Abstract
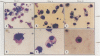
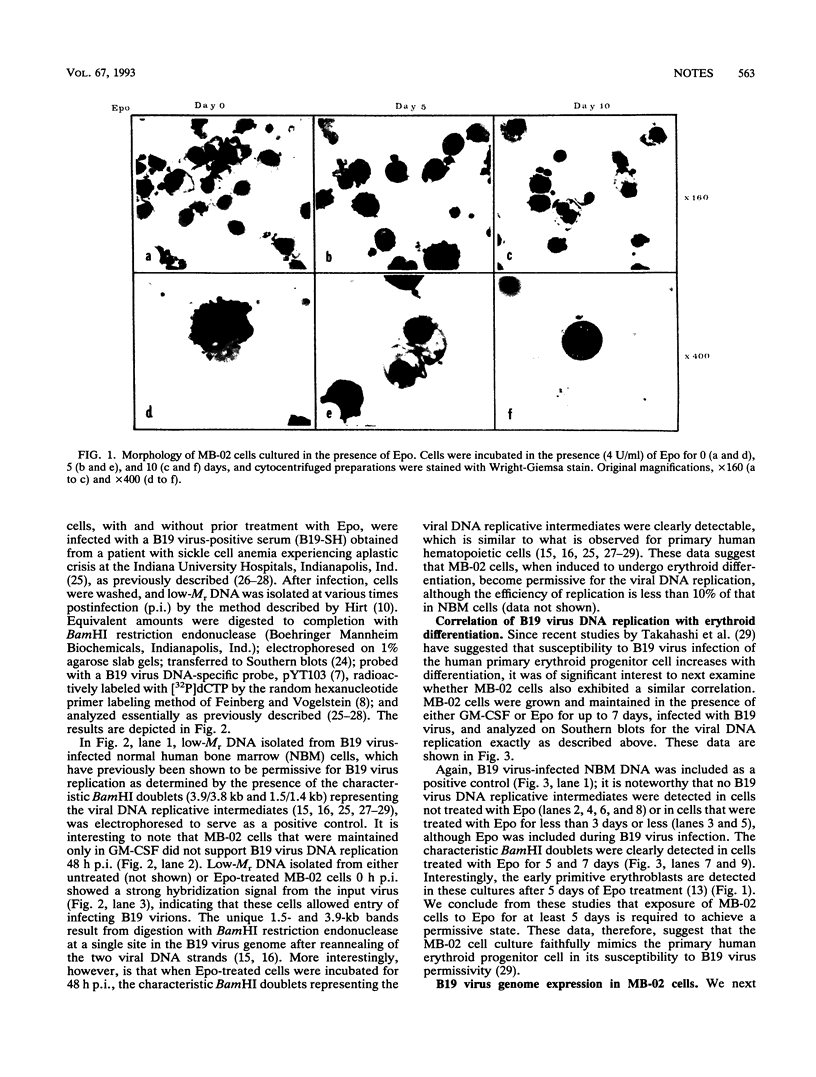
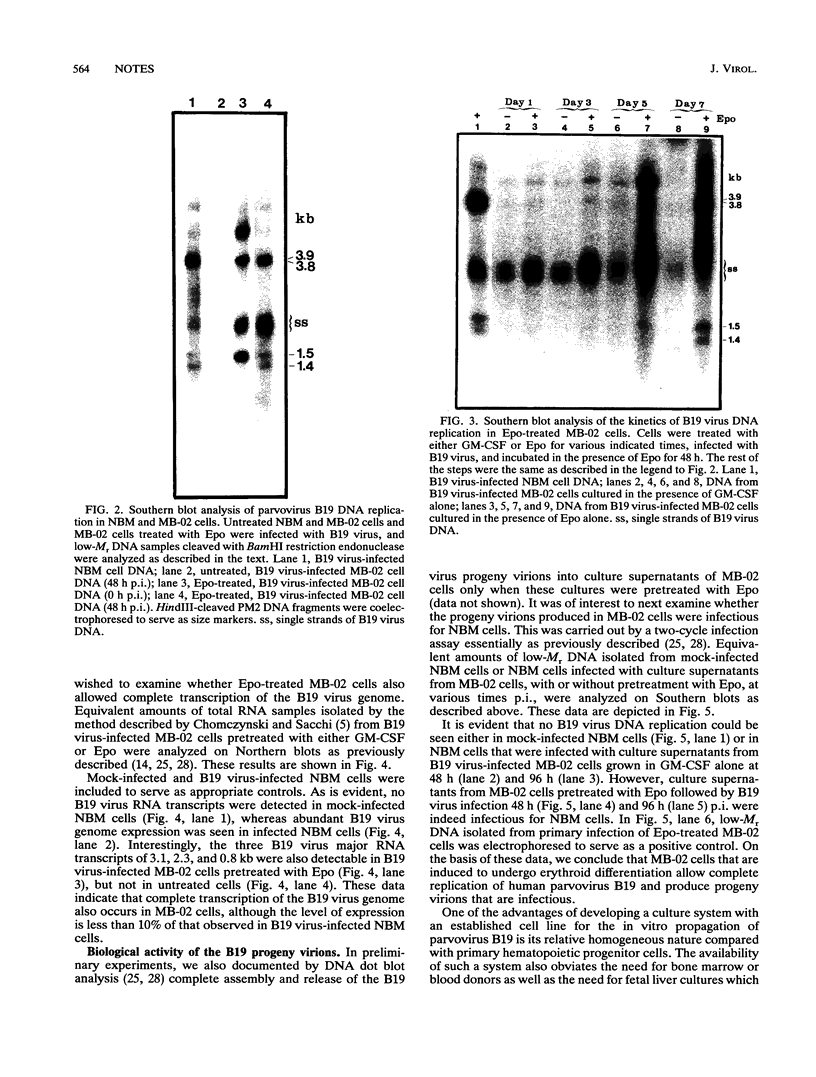
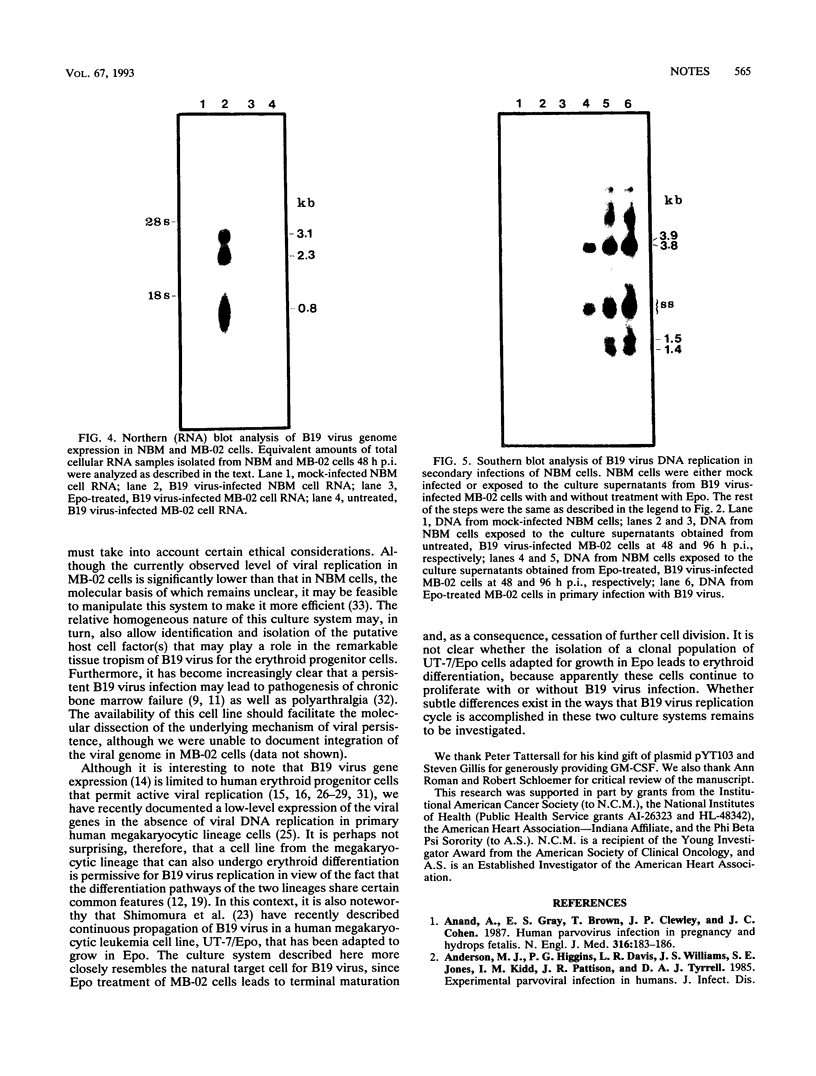
The pathogenic human parvovirus B19 has been shown to undergo productive replication in the erythroid lineage in primary normal human hematopoietic progenitor cells. However, none of the established erythroleukemia cell lines has allowed B19 virus replication in vitro. The remarkable erythroid tissue tropism of B19 virus was evaluated with a human megakaryocytic leukemia cell line, MB-02, which is dependent on the growth factor granulocyte-macrophage colony-stimulating factor but can be induced to undergo erythroid differentiation following treatment with erythropoietin (Epo). Whereas these cells did not support B19 virus DNA replication in the presence of granulocyte-macrophage colony-stimulating factor alone, active viral DNA replication was observed if the cells were exposed to Epo for 5 to 10 days prior to B19 virus infection, as detected by the presence of the characteristic B19 virus DNA replicative intermediates on Southern blots. No replication occurred if the cells were treated with Epo for 3 days or less. In addition, complete expression of the B19 virus genome also occurred in Epo-treated MB-02 cells, as detected by Northern blot analysis. B19 progeny virions were released into culture supernatants that were biologically active in secondary infection of normal human bone marrow cells. The availability of the only homogeneous permanent cell line in which induction of erythroid differentiation leads to a permissive state for B19 virus replication in vitro promises to yield new and useful information on the molecular basis of the erythroid tissue tropism as well as parvovirus B19-induced pathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand A., Gray E. S., Brown T., Clewley J. P., Cohen B. J. Human parvovirus infection in pregnancy and hydrops fetalis. N Engl J Med. 1987 Jan 22;316(4):183–186. doi: 10.1056/NEJM198701223160403. