Abstract
Tyrosine residues of neuroendocrine peptides are frequently the targets of oxidation reactions, one of which involves hydroxylation to peptidyl-3, 4-dihydroxy-phenyl-L-alanine (DOPA). The reactivity in vitro of peptidyl-DOPA in two neuroendocrine peptides, a neurotensin fragment (pELYENK) and proctolin (RYLPT), was investigated using ultraviolet-visible scanning spectrophotometry and matrix-assisted laser desorption ionization mass spectrometry following oxidation by tyrosinase and periodate. The peptides form covalently coupled dimers and trimers, and their masses are consistent with the presence of diDOPA cross-links. Lysine does not appear to participate in multimer formation because it is efficiently recovered in fragmentation ladders using subtilisin. While multimer formation in the neurotensin-derived peptide can be blocked effectively by adding N-acetyl-DOPA-ethylester to the reaction medium, the DOPA ethylester couples itself four to five times to each peptide.
Keywords: Neuroendocrine, tyrosine, DOPA peptides, cross-linking, tyrosinase
Tyrosine residues play a commanding role in the structure-activity relationships of many neuropeptides. β-Opioid peptides such as endorphin, antidiuretics (oxytocin, vasopressin), proctolin, neurotensin, angiotensin, and dynorphin, for example, all contain key tyrosine residues that tolerate little modification without loss of activity (e.g., Schröder and Hempel 1965). Peptidyl tyrosine is well-tailored to be a target of intermolecular recognition: hydrophobic, ionic (as the deprotonated phenolate), hydrogen bonding, π-π (Burley and Petsko 1985) and π-cation (Minoux and Chipot 1999) interactions all contrive to ensure many opportunities for highly specific interaction with receptors (Hofstädter et al. 1999). Given its tendency for one- and two-electron oxidations, however, the presence of tyrosine also marks those peptides and proteins bearing it with liability. Such oxidations can lead to loss of function through undesirable halogenation (Chowdhury et al. 1995), nitration (Busby and Gan 1975), hydroxylation (Gieseg et al. 1993; Cohen et al. 1998), and dityrosine formation (Souza et al. 2000; Michon et al. 1997).
We are interested here in exploring the reaction pathway of peptidyl tyrosine following its hydroxylation to peptidyl-3, 4-dihydroxy-phenyl-L-alanine (DOPA) in two neuropeptide sequences: neurotensin (Vincent et al. 1999) and proctolin (Konopinska and Rosinski 1999). DOPA is a major by-product in several proteins following hydroxyl radical attack (Cohen et al. 1998) or prolonged irradiation by ultraviolet (UV) light (Kato et al. 1995). DOPA formation, per se, may or may not compromise function of the neuropeptide. In proctolin, for example, the DOPA analog has higher activity than proctolin in certain target cell lines (Konopinska and Rosinski 1999). However, the facile oxidation of DOPA peptides leads to complications far beyond peptide function. Oxidation products of DOPA have been implicated in DNA damage (Spencer et al. 1994), excitotoxin formation (Newcomer et al. 1995), neuronal apoptosis (Walkinshaw and Waters 1995), and formation of pathoneuronal inclusions (Montine et al. 1995). Having said this, it must be added that the oxidation chemistry of DOPA is quite complicated, and often there has been more speculation than fact about the specific oxidation products involved. The most common naturally occurring DOPA-containing proteins are adhesives used by marine invertebrates (Waite 1990). In the course of maturation, these proteins become oxidized. Until recently, there has been some controversy regarding the chemical pathway of maturation (Rzepecki and Waite 1990). Much of this was put to rest by the noninvasive, nondestructive detection of diDOPA formation using solid-state nuclear magnetic resonance (McDowell et al. 1999). The present study attempts to determine whether diDOPA formation is limited specifically to marine adhesive proteins or has a broader relevance to oxidized tyrosine-containing proteins generally. Our results suggest the latter.
