comparing the impact of modifications in transduction protocols, for instance. Thus, we developed a novel “three-way” CRA using submyeloablated hosts in an attempt to further quantitate differences in repopulating ability caused by the transduction process. A schematic for this assay is shown in Figure 3. In contrast to the classic CRA in 1100 cGy-conditioned hosts, in the submyeloablative setting the test cells must compete for engraftment not only against the competitor cells but also host HSC which survive the conditioning regimen, requiring three markers to delineate test, competitor and donor cells. Thus, we chose to use Bl/6 (CD45.2+), Boy J (CD45.1+) and Bl/6 × Boy J F1 (CD45.1+/CD45.2+) mice to permit detection of test, competitor and host leukocytes by flow cytometry in this assay. The Bl/6 and Boy J strains have long been inbred on the Bl/6 background and are widely considered to have similar HSC function. Indeed, we previously demonstrated that these strains have comparable HSC function by obtaining similar results in classic (e.g., in ablated hosts) CRAs performed in parallel using each strain as the source of either test or competitor cells [17]. Thus, any of the three strains (Bl/6, Boy J or Bl/6 × Boy J F1) may be used as a source of test cells, competitor cells or hosts, as long as the strains remain consistent within a given experiment, so that the primary variable being examined is the impact of treatment on test cell repopulating ability (see Fig. 4 and Fig. 5 for examples). 550 cGy was chosen as the conditioning radiation dose for the submyeloablative CRAs since we empirically found that 550 cGy-conditioned hosts transplanted with reasonable (e.g., 106 lin− marrow cells) cell doses achieved moderate (~20–40%) levels of donor cell chimerism. This permitted detection of relatively equivalent test, competitor and host cell populations in control animals transplanted with similar numbers of fresh test and competitor cells (data not shown from pilot experiments, but see Fig. 4A and Fig. 5A for examples).
Figure 4.

Marrow cells transduced using a 5-FU-containing protocol display markedly impaired competitive repopulating ability in 550 cGy-conditioned hosts. (A) Representative scatter plots showing peripheral blood leukocyte analysis from 550 cGy-conditioned Boy J (CD45.1+) hosts which received a mixture of 2 × 106 fresh (left panel) or transduced (right panel) Bl/6 (CD45.2+) test cells and 2 × 106 Bl/6 × Boy J F1 (CD45.1+ and CD45.2+) competitor cells 5 months post-transplant. (B) Composite test cell chimerism 5 months post-transplant from 550 cGy-conditioned hosts transplanted with fresh or transduced test cells. On the left side of the graph, data were normalized per 106 transplanted cells; on the right side of the graph, chimerism was normalized per femur equivalent (FE) of cells transplanted, accounting for the enrichment of primitive-phenotype cells with this transduction protocol. N = 5 hosts for each group.
Figure 5.

Transduced lin− marrow cells display markedly impaired competitive repopulating ability in 550 cGy-conditioned hosts. (A) Representative scatter plots showing peripheral blood leukocyte analysis from 550 cGy-conditioned Bl/6 × Boy J F1 (CD45.1+ and 45.2+) hosts which received a mixture of fresh (left panel) or transduced (right panel) Boy J (CD45.1+) test cells and Bl/6 (CD45.2+) competitor cells 5 months post-transplant. (B) Test cell chimerism 5 months post-transplant from 550 cGy-conditioned hosts transplanted with fresh or transduced test cells is shown. N = 5 hosts for each group. *, P < 0.0001 comparing fresh test cell chimerism to that of transduced test cells.
