Abstract
A method is described for dissolving and disaggregating chemically synthesized polyglutamine peptides. Polyglutamine peptides longer than about Q20 have been reported to be insoluble in water, but dissolution in – and evaporation from - a mixture of trifluoroacetic acid and hexafluoroisopropanol converts polyglutamine peptides up to at least Q44 to a form readily soluble in aqueous buffers. This procedure also has a dramatic effect on peptides which appear to be completely soluble in water, by removing traces of aggregate that seed aggregation. The protocol makes possible solution studies—including in vitro aggregation experiments—on polyglutamine peptides with repeat lengths associated with increased risk of Huntington's Disease and other expanded CAG repeat diseases. It may also be useful in conducting reproducible, quantitative aggregation studies on other polypeptides.
Keywords: Polyglutamine, disaggregation, nucleation-dependent aggregation, seed, Huntington's disease
The misassembly of proteins and peptides into highly insoluble, noncovalent aggregates is a process of tremendous practical and fundamental importance. The formation of amyloid fibrils and other aggregates in vivo plays a role in a number of human neurodegenerative (Martin 1999) and other (Sipe 1992) diseases, as well as a growing list of non-Mendelian trait transfers in microbial genetics (Lindquist 1997). Aggregate formation is also important in biotechnology, controlling both inclusion body formation in recombinant expression (Mukhopadhyay 1997) and the efficiency with which dissolved and denatured inclusion bodies refold (Bernardez-Clark et al. 1999). The existence of the molecular chaperones (Fink and Goto 1998), a family of proteins responsible for suppressing aggregate formation in the cell, suggests that the efficiency of cellular protein biosynthesis is normally tempered by the intrinsic propensity of polypeptides to enter aggregation side reactions during the folding process. Many of these aggregation processes can be effectively modeled in simple, defined systems in vitro, making possible important studies on the mechanisms of assembly and the structures of the assembled products (Kelly and Lansbury 1994). In some cases, however, the apparent insolubility of the starting peptide in denaturing solvents places significant limitations on our ability to study fundamental aspects of aggregation. In other cases, studies of an apparently soluble peptide by different laboratories give dramatically different aggregation kinetics consistent with the presence of variable trace amounts of aggregation seeds in the starting materials.
The genetic defect in the expanded CAG repeat diseases involves a length increase of normally benign polyglutamine (polyGln) sequences in certain proteins, generally from a wild-type length <38 Gln residues to a pathological length greater than about 40 Gln residues (Cummings and Zoghbi 2000). Because one hypothesis of the disease mechanism is that polyGln aggregation is responsible for neurotoxicity (Cummings and Zoghbi 2000), it is possible that a demonstration of length dependence in polyGln aggregation might account for the length dependence of disease risk. In fact, studies using biosynthetic polyGln-containing fusion proteins, in which polyGln-rich peptide fragments are generated in situ by limited proteolysis, generally support this length dependence: PolyGln sequences of Q25 were found to aggregate inefficiently, while sequences of Q35 or higher aggregate readily (Scherzinger et al. 1999). In contrast to this data, however, experiments in other laboratories using chemically synthesized peptides suggest that a Q15 peptide is only modestly soluble and aggregates quickly at pH 7 (Perutz et al. 1994) and that peptides longer than Q22 are insoluble (Sharma et al. 1999). To date, it has not been possible to account for these two contrasting results on the solubility and aggregation tendencies of polyGln peptides in the Q15–Q20 range.
We describe here conditions that allow complete solubilization and disaggregation of chemically synthesized polyGln peptides up to repeat lengths of at least Q44, making controlled studies on the kinetics of formation and morphologies of polyGln aggregates from simple well-defined peptides possible for the first time. The results generally support conclusions about the length dependence of polyGln solubility based on studies of polyGln protein fragments (Scherzinger et al. 1999). We expect that this protocol may also prove useful in the complete solubilization and disaggregation of other amyloidogenic peptides.