Results
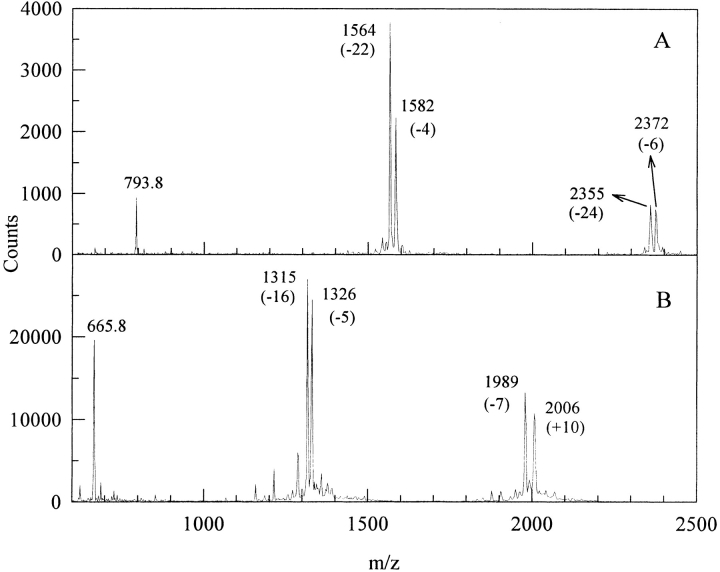
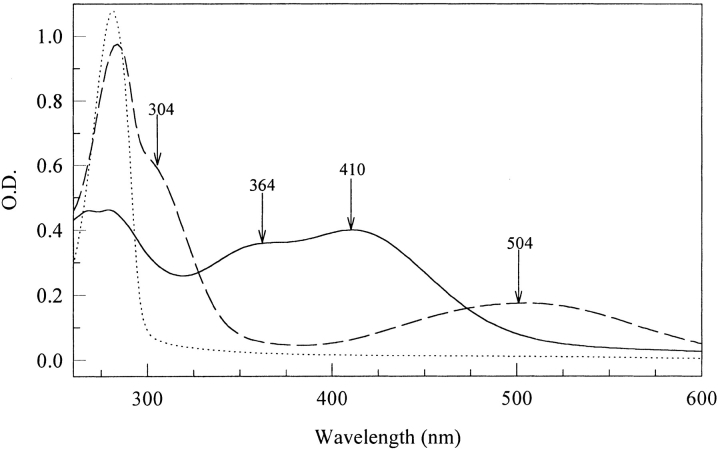
Tyrosine residues in model neuropeptides can be converted to DOPA residues in several ways including hydroxyl radical attack, UV irradiation and with enzymes. Tyrosinase-catalyzed DOPA formation was chosen here because it is certainly the gentlest and most specific method. In vitro oxidation of the DOPA-analogs of neurotensin N6 and proctolin leads to rapid multimer formation as measured by MALDI TOF (Matrix Assisted Laser Desorption/Ionization − Time of Flight) mass spectrometry (Fig. 1 ▶). Singly charged [M+H] + species for the peptides are converted to dimeric and trimeric forms, which lie 2–5 Da below the expected mass gain per noncovalently coupled multimer, e.g., [2M+H] + (Table 1). Another peak occurs at 18 Da lower than the multimer peak (Fig. 1A ▶). UV-Vis scans taken during the course of the enzyme or periodate-induced oxidation exhibit the standard attributes of quinones (λ 305 nm) and quinone-amine adducts (500 nm) (Fig. 2 ▶). The two peptides differ notably by the 350 nm shoulder in tyrosinase-oxidized DOPA-neurotensin N6 (Fig. 2A ▶). This is reminiscent of dehydroDOPA derivatives, which form by tautomerization from quinone methides (Taylor et al. 1991; Rzepecki et al. 1991).
Fig. 1.
MALDI TOF of the DOPA analogs of neurotensin N6 (A) and proctolin (B) cross-linked with tyrosinase. The peptides were incubated at a 50:1 peptide to enzyme weight ratio in a 10-mM HEPES pH 7.5 with stirring at room temperature. These spectra correspond to a 30-min incubation period. Parenthetical numbers correspond to the mass loss as a result of cross-linking and oxidation of one or more DOPA functionalities in the multimers.
Table 1.
Chemical data for neurotensin N6 derivatives and oligomers
| N6 Derivative | Mass (calculated) | Mass (observed) | λmax (observed) nm |
| Neurotensin N6 | 776.8 | 776.8 | 276 |
| DOPA-N6 | 792.8 | 792.8 | 280 |
| Oxidised DOPA-N6 | 790.8 | 790.5 | 502/305 |
| Dimer | 1585.6 | 1581.0 | 410/364 |
| Trimer | 2378.4 | 2371.0 | 410/364 |
Fig. 2.
Ultraviolet (UV)-visible spectra of DOPA-neurotensin N6 incubated with tyrosinase (A) and periodate (B). In both cases, the peptide was incubated in a 10-mM HEPES pH 7.5 buffer at room temperature. Tyrosinase was added at a 50:1 peptide to enzyme ratio. The scans correspond to 0, 5, 15, 30, 60, and 120 min. Periodate was added at different molar ratios to the DOPA content. Scans correspond to 0:1, 1:1, 10:1, 20:1, 30:1, and 50:1 periodate:DOPA molar ratios.