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Jones S. E., Fisher-Hoch S. P., Lewis E., Hall S. M., Bartlett C. L., Cohen B. J., Mortimer P. P., Pereira M. S. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet. 1983 Jun 18;1(8338):1378–1378. doi: 10.1016/s0140-6736(83)92152-9. [DOI] [PubMed] [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984 Nov 3;2(8410):1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frickhofen N., Abkowitz J. L., Safford M., Berry J. M., Antunez-de-Mayolo J., Astrow A., Cohen R., Halperin I., King L., Mintzer D. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med. 1990 Dec 15;113(12):926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kurtzman G. J., Ozawa K., Cohen B., Hanson G., Oseas R., Young N. S. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987 Jul 30;317(5):287–294. doi: 10.1056/NEJM198707303170506. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Gumucio D. L., Brodsky I. Granulocyte-macrophage colony-stimulating factor-dependent growth and erythropoietin-induced differentiation of a human cell line MB-02. Blood. 1991 Dec 1;78(11):2860–2871. [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Hao Y. S., Kurtzman G., Shimada T., Young N. Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol. 1987 Aug;61(8):2395–2406. doi: 10.1128/jvi.61.8.2395-2406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987 Aug;70(2):384–391. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Reid D. M., Reid T. M., Brown T., Rennie J. A., Eastmond C. J. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet. 1985 Feb 23;1(8426):422–425. doi: 10.1016/s0140-6736(85)91146-8. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Saarinen U. M., Chorba T. L., Tattersall P., Young N. S., Anderson L. J., Palmer E., Coccia P. F. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986 May;67(5):1411–1417. [PubMed] [Google Scholar]