Results and Discussion
Previously, Zagorski and colleagues noted that synthetic samples of the Alzheimer's peptide Aβ can be effectively disaggregated through a sequential treatment with the volatile solvents TFA and HFIP (Zagorski et al. 1999). In this protocol, synthetic peptide is dissolved in TFA, after which the TFA is volatilized. After one or more such treatments, a similar dissolution/evaporation cycle is performed with HFIP. The peptide residue from this treatment, dissolved in aqueous buffer, has improved solubility and shows no evidence of aggregates. Perhaps the most sensitive test for the presence of peptide aggregates is aggregation kinetics. Thus, freshly dissolved samples from some batches of Aβ(1–40) rapidly aggregate to amyloid fibrils at pH 7 because of the presence of preexisting aggregates acting as seeds. If the same peptide is first exposed to HFIP treatment, however, it requires days for initiation of aggregation under the same conditions (Wood et al. 1996). Disaggregation treatments employing removable, volatile solvents are potentially more robust than the use of nonvolatile disaggregating solvents like DMSO, as use of the latter requires permanent introduction of the disaggregant as a cosolvent in the final reaction mixture, which, for example, can bias aggregation kinetics (Zagorski et al. 1999). TFA treatment is also more effective than DMSO at eliminating residual levels of soluble aggregates, as evidenced by reduced line broadening in NMR spectra (Zagorski et al. 1999).
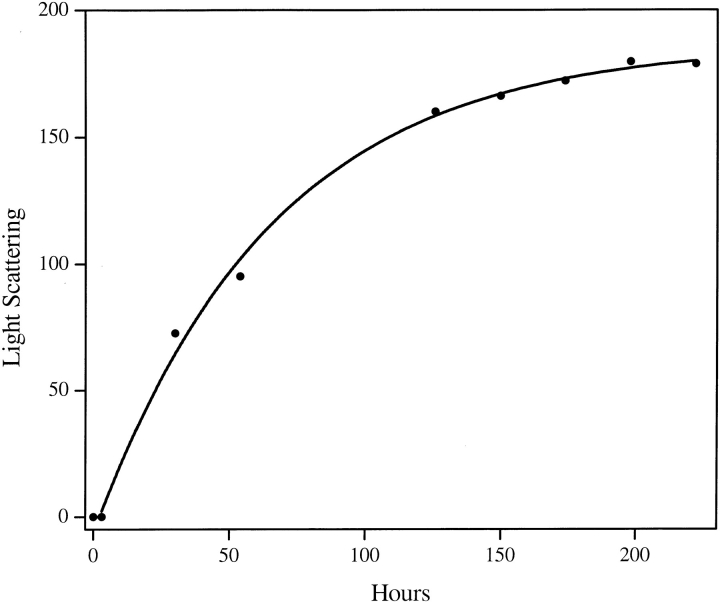
We obtained mixed results in initial attempts to apply the published protocol, involving sequential TFA–HFIP treatment (Zagorski et al. 1999), to our polyGln peptides. While this protocol is effective at solubilizing and disaggregating peptides in the range Q15–Q35, we found it to be poorly effective with peptides >Q35 because of their limited solubilities in 100% TFA. In an attempt to improve solubility in the organic solvent, we exposed polyGln peptides to a 1 : 1 mixture of TFA and HFIP. We found that peptides like K2Q44K2 are poorly soluble in pH 3 water and incompletely soluble in 100% TFA but are completely dissolved by a 1 : 1 mixture of TFA and HFIP. We also found that volatilization of this solvent mixture provides a Q44 peptide that is readily soluble in pH3 water and, once dissolved, is devoid of detectable aggregates. Figure 1 ▶ shows the aggregation kinetics of this rigorously disaggregated K2Q44K2.
Fig. 1.
Time course of aggregation for a 57-μg/mL (9.2 μM) solution of K2Q44K2 in pH 7.5 PBS at 37°C. Lyophilized peptide was pretreated with a 1 : 1 mixture of TFA and HFIP as described in the Materials and Methods section. The reaction was monitored by 90° light scattering in a fluorometer with excitation and emission wavelengths set to 450 nm. The curve shown derives from a fit of the data to a first order reaction equation. The zero hour time point was not included in this fit, reflecting a short lag time for this peptide before onset of aggregation.