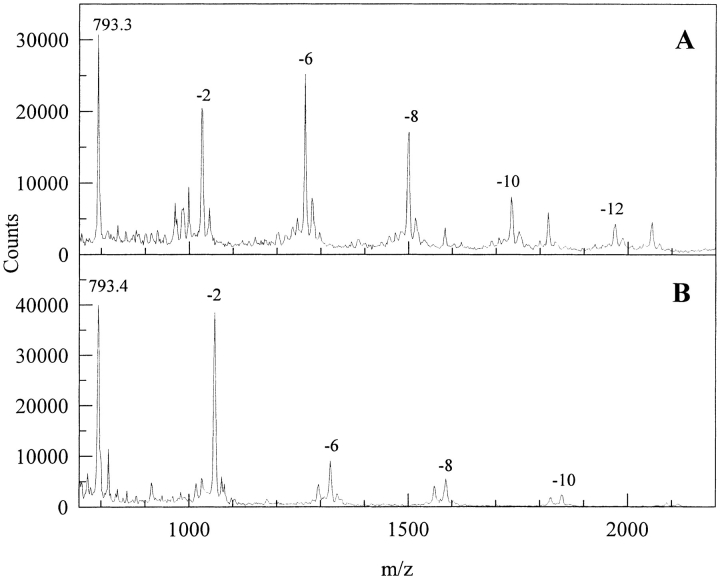
The extreme sensitivity of multimer formation to the addition of simple DOPA analogs is revealed in Figure 3A and 3B ▶. Here, all evidence of dimers and trimers has been supplanted by the multiple (up to 5Xs) coupling of low-molecular-weight DOPA analogs to the peptide. Again, each step in the coupling ladder is marked by a 2-Da loss.
Fig. 3.
The tyrosinase-catalyzed oxidation of the DOPA analog of neurotensin N6 in the presence of N-acetyl DOPA amide (A) and N-acetyl DOPA ethylester (B). Reaction conditions are as in Figure 1 ▶. Competitive inhibitors were added in a 10-molar excess to the peptide-DOPA content. Numbers indicate the mass lost with each addition of the DOPA analog.
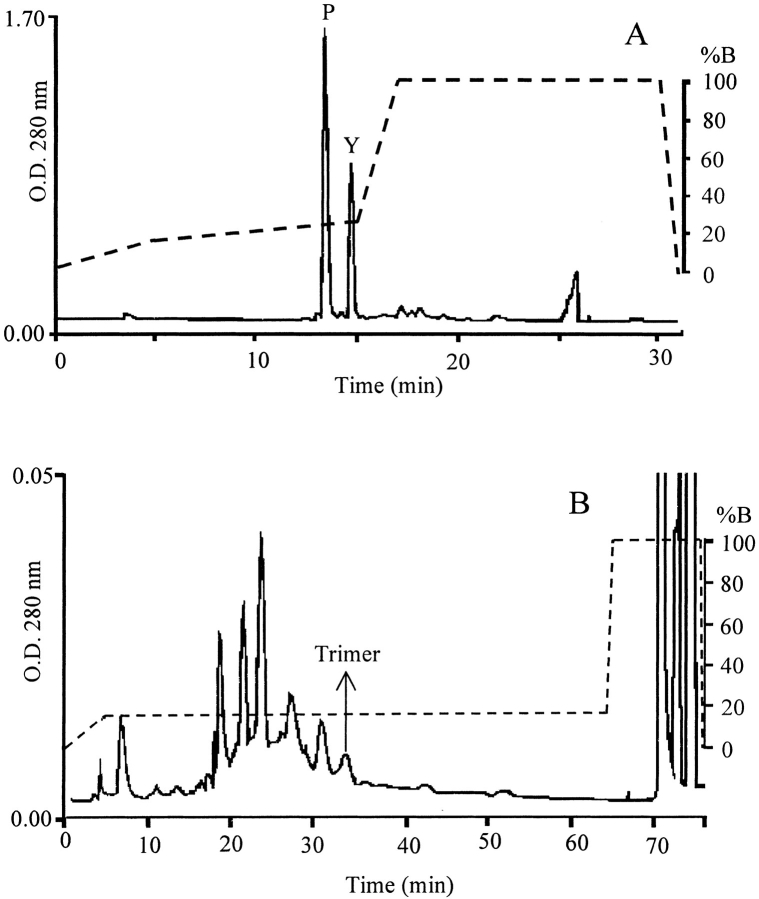
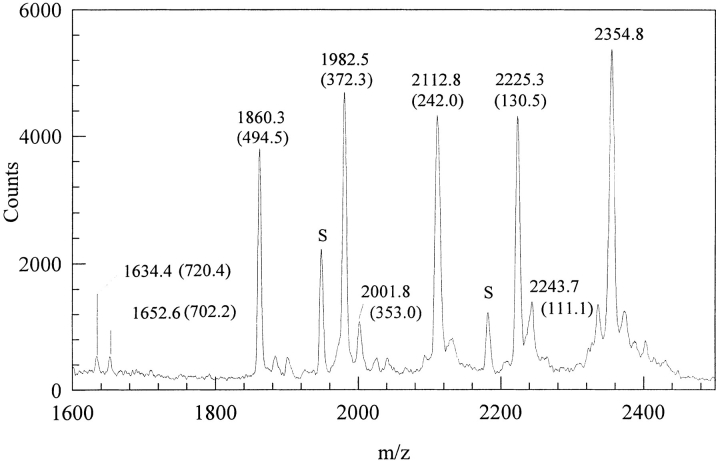
Given the rapid and extensive conversion of oxidized DOPA-neurotensin N6 to dimeric and trimeric multimers, we attempted a purification of the multimers with the aim of identifying the coupling site in a multimer. Two peaks are separated by reverse-phase high-pressure liquid chromatography (HPLC) (Fig. 4A ▶); these show a limited separation of monomer and oxidized monomer (-2 Da) for the first peak, and dimers (−3 Da and -22 Da) as well as trimers in the second (Table 1; Fig. 5 ▶). The UV-Vis