We first used the submyeloablative CRA to assess the repopulating ability of marrow transduced using the 5-FU protocol relative to that of fresh WBM. In this experiment, 2 × 106 fresh or transduced test cells from Bl/6 donors were mixed with 2 × 106 fresh competitor cells from F1 donors, and the cell mixture was transplanted into each of five 550 cGy-conditioned Boy J hosts. The transduction efficiency with MSCV-m91Neo (the same vector as in our prior studies [17]) was 45% at the time of transplant. In both arms of the experiment (fresh and transduced test cells), the competitor cells came from a common pool to permit direct comparison between the transduced and fresh test cell arms; furthermore, donor and host mice were age- and sex-matched, so that the primary variable being examined was the impact of the transduction protocol on test cell engraftment. Representative flow cytometry analyses from hosts five months post-transplant are shown in Figure 4A. The left panel shows the relative engraftment of fresh test cells, competitor cells and residual host cells. In the right panel, hosts were transplanted with a mixture of competitor cells, and test cells which were transduced using the 5-FU protocol. The composite data from the complete experiment is shown in Fig. 4B. On the left side of the graph is the “raw” test cell chimerism data, normalized per 106 test cells transplanted; thus, the repopulating ability of the transduced cells appears to compare favorably to that of fresh WBM on a “per-cell” basis. However, the 5-FU-transduced marrow used in this experiment is highly enriched for primitive cells compared to fresh WBM. The 5-FU-transduced marrow contained nearly 11-fold more SLK cells than did fresh WBM (2.22% vs. 0.25% SLK cells at the time of transplant). Furthermore, 2 × 106 5-FU-transduced marrow cells in this experiment represented 1.85 femur equivalents (FE) of marrow, whereas 2 × 106 fresh WBM cells represented only 0.18 FE. When test cell chimerism is normalized per FE of cells transplanted (Fig. 5B, right panel), it is evident that the transduced cells engrafted only 10.7% as well as fresh test cells. Thus, from this experiment we conclude that transduction with the 5-FU protocol impairs engraftment ~10-fold in 550 cGy-conditioned hosts, compared to fresh marrow.
Next, we employed this submyeloablative CRA to determine the repopulating ability of the clinically-relevant target of lin− marrow transduced with the lineage depletion protocol relative to that of fresh lin− cells. In this experiment, 106 fresh or transduced test cells from Boy J donors were mixed with 106 competitor cells from Bl/6 donors and transplanted into each of five 550- cGy-conditioned F1 hosts. The fraction of test cells expressing GFP at the time of transplant was 76%. A representative flow cytometry analysis is shown in Fig. 5A: in the left panel, the fresh test cells, competitor cells and residual host cells compete similarly for engraftment. The right panel, in which transduced test cells are utilized, demonstrates impaired engraftment of the transduced test cells, and enhanced engraftment of the fresh competitor cells. Figure 4B shows the cumulative results from the submyeloablative CRA experiment. The mean fresh test cell chimerism was 38 ± 1.1%, whereas the mean transduced test cell chimerism was 3 ± 0.7% (P < 0.0001) five months post-transplant. These data indicate that the transduction protocol used here decreases test cell competitiveness in submyeloablated hosts by ~10-fold, similar to that observed in 1100 cGy-conditioned hosts (Fig. 2).
DISCUSSION
We previously reported that retroviral transduction of 5-FU-treated murine marrow cells leads to an engraftment defect apparent only after transplantation into submyeloablated hosts, despite the ability of transduced cells to engraft in lethally-irradiated hosts equally well as cells treated only with 5-FU [17]. In the present work, we focused on quantifying the engraftment of transduced marrow cells in submyeloablated hosts. We developed an innovative “three-way” CRA assay (Fig. 3), which permits assessment of test, competitor and host cell chimerism, with which the engraftment of ex vivo cultured marrow cells could be quantitatively compared to fresh unmanipulated cells in submyeloablated hosts. We showed, when differences in cell phenotype and enrichment of more primitive cells were accounted for, that the engraftment of marrow transduced using a standard 5-FU-containing protocol was ~10-fold less than fresh marrow (Fig. 4). We also sought to determine if cells transduced using a more clinically-relevant transduction protocol lacking 5-FU treatment of donor mice and culture with bovine serum [18] would result in improved engraftment in submyeloablated hosts. On the contrary, despite the fact that cells transduced in this manner effectively repopulated lethally-irradiated hosts, the engraftment of transduced cells in submyeloablated hosts was significantly lower than freshly-isolated cells (Fig. 1). Furthermore, we determined that the repopulating ability of HSC transduced in this fashion was diminished ~10-fold compared to freshly isolated cells in ablated (Fig. 2) and in submyeloablated (Fig. 5) hosts. Our submyeloablative CRA provides a means with which to evaluate the effect of various aspects of ex vivo culture and/or transduction (e.g., medium, cytokines, viral vectors, etc.) upon engraftment in submyeloablated hosts.
