Abstract
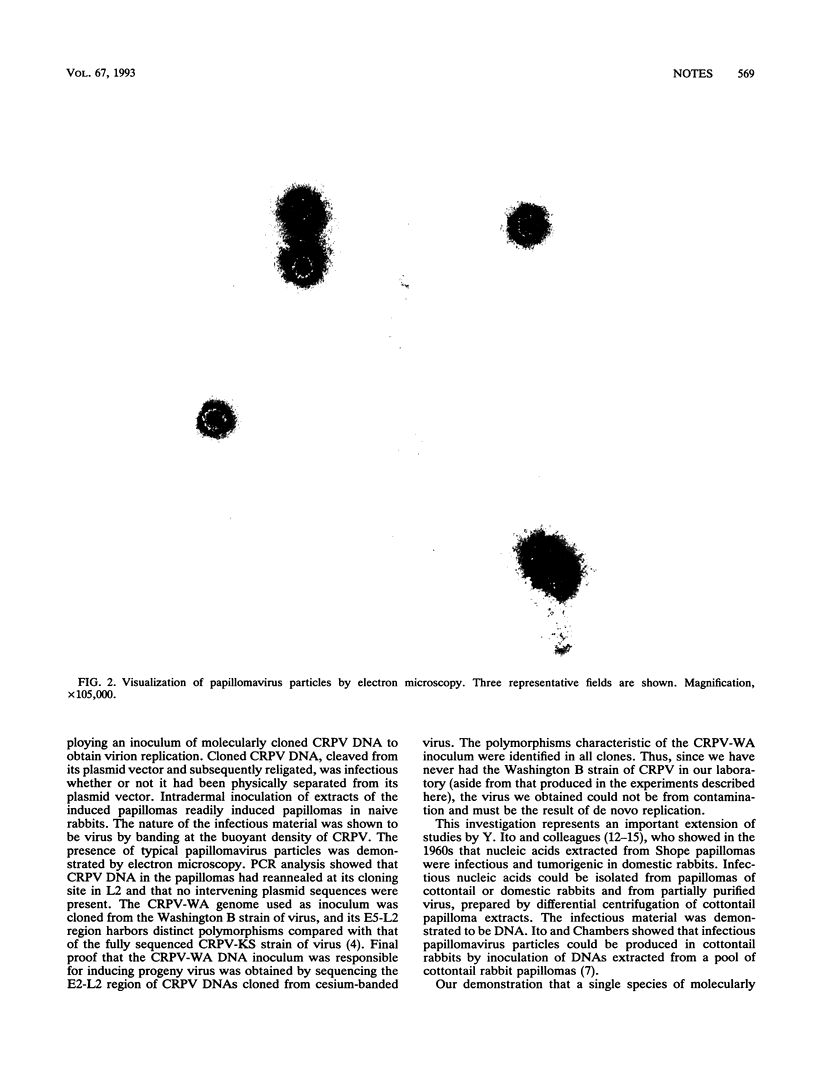
The ability to obtain infectious papillomavirus virions from molecularly cloned DNA has not been previously reported. We demonstrate here that viral genomes isolated from a recombinant++ DNA clone of cottontail rabbit papillomavirus (CRPV) gave rise to infectious virus when inoculated into cottontail rabbit skin. Replication occurred in papillomas that formed at inoculation sites. Extract of a DNA-induced papilloma was serially passaged to naive rabbits with high efficiency. Complete virus was fractionated on cesium chloride density gradients, and papillomavirus particles were visualized by electron microscopy. CRPV DNA isolated from virions contained DNA sequence polymorphisms that are characteristic of the input CRPV-WA strain of virus, thereby proving that the newly generated virus originated from the molecularly cloned viral genome. These findings indicate that this will be a useful system in which to perform genetic analysis of viral gene functions involved in replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREEDIS C., BERWICK L., ANDERSON T. F. Fractionation of Shope papilloma virus in cesium chloride density gradients. Virology. 1962 May;17:84–94. doi: 10.1016/0042-6822(62)90084-3. [DOI] [PubMed] [Google Scholar]
- Brandsma J. L., Abramson A. L. Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg. 1989 May;115(5):621–625. doi: 10.1001/archotol.1989.01860290079018. [DOI] [PubMed] [Google Scholar]
- Brandsma J. L., Yang Z. H., Barthold S. W., Johnson E. A. Use of a rapid, efficient inoculation method to induce papillomas by cottontail rabbit papillomavirus DNA shows that the E7 gene is required. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J. L., Yang Z. H., DiMaio D., Barthold S. W., Johnson E., Xiao W. The putative E5 open reading frame of cottontail rabbit papillomavirus is dispensable for papilloma formation in domestic rabbits. J Virol. 1992 Oct;66(10):6204–6207. doi: 10.1128/jvi.66.10.6204-6207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS V. C., ITO Y. MORPHOLOGY OF SHOPE PAPILLOMA VIRUS ASSOCIATED WITH NUCLEIC ACID-INDUCED TUMORS OF COTTONTAIL RABBITS. Virology. 1964 Jul;23:434–436. doi: 10.1016/0042-6822(64)90269-7. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollard S. C., Wilson J. L., Demeter L. M., Bonnez W., Reichman R. C., Broker T. R., Chow L. T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992 Jul;6(7):1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- Giri I., Danos O., Yaniv M. Genomic structure of the cottontail rabbit (Shope) papillomavirus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1580–1584. doi: 10.1073/pnas.82.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO Y. A tumor-producing factor extracted by phenol from papillomatous tissue (Shope) of cottontail rabbits. Virology. 1960 Dec;12:596–601. doi: 10.1016/0042-6822(60)90182-3. [DOI] [PubMed] [Google Scholar]
- Ito Y. HEAT-RESISTANCE OF THE TUMORIGENIC NUCLEIC ACID OF SHOPE PAPILLOMATOSIS. Proc Natl Acad Sci U S A. 1961 Dec;47(12):1897–1900. doi: 10.1073/pnas.47.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Patrick S. D., Cladel N. M., Welsh P. A. Experimental infection with human papillomavirus type 1 of human hand and foot skin. Virology. 1990 Jul;177(1):415–417. doi: 10.1016/0042-6822(90)90503-j. [DOI] [PubMed] [Google Scholar]
- Krieg P., Amtmann E., Sauer G. The simultaneous extraction of high-molecular-weight DNA and of RNA from solid tumors. Anal Biochem. 1983 Oct 15;134(2):288–294. doi: 10.1016/0003-2697(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Meyers C., Frattini M. G., Hudson J. B., Laimins L. A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992 Aug 14;257(5072):971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Meyers C., Wettstein F. O. Genetic analysis of CRPV pathogenesis: the L1 open reading frame is dispensable for cellular transformation but is required for papilloma formation. Virology. 1989 May;170(1):321–325. doi: 10.1016/0042-6822(89)90388-7. [DOI] [PubMed] [Google Scholar]
- SYVERTON J. T. The pathogenesis of the rabbit papilloma-to-carcinoma sequence. Ann N Y Acad Sci. 1952 Jul 10;54(6):1126–1140. doi: 10.1111/j.1749-6632.1952.tb39983.x. [DOI] [PubMed] [Google Scholar]
- Sterling J., Stanley M., Gatward G., Minson T. Production of human papillomavirus type 16 virions in a keratinocyte cell line. J Virol. 1990 Dec;64(12):6305–6307. doi: 10.1128/jvi.64.12.6305-6307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]