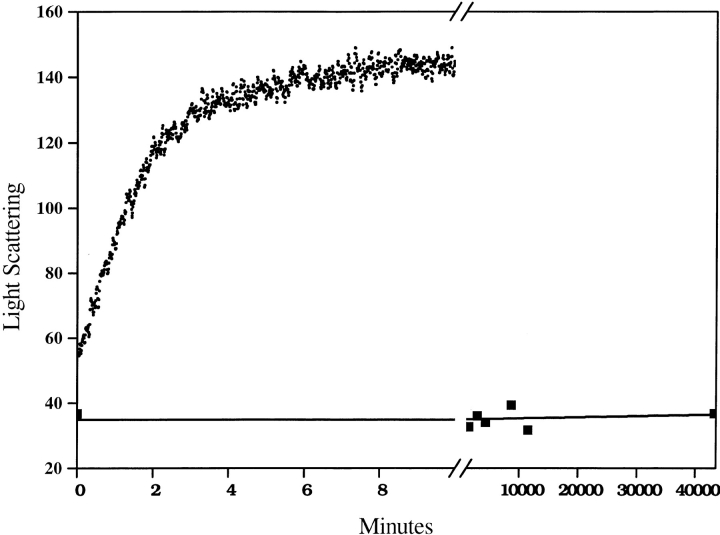
Besides improving recovery of soluble peptide, and hence expanding the range of peptides whose aggregation properties can be studied, the protocol described here also produces dramatic changes in the aggregation kinetics of peptides that at first appearance do not seem to require pretreatment for solubility. Figure 2 ▶ shows the aggregation of the previously described (Perutz et al. 1994) model peptide D2Q15K2. When this lyophilized peptide is directly brought up in pH 3 water, it appears to be dissolved by both visual inspection and by a 90° light scattering measurement (not shown). When the peptide is adjusted to pH 7.5 with 10× PBS, rapid aggregation results (Fig. 2 ▶). However, when the lyophilized peptide is pretreated with TFA/HFIP, as described here, dissolved in pH 3 water, and then adjusted to pH 7.5, the resulting solution is stable for at least 1 mo at 37°C, exhibiting no aggregation as judged by light scattering and by HPLC (Fig. 2 ▶). This implies that when the lyophilized D2Q15K2 peptide is directly dissolved in pH 3 water without prior treatment, it contains contaminating seed aggregates and/or residual secondary structure within the peptide that render it susceptible to rapid aggregation.
Fig. 2.
Time course of aggregation of a 7-μM solution of D2Q15K2 at pH 7.5 and 37°C, when synthetic peptide was dissolved directly in pH 3 water before adjusting to 7.5 (top curve, rapid aggregation) or pretreated with the TFA-HFIP protocol before dissolving in pH 3 water and then adjusting to pH 7.5 (bottom curve, closed squares, no aggregation). The reaction is monitored by 90° light scattering as in Figure 1 ▶. A colorimetric protein assay (BCA reagent, Pierce Chemical) confirmed that at least 90% of the untreated peptide (top curve) was aggregated after several minutes at pH 7.5. HPLC analysis confirmed that 93% of the TFA/HFIP treated peptide (bottom curve) remained in solution after 28 d.
These results on a rigorously disaggregated, chemically synthesized Q15 peptide are consistent with the results from biosynthetic polyGln-containing proteins (Scherzinger et al. 1999), suggesting that only polyGln peptides significantly longer than Q15 spontaneously aggregate readily at μM concentrations. (After 7 mo incubation at ph 7 and 37°C, a disaggregated Q15 sample is partially aggregated, implying that aggregation under these conditions is thermodynamically favored, despite the significant kinetic barrier.) The radically different aggregation kinetics for Q15 versus Q44 polyGln peptides reported here and elsewhere (Scherzinger et al. 1999) are consistent with hypothetical disease mechanisms connecting the length dependence of disease risk to the length dependence of polyGln aggregation.
To assess whether prolonged exposure of polyGln peptides to the solvent disaggregation protocol might introduce any chemical changes in the peptide, we incubated samples of the peptide K2Q15K2 overnight at RT in a 50 : 50 mixture of TFA and HFIP, as well as TFA alone and HFIP alone. In excellent agreement with the calculated MW of 2453.68, the reconstructed +1 state parent ion of the starting peptide from electrospray mass spectrometry was calculated to be 2352.50. The corresponding reconstructed +1 states after the three solvent treatments were 2352.40, 2352.40, and 2352.40, respectively. No new fragments were observed in the mass spectra after these solvent treatments.
We have not conducted an exhaustive survey of solubilization conditions for polyGln peptides. As reviewed above, long polyGln peptides obtained directly as lyophilized solid phase synthesis products are insoluble in pH 3 aqueous TFA and are only partially soluble in neat TFA. These peptides are insoluble in methanol and ethanol. A K2Q41K2 peptide is soluble up to at least 1 mg/mL in 100% DMSO, and time-dependent aggregation, including a short lag phase, is observed when this stock solution is diluted 20-fold into PBS. However, we have not conducted a detailed analysis of reaction kinetics and product structure from polyGln solubilized in DMSO and aggregated in DMSO-containing solutions.