Only a few published studies have attempted to quantitate the engraftment defect induced by ex vivo culture and/or retroviral transduction; these studies, however, were performed solely in ablated hosts. Qin et al. [28] were among the first to report that transduced HSC were at a relative disadvantage for engraftment, compared to fresh HSC, in ablated hosts. Bryder et al. [29] showed that transduction of murine HSC using an optimized protocol lacking the use of 5-FU and bovine serum markedly reduced competitive engraftment in ablated hosts, but that further ex vivo culture with cytokines could in part reverse the engraftment defect. Our results confirm that transduction with a clinically-relevant protocol impairs engraftment in ablated hosts (Fig. 2) and extend the findings of Bryder et al. [29] to additionally show a marked engraftment impairment in submyeloablated hosts (Fig. 4).
We used SCF and IL-6 in both transduction protocols in this work because this combination is known to enhance HSC self-renewal and mediates efficient retroviral transduction [30], and because of our past experience with this cocktail [17, 24, 27]. Of note, Li et al. [18] and Bryder et al. [29] used different cytokine cocktails for their work; numerous other cocktails have also been reported to maintain and/or expand HSC function in culture [31, 32] or augment retroviral transduction [18, 21, 33, 34]. The use of alternative cytokine cocktails may lead to disparate transduction and/or engraftment results, as could the use of producer cells or RetroNectin for transduction; determination of the impact of such factors remains to be determined. Furthermore, it seems reasonable that minimal ex vivo manipulation and transduction with non-retroviral vectors may result in superior levels of engraftment. Both lentiviral and foamy viral vectors transduce quiescent cells [35, 36], although less efficiently than dividing cells, and may allow transduced HSC to retain engraftment capabilities similar to unmanipulated cells. Mostoslavsky and colleagues [37] showed high levels (≥60%) of GFP expression in highly-purified murine side-population HSC transduced with lentiviral supernatant using a 4 hour incubation in SFM with SCF and thrombopoietin. Importantly, these authors demonstrated that cells transduced in this manner with lentiviral supernatant displayed repopulating ability similar to that of unmanipulated side-population cells in CRAs in ablated hosts [37]. The submyeloablative CRA described here is ideal for investigating the impact of various cytokine combinations, as well as the effect of post-transduction manipulations such as further culture with cytokines after transduction [29, 31] on engraftment.
In summary, we developed a novel CRA in submyeloablated hosts to quantify engraftment of transduced marrow cells. Engraftment in submyeloablated hosts is more stringent than engraftment in ablated hosts since clonal dynamics studies have shown that sublethal irradiation primarily affects progenitors and not HSC [38], resulting in host HSC recovery and competition with donor cells for engraftment; competition from host cells is lacking in ablated hosts. Our data underline the importance of studying engraftment with submyeloablative conditioning, in part because of the clinical implications for human gene therapy, but also because these studies can reveal engraftment defects which are not apparent in ablated hosts (Fig. 5; [17]). The use of CRAs in submyeloablated hosts will permit more thorough investigation of HSC repopulating ability following differing transduction protocols and conditioning regimens in future studies.