spectra for the oxidized monomer and dimer show significant differences. The monomer shows a quinone absorbance at 305 nm and is pink (504 nm). In contrast, the dimer/trimer is yellow (410 nm) with a shoulder at 364 nm (Fig. 6 ▶). Better resolution of multimers is afforded by a slight reduction in the acetonitrile gradient (Fig. 4B ▶) resulting in fractions with homogeneous oligomers. A trimer was used for sequencing with MALDI TOF (Fig. 7 ▶). Upon digestion with subtilisin, the trimer showed mass changes consistent with the removal of K, N, and E from the C-terminus, and pyroglutamate (pE) from the N-terminus (Fig. 7 ▶ and Table 2). There was no evidence of Leu or DOPA release.
Fig. 4.
HPLC of monomer and oligomer separation (A). HPLC of dimers and trimers (B). Columns and elution profiles (corresponding to the dashed line) are as described in Materials and Methods. Absorbance was measured at 280 nm. In A, P is pink peak and Y is yellow peak. In B, arrow indicates fraction corresponding to the sequenced trimer.
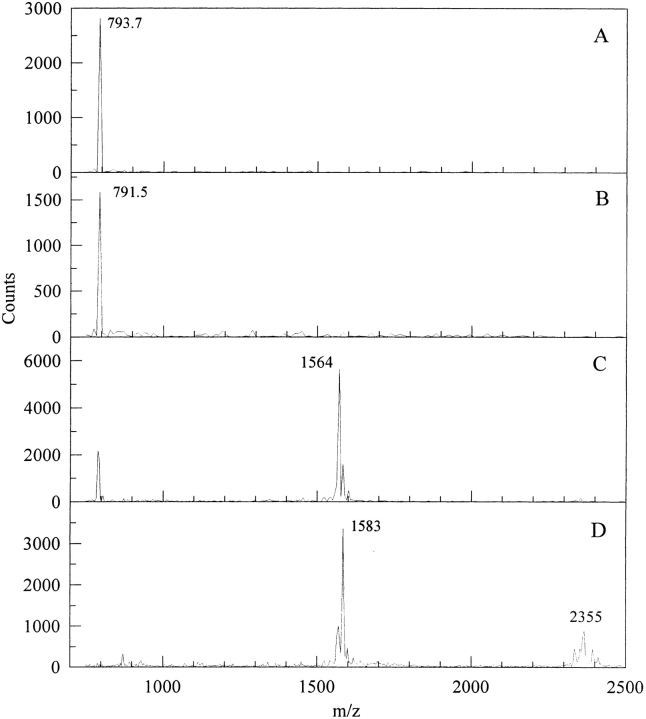
Fig. 5.
MALDI TOF spectrum of fractions from the fractions of the peaks from Figure 4A ▶. (A) and (B) correspond to fractions of the pink peak, (C) and (D) correspond to those of the yellow peak. Reaction conditions are as in Figure 1 ▶.