The ability of the protocol reported here to solubilize longer polyGln peptides will allow generation of soluble polyGln peptides in the pathological length range of Q35, and higher, as subjects for biophysical and kinetic analysis, which will open up new avenues for the study of the molecular mechanisms of expanded CAG repeat diseases. It should now be possible to conduct spectroscopic studies to characterize the monomeric states of these peptides as well as their structural transitions as they progress into the aggregated state. Several other aggregation-prone peptides, including sequence variants of Aβ, have been effectively disaggregated by this protocol in our laboratory (data not shown), suggesting that the protocol may be useful in the analysis of aggregation phenomena exhibited by other polypeptides. It is not clear, however, whether this treatment will prove to be equally effective on all poorly soluble, aggregation-prone polypeptides, especially larger protein molecules. In the case of globular proteins, which have highly populated native folded states that are optimized for aqueous solubility, treatment with organic solvents may induce aggregation-prone misfolded states. Further experiments will be required to explore the wider application of the solvent treatment described here to aggregated globular proteins.
The success of this protocol with polyGln peptides seems to depend on the ability of the TFA/HFIP solvent mixture to effectively break some resistant internal structure within polyGln peptide aggregates. The nature of this structure and the mechanism by which it is broken by mixed TFA/HFIP treatment is at present not known. The known ability of TFA to provide special properties to peptides as a counter ion (Pearson and McCroskey 1996) cannot explain these results, as TFA is present in both experiments shown in Figure 2 ▶. It is possible that some lyophilized synthetic peptides may contain extensively intra- and/or intermolecular H-bonded monomers and/or aggregates, in analogy to many aggregates of misfolded proteins (Clark et al. 1981), and that mixed TFA-HFIP is particularly effective at disrupting this H-bonded structure.
These results may have implications beyond the field of protein aggregation studies per se. Experiments using chemically synthesized peptides to model portions of protein structures are often defeated because of peptide insolubility. The experiments described here suggest that some peptides that appear completely insoluble in aqueous buffers can be rendered at least transiently soluble by a rigorous disaggregation protocol. In fact, depending on the magnitude of its characteristic critical concentration (Harper and Lansbury 1997), some "problem" peptides—once they are solubilized and disaggregated—may be stable in solution at modest concentrations almost indefinitely.
Materials and methods
Materials and general methods
Peptides were purchased as unpurified custom peptides from the Keck Biotechnology Center of Yale University. The flanking lysine pairs were added to the peptide design to bestow a net charge on the peptide at pH 7 and thus improve initial solubility; no experiments were conducted, however, to test the solubility of polyglutamine peptides lacking such flanking charged groups. While shorter polyGln peptides like K2Q15K2 were essentially pure by HPLC and MS analysis, K2Q44K2 contained, as expected, some Gln deletion peptides derived from incomplete coupling in the solid phase synthesis. Peptide amounts were established with the aid of a standard polyGln peptide prepared as follows. A sample of a K2Q15K2 peptide was dissolved in aqueous buffer by the disaggregation protocol described in this article, and its was concentration determined by amino acid composition analysis (Commonwealth Biotechnologies). This standard was aliquoted and frozen at −80°C for future use. The area under the A215 peak in the HPLC profile obtained from a known volume of the K2Q15K2 standard was used to generate a conversion factor to calculate the weight concentrations of other polyGln peptides. HPLC analysis was used to adjust starting concentrations and to monitor aggregation reactions by analysis of centrifugation supernatants of reaction aliquots. Mass spectrometry was conducted on a Sciex 150EX (Perkin Elmer) at an orifice voltage of 55 V.
Methods
All steps involving TFA and HFIP should be performed in a fume hood using appropriate protection. The Erlenmeyer flask should be covered with a cap or parafilm during mixing.
The basic protocol for peptide disaggregation is as follows. To 1–5 mg of peptide (lyophilized powder from solid-phase peptide synthesis) is added 1 : 1 TFA/HFIP to generate a 0.5 mg/mL suspension in a 20-mL glass Erlenmeyer flask. The suspension is incubated at room temperature (RT) with vortexing until visual inspection indicates the aggregate has dissolved and then further incubated, for a total time of 0.5–4 h, as discussed below. Solvent is then removed at RT under a stream of argon gas directed through a Pasteur pipet into the flask. Gas flow is continued 15–30 min after visible removal of solvent. Immediately after gas flow is terminated, distilled water, previously adjusted to pH 3 with TFA, is added, to give a concentration of 100–200 μM. The pH 3 solution of the peptide can be transferred to Eppendorf tubes for storage frozen (−80°C) or immediately used for aggregation kinetics. For the former, aliquots should be snap frozen in liquid nitrogen or dry ice/ethanol before storage at −80°C.