ACKNOWLEDGMENTS
This work was supported by American Cancer Society grant IRG-84-002-19, Clarian Health Partners Values Fund for Research grant VFR-145, and National Institutes of Health grant K08 HL75253 (to WSG); a Hope Street Kids grant (to KEP); and the Riley Children’s Foundation. The Indiana University Cancer Center Flow Cytometry Resource Facility is supported by the National Cancer Institute (P30 CA082709). The authors thank Dr. Mervin C. Yoder for many helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 2.Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- 3.Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Down J, Ploemacher R. Transient and permanent engraftment potential of murine hematopoietic stem cell subsets: differential effects of host conditioning with gamma radiation and cytotoxic drugs. Exp Hematol. 1993;21:913–921. [PubMed] [Google Scholar]
- 5.Tomita Y, Sachs D, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]
- 6.Stewart FM, Zhong S, Wuu J, Hsieh C, Nilsson SK, Quesenberry PJ. Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood. 1998;91:3681–3687. [PubMed] [Google Scholar]
- 7.Weinberg K, Kohn D. Gene therapy for congenital immunodeficiency diseases. Immunology and Allergy Clinics of North America. 1996;16:453–476. [Google Scholar]
- 8.Dunbar C. Gene transfer to hematopoietic stem cells: Implications for gene therapy of human disease. Annu Rev Med. 1996;47:11–20. doi: 10.1146/annurev.med.47.1.11. [DOI] [PubMed] [Google Scholar]
- 9.Kume A, Hanazono Y, Mizukami H, Urabe M, Ozawa K. Hematopoietic stem cell gene therapy: a current overview. Int J Hematol. 1999;69:227–233. [PubMed] [Google Scholar]
- 10.Schiffmann R, Medin J, Ward J, Stahl S, Cottler-Fox M, Karlsson S. Transfer of the human glucocerebrosidase gene into hematopoietic stem cells of nonablated recipients: Successful engraftment and long-term expression of the transgene. Blood. 1995;86:1218–1227. [PubMed] [Google Scholar]
- 11.Kittler E, Peters S, Crittenden R, et al. Cytokine-facilitated transduction leads to low-level engraftment in nonablated hosts. Blood. 1997;90:865–872. [PubMed] [Google Scholar]
- 12.Mardiney M, Jackson S, Spratt S, Li F, Holland S, Malech H. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood. 1997;89:2268–2275. [PubMed] [Google Scholar]
- 13.Bienzle D, Abrams-Ogg A, Kruth S, et al. Gene transfer into hematopoietic stem cells: Long-term maintenance of in vitro activated progenitors without marrow ablation. Proc Natl Acad Sci USA. 1994;91:350–354. doi: 10.1073/pnas.91.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barquinero J, Kiem H, von Kalle C, et al. Myelosuppressive conditioning improves autologous engraftment of genetically marked hematopoietic repopulating cells in dogs. Blood. 1995;85:1195–1201. [PubMed] [Google Scholar]
- 15.Kiem H, Andrews R, Morris J, et al. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 16.Tisdale JF, Hanazono Y, Sellers SE, et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 17.Goebel WS, Yoder MC, Pech N, Dinauer MC. Donor chimerism and stem cell function in a murine congenic transplantation model following low dose radiation conditioning: Effects of a retroviral-mediated gene transfer protocol and implications for gene therapy. Exp Hematol. 2002;30:1324–1332. doi: 10.1016/s0301-472x(02)00927-x. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Schwieger M, Lange C, et al. Predictable and efficient retroviral gene transfer into murine bone marrow repopulating cells using a defined vector dose. Exp Hematol. 2003;31:1206–1214. doi: 10.1016/j.exphem.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Ding C, Kume A, Bjorgvinsdottir H, Hawley R, Pech N, Dinauer M. High level reconstitution of respiratory burst activity in human X-linked chronic granulomatous disease (X-CGD) cell line and correction of murine X-CGD bone marrow cells by retroviral-mediated gene transfer of human gp91phox. Blood. 1996;88:1834–1840. [PubMed] [Google Scholar]