Fig. 6.
Ultraviolet (UV)-Vis spectra of HPLC peaks from Figure 4A ▶. Unoxidized DOPA-neurotensin N6 (|) yellow (—) and pink (– –) peaks.
Fig. 7.
MALDI TOF sequencing of a trimer of oxidized DOPA-neurotensin N6. The trimer was incubated with a 10:1 peptide:subtilisin ratio (w/w) in a 10-mM HEPES pH7.5 buffer. The spectrum corresponds to a 30-min digest. Peak labels in parenthesis reflect the mass lost to digestion, and are explained further in Table 2. Peaks labeled "S" correspond to subtilisin fragments.
Table 2.
Mass losses calculated and observed for the subtilisin degradation of a trimer of DOPA modified neurotensin N6
| Digested Fragment | Mass Loss (calculated) | Mass Loss (observed) |
| pE | 111.1 | 111.1 |
| K | 128.2 | 130.5 |
| -N-K | 242.3 | 242.0 |
| pE-, -N-K | 353.4 | 353.0 |
| -E-N-K | 371.4 | 372.3 |
| pE-, 3(-K) | 495.7 | 494.5 |
| 3(pE-), -L-, 2(-K) | 702.8 | 702.2 |
| 2(pE-), 3(-K), -N- | 720.9 | 720.4 |
The observed masses are those from the parenthetical numbers of Figure 7. ▶
Discussion
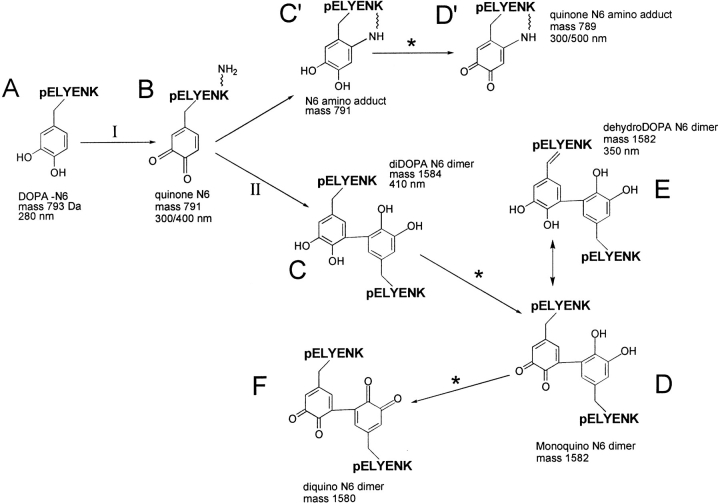
The DOPA analogs of neurotensin and proctolin undergo covalent coupling during oxidation by tyrosinase or periodate. This was to be expected based on an earlier study of DOPA-containing adhesive protein-derived decapeptides (Burzio and Waite 2000); upon oxidation, decapeptides formed multimers as high as 8-mers, while neurotensin-N6 and proctolin are both limited to trimers and dimers. The bigger question has to do with how the peptides are coupled. There are two common coupling paradigms: 1) amine-quinone adducts (Michael-type additions) such as dopachrome (Nagatsu 1973) and lysyl-dopaquinone (Wang et al. 1996) with λmax at ∼500 nm, and 2) polyphenolic coupling products such as 5, 5′diDOPA (McDowell et al. 1999) with λmax at ∼420 nm (Andersen et al. 1992) (Fig. 8 ▶). Curiously, there is evidence for both forms in the present study: a 500 nm peak was detected in the oxidized neurotensin N6 (Fig. 3 ▶) and in the oxidized purified monomer (Fig. 6 ▶). This cannot be dopachrome because DOPA's α-amino group is blocked in a peptide bond. Detection of the pink oxidized monomer is especially intriguing because it might represent a stable intramolecular adduct of Lys-6 and the quinone at DOPA-3 such as that found in lysyl oxidase (Wang et al 1996) (Fig. 8D ▶). Its structure needs further characterization. Dimers (Fig. 8C ▶–F) and trimers, on the other hand, absorb maximally at 420 and 334 nm, but not at 500 nm. Accordingly, there is a decrease of 2 Da per each ring-C to ring-C bond formed (Fig. 8 ▶). Another 2 Da would be lost for each coupled DOPA that is reoxidized to o-Dopaquinone (Fig. 8D and F ▶) or DOPA-quinone methide. Moreover, the absorbance shoulder at 350 to 364 nm in neurotensin suggests that some dehydroDOPA (E) is formed from the quinone by tautomerization (Rzepecki et al. 1991). Reoxidation of this to quinone would entail the loss of an additional 2 Da. Subtilisin digestion of the trimer indicates that there are no hitches in peptide bond cleavage until the Leu-DOPA sequence is encountered. This blockage may be steric as a result of peptide coupling through the DOPA residues, or, in the case of dehydroDOPA formation, to the planar α-C (enamide) of DOPA.