- Schwarz T. F., Serke S., Hottenträger B., von Brunn A., Baurmann H., Kirsch A., Stolz W., Huhn D., Deinhardt F., Roggendorf M. Replication of parvovirus B19 in hematopoietic progenitor cells generated in vitro from normal human peripheral blood. J Virol. 1992 Feb;66(2):1273–1276. doi: 10.1128/jvi.66.2.1273-1276.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Shimomura S., Komatsu N., Frickhofen N., Anderson S., Kajigaya S., Young N. S. First continuous propagation of B19 parvovirus in a cell line. Blood. 1992 Jan 1;79(1):18–24. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Bruno E., Briddell R., Cooper R., Srivastava C., van Besien K., Hoffman R. Parvovirus B19-induced perturbation of human megakaryocytopoiesis in vitro. Blood. 1990 Nov 15;76(10):1997–2004. [PubMed] [Google Scholar]
- Srivastava A., Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol. 1988 Aug;62(8):3059–3063. doi: 10.1128/jvi.62.8.3059-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava C. H., Samulski R. J., Lu L., Larsen S. H., Srivastava A. Construction of a recombinant human parvovirus B19: adeno-associated virus 2 (AAV) DNA inverted terminal repeats are functional in an AAV-B19 hybrid virus. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8078–8082. doi: 10.1073/pnas.86.20.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava C. H., Zhou S., Munshi N. C., Srivastava A. Parvovirus B19 replication in human umbilical cord blood cells. Virology. 1992 Aug;189(2):456–461. doi: 10.1016/0042-6822(92)90569-b. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Ozawa K., Takahashi K., Asano S., Takaku F. Susceptibility of human erythropoietic cells to B19 parvovirus in vitro increases with differentiation. Blood. 1990 Feb 1;75(3):603–610. [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A. Human parvovirus arthropathy. Lancet. 1985 Feb 23;1(8426):419–421. doi: 10.1016/s0140-6736(85)91145-6. [DOI] [PubMed] [Google Scholar]
- Yaegashi N., Shiraishi H., Takeshita T., Nakamura M., Yajima A., Sugamura K. Propagation of human parvovirus B19 in primary culture of erythroid lineage cells derived from fetal liver. J Virol. 1989 Jun;63(6):2422–2426. doi: 10.1128/jvi.63.6.2422-2426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]