Aggregates can persist in the TFA/HFIP mixture after all visible traces of insoluble peptide have disappeared. To check for this, after apparent solubilization has been achieved, a test aliquot of the solution is dried under argon and resuspended in pH 3 water according to the above protocol. The aqueous 100–200 μM solution should exhibit low 90° light scattering, comparable to that of water alone, in a fluorometer with excitation and emission wavelengths set to 450 nm. If scattering is high, incubation of the main solubilization reaction in 1 : 1 TFA : HFIP is continued until light scattering in this test is reduced to the background level of aqueous buffer alone. Alternatively, peptide can be incubated in the TFA/HFIP buffer, with occasional swirling, for 4 h or more to insure complete solubilization.
Residual aggregates are removed by centrifugation of the pH 3 aqueous solution for 3 h at 50,000g and 4°C, and the top two-thirds of the solution are decanted and used for kinetic experiments. PolyGln peptides aggregate faster at pH 7 than at pH 3, so it is possible to clear aggregates by centrifugation at pH 3. This step may not be feasible for other peptides, depending on the pH dependence of their aggregation kinetics.
Caution should be taken in storing disaggregated peptides in pH 3 buffer; we found that polyGln peptides can exhibit a time-dependent aggregation in the pH 3 buffer, even at 4°C. Immediate snap freezing of peptides in liquid nitrogen, followed by storage at −80°C, preserves the disaggregated state of dilute polyGln solutions for at least several months of storage (V. Berthelier, unpubl.). Storage at −20°C is not sufficient to protect against aggregation.
Acknowledgments
R.W. acknowledges support from the Lindsay Young Alzheimer's Disease Gift Fund and from the Hereditary Disease Foundation Lieberman Award. We thank Angela Williams and Charles Murphy for the mass spectrometric analysis.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.42301
References
- Bernardez-Clark, E.D., Schwarz, E., and Rudolph, R. 1999. Inhibition of aggregation side reactions during in vitro protein folding. Meths. Enzymol. 309 217–236. [DOI] [PubMed] [Google Scholar]
- Clark, A.H., Saunderson, D.H., and Suggett, A. 1981. Infrared and laser-Raman spectroscopic studies of thermally-induced globular protein gels. Int. J. Pept. Protein Res. 17 353–364. [DOI] [PubMed] [Google Scholar]
- Cummings, C.J. and Zoghbi, H.Y. 2000. Fourteen and counting: Unraveling trinucleotide repeat diseases. Hum. Mol. Genet. 9 909–916. [DOI] [PubMed] [Google Scholar]
- Fink, A.L. and Goto Y. (Eds.) 1998. Molecular chaperones in the life cycle of proteins. Dekker, New York.
- Harper, J.D. and Lansbury Jr., P.T. 1997. Models of amyloid seeding in Alzheimer's disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66 385–407. [DOI] [PubMed] [Google Scholar]
- Kelly, J.W. and Lansbury Jr., P.T. 1994. A chemical approach to elucidate the mechanism of transthyretin and β-protein amyloid fibril formation. Amyloid 1 186–205. [Google Scholar]
- Lindquist, S. 1997. Mad cows meet psi-chotic yeast: The expansion of the prion hypothesis. Cell 89 495–498. [DOI] [PubMed] [Google Scholar]
- Martin, J.B. 1999. Molecular basis of the neurodegenerative disorders. N. Engl. J. Med. 340 1970–1980. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, A. 1997. Inclusion bodies and purification of proteins in biologically active forms. Adv. Biochem. Eng. Biotechnol. 56 61–109. [DOI] [PubMed] [Google Scholar]
- Pearson, J.D. and McCroskey, M.C. 1996. Perfluorinated acid alternatives to trifluoroacetic acid for reversed-phase high-performance liquid chromatography. J. Chromatogr. A 746 277–281. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F., Johnson, T., Suzuki, M., and Finch, J.T. 1994. Glutamine repeats as polar zippers: Their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. 91 5355–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger, E., Sittler, A., Schweiger, K., Heiser, V., Lurz, R., Hasenbank, R., Bates, G.P., Lehrach, H., and Wanker, E.E. 1999. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: Implications for Huntington's disease pathology. Proc. Natl. Acad. Sci. 96 4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe, J.D. 1992. Amyloidosis. Annu. Rev. Biochem. 61 947–975. [DOI] [PubMed] [Google Scholar]
- Wood, S.J., Chan, W., and Wetzel, R. 1996. Seeding of Aβ fibril formation is inhibited by all three isotypes of apolipoprotein E. Biochemistry 35 12623–12628. [DOI] [PubMed] [Google Scholar]
- Zagorski, M.G., Yang, J., Shao, H., Ma, K., Zeng, H., and Hong, A. 1999. Methodological and chemical factors affecting amyloid-β amyloidogenicity. Meths. Enzymol. 309 189–204. [DOI] [PubMed] [Google Scholar]