Fig. 8.
Proposed pathway of DOPA neurotensin N6 cross-linking. Step I is catalyzed by tyrosinase in the presence of O2. Reoxidations at steps marked * may also involve the enzyme. The aryl coupling step at II requires one equivalent each of DOPA-N6 and quinone-N6, which dismutate to two DOPA-semiquinones. Specific ring positions coupled remain to be determined; they were 5, 5′ in McDowell et al. 1999.
Neurotensin-N6 coupling through DOPA is the most reasonable explanation for the present results, and diDOPA is a good candidate for the cross-link. The fact that dimers and trimers of oxidized neurotensin N6 were competitively inhibited by low-molecular-weight DOPA analogs adds weight to this. 5, 5′-DiDOPA was detected in DOPA-containing adhesive proteins (McDowell et al. 1999) and in mussel adhesive decapeptides oxidized in vitro (Burzio and Waite 2000). Notably, one of the aromatic partners in the DOPA-derived cross-links of neurotensin-N6 would appear to be dehydroDOPA (λmax 364 nm); this was not observed in the decapeptides, but it was reported previously in oxidized N-acetylDOPA-ethyl ester (Rzepecki et al. 1991; Taylor et al. 1991) and in intact oxidized mefp-1 (Burzio 1999). In summary, these results are consistent with aryl (diDOPA) coupling as the cause of multimer formation in neuropeptide-derived sequences. The specific details of substitution remain to be determined. DiDOPA coupling chemistry is likely to be much more common than previously thought; it certainly is not limited to marine adhesive proteins.
Materials and methods
Neurotensin fragment 1–6 (N6) [pELYENK] and proctolin [RYLPT] were purchased from Sigma Chemical Corp. (St. Louis, MO). Sodium meta-periodate and mushroom tyrosinase were both from Sigma Chemical Corp. The latter had a specific activity of 3400 units/mg−1. Subtilisin, a nonspecific endoprotease, was obtained from Roche Diagnostics (Indianapolis, IN), and was used to sequence peptide oligomers by MALDI-MS. Human angiotensin and a fragment of ACTH (18–39), used as calibration standards for MALDI TOF MS, were obtained from Sigma Chemical Corp.
Peptide hydroxylation
The hydroxylation of the Tyr residues of the peptides was done according to published procedure (Marumo and Waite 1986). Briefly, the peptides were dissolved in 4 mL of 100 mM phosphate pH 7.5 with 25 mM ascorbic acid at room temperature with constant stirring and aeration. Tyrosinase (for the monophenolase activity, a unit is defined as a change in absorbance of 0.001 min-1 mL-1 at 280 nm with L-tyrosine as the substrate) was added and the reaction was allowed to proceed for 60 min. The reaction was terminated by adding 25 μL 6 N HCl/mL.
The differentially hydroxylated peptides were separated by reverse-phase HPLC on a C-8 column (Brownlee RP-300) using the following gradient made by mixing 0.1% (v/v) trifluoroacetic acid (TFA) in water with 0.1% (v/v) TFA in acetonitrile (B): 0 to 15% in 45 min, to 100% at 46 min. 1-mL fractions were collected, lyophilized, and reconstituted in water. The extent of hydroxylation was determined by MALDI TOF.
Peptide oxidation
Mushroom tyrosinase also was used as an oxidant (SA = 3400 units/mg where each unit equals a difference in absorbance at 265 nm of 0.001 min-1 mL-1 at pH 6.5 at 25°C in a reaction mixture containing either L-DOPA or catechol and ascorbate). The oxidation reaction was carried out on hydroxylated peptides in the absence of ascorbate by adding 0.5 mg enzyme/mL at a 50:1 peptide:enzyme weight ratio in 10 mM HEPES pH 7.5 with stirring at room temperature.
Oxidation with periodate was carried out in the same incubation conditions. Periodate addition was done at different molar ratios to the DOPA present.