- 20.Bjorgvinsdottir H, Zhen L, Dinauer M. Cloning of murine gp91phox cDNA and functional expression in a human X-linked chronic granulomatous disease cell line. Blood. 1996;87:2005–2010. [PubMed] [Google Scholar]
- 21.Pollok KE, van Der Loo JC, Cooper RJ, et al. Differential transduction efficiency of SCID-repopulating cells derived from umbilical cord blood and granulocyte colony-stimulating factor-mobilized peripheral blood. Hum Gene Ther. 2001;12:2095–2108. doi: 10.1089/10430340152677430. [DOI] [PubMed] [Google Scholar]
- 22.Sadat MA, Pech N, Saulnier S, et al. Long-Term High-Level Reconstitution of NADPH Oxidase Activity in Murine X-Linked Chronic Granulomatous Disease Using a Bicistronic Vector Expressing gp91phox and a ΔLNGFR Cell Surface Marker. Hum Gene Ther. 2003;14:651–666. doi: 10.1089/104303403321618164. [DOI] [PubMed] [Google Scholar]
- 23.Wahlers A, Schwieger M, Li Z, et al. Influence of multiplicity of infection and protein stability on retroviral vector-mediated gene expression in hematopoietic cells. Gene Ther. 2001;8:477–486. doi: 10.1038/sj.gt.3301426. [DOI] [PubMed] [Google Scholar]
- 24.Goebel WS, Pech N, Meyers JL, Srour EF, Yoder MC, Dinauer MC. A murine model of antimetabolite-based, submyeloablative conditioning for bone marrow transplantation: biologic insights and potential applications. Exp Hematol. 2004;32:1255–1264. doi: 10.1016/j.exphem.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 26.Harrison DE, Jordan CT, Zhong RK, Astle CM. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 27.Barese C, Pech N, Dirscherl S, et al. Granulocyte colony-stimulating factor prior to nonmyeloablative irradiation decreases murine host hematopoietic stem cell function and increases engraftment of donor marrow cells. Stem Cells. 2007;25:1578–1585. doi: 10.1634/stemcells.2006-0808. [DOI] [PubMed] [Google Scholar]
- 28.Qin S, Ward M, Raftopoulos H, et al. Competitive repopulation of retrovirally transduced haemopoietic stem cells. Br J Haematol. 1999;107:162–168. doi: 10.1046/j.1365-2141.1999.01664.x. [DOI] [PubMed] [Google Scholar]
- 29.Bryder D, Bjorgvinsdottir H, Sasaki Y, Jacobsen SE. Deficiency of oncoretrovirally transduced hematopoietic stem cells and correction through ex vivo expansion. J Gene Med. 2005;7:137–144. doi: 10.1002/jgm.658. [DOI] [PubMed] [Google Scholar]
- 30.Luskey BD, Rosenblatt M, Zsebo K, Williams DA. Stem cell factor, interleukin-3, and interleukin-6 promote retroviral-meditated gene transfer into murine hematopoietic stem cells. Blood. 1992;80:396–402. [PubMed] [Google Scholar]
- 31.Takatoku M, Sellers S, Agricola BA, et al. Avoidance of stimulation improves engraftment of cultured and retrovirally transduced hematopoietic cells in primates. J Clin Invest. 2001;108:447–455. doi: 10.1172/JCI12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CC, Lodish HF. Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood. 2005;105:4314–4320. doi: 10.1182/blood-2004-11-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wognum AW, Visser TP, Peters K, Bierhuizen MF, Wagemaker G. Stimulation of mouse bone marrow cells with kit ligand, FLT3 ligand, and thrombopoietin leads to efficient retrovirus-mediated gene transfer to stem cells, whereas interleukin-3 and interleukin-11 reduce transduction of short- and long-term repopulating cells. Hum Gene Ther. 2000;11:2129–2141. doi: 10.1089/104303400750001435. [DOI] [PubMed] [Google Scholar]
- 34.Wu T, Kim HJ, Sellers SE, et al. Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods. Mol Ther. 2000;1:285–293. doi: 10.1006/mthe.2000.0034. [DOI] [PubMed] [Google Scholar]
- 35.Naldini L, Blomer U, Gallay P, et al. In vivo gene therapy and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 36.Trobridge G, Russell DW. Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors. J Virol. 2004;78:2327–2335. doi: 10.1128/JVI.78.5.2327-2335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Laukkanen MO, Kuramoto K, Calmels B, et al. Low-dose total body irradiation causes clonal fluctuation of primate hematopoietic stem and progenitor cells. Blood. 2005;105:1010–1015. doi: 10.1182/blood-2004-04-1498. [DOI] [PubMed] [Google Scholar]