Separation of monomer from oligomers
The N16-D peptide was cross-linked as described in the previous section. To separate the dimer and trimer from any monomer the polymerized peptide mix was run on a Brownlee Aquapore analytical C-4 HPLC column (40 mm × 250 mm) (Rainin Medical Instruments, Woburn, MA). Two different gradients were employed: one to separate the oligomers from the monomers (0%–10%B in 5 min, 10%–20%B in 10 min, and a wash at 100% for 5 min) and another for separating the dimers and trimers (0%–16%B in 5 min, 16%–21%B in 50 min, and a wash at 100%B for 10 min).
Product characterization
UV-Vis absorbance was measured on a Hewlett-Packard diode array spectrophotometer (HP Model 8453) equipped with interface 35900E and ChemStation software.
Matrix-assisted laser desorption ionization mass spectrometry with time-of-flight analysis (MALDI TOF) was done using a PE Biosystems Voyager DE instrument with delayed extraction. Matrix (α-cyano-3-hydroxycinnamic acid) was prepared as a saturated solution in 1:1 (v/v) water and acetonitrile with 0.1% TFA. All samples were run in linear mode with 20 kV accelerating voltage, 18.96 kV grid voltage, and 2 V guide wire voltage. Typical relative laser power was 1800. Neurotensin fragment 1–6 (MW 776.8), human angiotensin (MW 1297.5) and ACTH fragment 18–39 (MW 2465.7) were used as internal and external calibrants.
Subtilisin digestion
Subtilisin digestion was done on purified dimers and trimers of cross-linked DOPA-N6. The peptides were incubated with the enzyme at a weight ratio of 1:10 (enzyme:peptide) in a 10-mM HEPES pH 7.5 buffer. Aliquots were taken at different times, mixed with MALDI matrix, and deposited on the MALDI sample plate. The samples then were analyzed by MALDI TOF. Cleaved amino acids were identified by the mass differences when compared to the original mass of the oligomer.
Acknowledgments
We thank Dr. G. Nicol for his mass spectrometry assistance and discussions. Dr. L. Rzepecki synthesized and generously donated the DOPA analogs. This work was funded by the Office of Naval Research and the Biomaterials Program at National Institutes of Dental Craniofacial Research (NIDCFR).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.44201.
References
- Burley, S.K. and Petsko, G.A. 1985. Aromatic-aromatic interaction: A mechanism of protein structure stabilization. Science 229 23–28. [DOI] [PubMed] [Google Scholar]
- Burzio, L.A. 1999. Formation of DOPA derived cross-links in mussel byssal proteins and peptides. Ph.D. Thesis. University of Delaware, Newark, DE.
- Burzio, L.A. and Waite, J.H. 2000. Cross-linking in adhesive quinoproteins: Studies with model decapeptides. Biochemistry 39 11147–11153. [DOI] [PubMed] [Google Scholar]
- Busby, T.F. and Gan, J.C. 1975. Reaction of tetranitromethane with human plasma α1-antitrypsin. Int J Biochem 6 835–841. [Google Scholar]
- Chowdhury, S.K., Eshraghi, J., Wolfe, H., Forde, D., Hlavac, A.G., and Johnston, D. 1995. Mass spectrographic identification of amino acid transformations during oxidation of peptides and proteins: Modifications of methionine and tyrosine. Anal Chem 34 390–398. [DOI] [PubMed] [Google Scholar]
- Cohen, G., Yakushin, S., and Dembiec-Cohen, D. 1998. Protein Dopa as an index of hydroxyl radical attack on protein tyrosine. Anal Biochem 263 232–239. [DOI] [PubMed] [Google Scholar]
- Gieseg, S.P., Simpson, J.A., Charlton, T.S., Duncan, M.W., and Dean, R.T. 1993. Protein bound DOPA is a major reductant formed during hydroxyl damage to proteins. Biochemistry 32 4780–4786. [DOI] [PubMed] [Google Scholar]
- Hofstädter, K., Stuart, F., Jiang, L., Vrijbloed, J.W., and Robinson, J.A. 1999. On the importance of being aromatic at an antibody-protein antigen interface. J Mol Biol 285 805–815. [DOI] [PubMed] [Google Scholar]
- Kato, Y., Nishikawa, T., and Kawakishi, S. 1995. Formation of protein-bound 3, 4-dihydroxyphenylalanine in collagen types I and IV exposed to UV light. Photochem Photobiol 61 367–372. [DOI] [PubMed] [Google Scholar]
- Konopinska, D. and Rosinski, G. 1999. Proctolin, an insect neuropeptide. J Peptide Sci 5 533–546. [DOI] [PubMed] [Google Scholar]
- Marumo, K. and Waite, J.H. 1986. Optimization of hydroxylation of tyrosine and tyrosine-containing peptides by mushroom tyrosinase. Biochem Biophys Acta 872 98–103 [DOI] [PubMed] [Google Scholar]
- McDowell, L.M., Burzio, L.A., Waite, J.H., and Schaefer, J. 1999. Rotational echo double resonance detection of cross-links formed in mussel byssus under high-flow stress. J Biol Chem 274 20293–20295. [DOI] [PubMed] [Google Scholar]
- Michon, T., Chenu, M., Kellershon, N., Desmadril, M., and Gueguen, J. 1997. Horseradish peroxidase oxidation of tyrosine-containing peptides and their subsequent polymerization: A kinetic study. Biochemistry 36 8504–8513. [DOI] [PubMed] [Google Scholar]
- Minoux, H. and Chipot, C. 1999. Cation-π interactions in proteins: Can simple models provide an accurate description. J Am Chem Soc 121 10366–10372. [Google Scholar]
- Montine, T.J., Farris, D.B., and Graham, D.G. 1995. Covalent cross-linking of neurofilament proteins by oxidized catechols as a potential mechanism of Lewy body formation. J Neuropath Exp Neurol 54 311–319. [DOI] [PubMed] [Google Scholar]
- Nagatsu, T. 1973. Biochemistry of Catecholamines. University Park Press, Baltimore, MD.
- Newcomer, T.A., Rosenberg, P.A., and Aizenman, E. 1995, Iron-mediated oxidation of DOPA to an excitotoxin. J Neurol 64:1742–1748. [DOI] [PubMed] [Google Scholar]
- Rzepecki, L.M. and Waite, J.H. 1991. DOPA proteins: Versatile varnishes and adhesives from marine fauna. Bioorgan Mar Chem 4 119–148. [Google Scholar]
- Rzepecki, L.M., Nagafuchi, T., and Waite, J.H. 1991. α, β-Dehydro-3, 4-dihydroxyphenylalanine derivatives: Potential sclerotization intermendiates in natural composite materials. Arch. Biochem Biophys 285 17–26. [DOI] [PubMed] [Google Scholar]
- Schröder, E. and Hempel, R. 1965. Über Peptidylsynthesen. Über Angiotensin-Analoga. Justus Liebigs Ann Chem 684 243–251. [DOI] [PubMed] [Google Scholar]
- Smith, G.J. and Haskell, T.G. 2000. The fluorescent oxidation productions of dihydroxyphenylalanine and its esters. J Photochem Photobiol B Biol 55 103–108. [DOI] [PubMed] [Google Scholar]
- Spencer, J.P.E., Jenner, A., Aruoma, O.I., Evans, P.J., Kaur, H., Dexter, D.T., Jenner, P., Lees, A.J., Marsden, D.C., and Halliwell, B. 1994. Intense oxidative DNA damage promoted by L-DOPA and its metabolites: Implications for neuro-degenerative disease. FEBS Lett 353 246–250. [DOI] [PubMed] [Google Scholar]
- Souza, J.M., Giasson, B.I., Chen, Q., Lee, V.M.Y., and Ischiropoulos, H. 2000. Dityrosine cross-linking promotes formation of stable α-synuclein polymers. J Biol Chem 275 18344–18349. [DOI] [PubMed] [Google Scholar]
- Taylor, S.W., Molinski, T.F., Rzepecki, L.M., and Waite, J.H.. 1991. Oxidation of peptidyl-DOPA analogues: Implications for the synthesis of tunichromes and related oligopeptides. J Nat Prod 54 918–922. [DOI] [PubMed] [Google Scholar]
- Vincent, J.P., Mazella, J., and Kiabgi, P. 1999. Neurotensin and neurotensin receptors. TiPS 20 302–309. [DOI] [PubMed] [Google Scholar]
- Waite, J.H. 1990. The phylogeny and chemical diversity of quinone-tanning: A review. Comp Biochem Physiol 97B 19–29. [DOI] [PubMed] [Google Scholar]
- Walkinshaw, G. and Waters, C.M. 1995. Induction of apoptosis in catecholaminergic PC12 cells by L-Dopa. J Clin Invest 95 2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.X., Mure, M., Medzihradsky, K.F., Burlingame, A.L., Brown, D.E., Dooley, D. M., Smith, A.J., Kagan, H.K., and Klinman J.P. 1996. A cross-linked cofactor in lysyl oxidase: redox function for amino acid side chains. Science 273 1078–1084. [DOI] [PubMed] [Google Scholar]