Abstract
Objective
We report the effects of several different measures of body size at baseline on the subsequent development of diabetes. High levels of body fat predict the onset of diabetes, but this association has not been previously reported in a large multiethnic population of overweight or obese people with impaired glucose tolerance.
Research Methods and Procedures
Height, weight, waist circumference, hip circumference, and skinfolds were measured at baseline in 3234 participants enrolled in a randomized clinical trial to treat individuals with impaired glucose tolerance with placebo, metformin, or a lifestyle modification program. Cox proportional hazards models were used to assess the effect of baseline body size variables on the development of diabetes.
Results
Over an average of 3.2 years in both the placebo and lifestyle groups, baseline waist circumference had the highest or second highest R2 value for predicting diabetes in both sexes. Cox hazard ratios per 1 standard deviation were 1.43 and 1.49 for men in the placebo or lifestyle groups, respectively, and 1.29 and 1.53 for women in the placebo and lifestyle groups, respectively, adjusted for age and self-reported race/ethnicity. The c-statistic from the receiver operating characteristic curves also favored the waist circumference in men and women in the lifestyle group and men in the placebo group. No components of body size were predictive in the metformin-treated group, and metformin compared with the placebo group was effective in preventing diabetes only in individuals with a BMI ≥35 kg/m2 or a waist circumference ≥98.0 cm.
Discussion
Large waist circumference was a better predictor of risk for developing diabetes than most other measures in the placebo and lifestyle groups. No baseline measure of body size or shape predicted risk of diabetes in the metformin-treated group.
Keywords: BMI, waist circumference, skinfolds, life-style, metformin
Introduction
Obesity (1-9) and central adiposity (10-25) are risk factors for type 2 diabetes mellitus. Although the mechanisms for this relationship are not entirely understood, one hypothesis suggests that the release of fatty acids and cytokines from the increased amounts of visceral fat into the portal circulation affects hepatic metabolism of insulin and inflammatory markers. Ethnic differences have been identified in the study of adipose tissue and visceral fat (26-33), and this may be reflected in their effect on the development of diabetes.
The Diabetes Prevention Program (DPP)1 is a multicenter randomized clinical trial comparing an open-label lifestyle arm against a double-blind placebo-controlled metformin or placebo intervention in a multiethnic cohort with impaired glucose tolerance (34-36). The development of diabetes was determined by an annual oral glucose tolerance test or semiannual fasting glucose measurements using the criteria of the American Diabetes Association. We report the effects of several different measures of body size at baseline on the subsequent development of diabetes.
Research Methods and Procedures
Participant Eligibility and Recruitment
The design and methods of the DPP (34), the baseline characteristics of the cohort (35), and the primary outcomes (36) have been published previously and are summarized briefly here. Participants were recruited from 1996 to 1999 by 27 clinical centers located throughout the United States. Key eligibility requirements were age ≥25 years, BMI ≥24 kg/m2 (≥22 kg/m2 for Asian/Pacific Islander Americans), a fasting plasma glucose of 5.3 to 7.0 mM (95 to 125 mg/dL) [<7.0 mM (<125 mg/dL) in the American Indian Centers], and a 2-hour plasma glucose level of 7.8 to 11.1 mM (140 to 199 mg/dL) after a 75-g oral glucose load. Individuals were excluded if they had diabetes, if they had any condition likely to limit life span or increase risk of intervention, if they had any condition that would likely affect our ability to conduct the trial as designed (e.g., unwilling to accept treatment assignment by randomization), or if they were taking any medication or had any medical condition likely to confound the assessment of diabetes status (e.g., thiazide diuretics). Self-reported race/ethnicity was assessed using the 1990 U.S. Census questions.
Of the 3234 participants enrolled in the study, 54.7% were white, 19.9% African-American, 15.7% Hispanic, 5.3% American Indian, and 4.4% Asian/Pacific Islander. Women comprised 67.7% of the participants.
Intervention Groups
The participants were randomized to one of three treatment groups: placebo, metformin (850 mg twice daily), or an intensive lifestyle modification program (37). After randomization, all participants, regardless of treatment assignment, received written information and a 20- to 30-minute individual session with their case manager to address the importance of a healthy lifestyle for the prevention of type 2 diabetes. These recommendations were reviewed annually with all participants.
The goals of the intensive lifestyle intervention were: 1) to achieve and maintain a weight reduction of at least 7% of initial body weight through healthy eating and physical activity; and 2) to achieve and maintain a level of physical activity of at least 150 minutes per week (equivalent to ~700 kcal/wk) through moderate-intensity activity (such as walking or bicycling) (37). Key elements of the intervention included training in diet, exercise, and behavior modification skills, frequent contact with the interventionist (no less than monthly), and cultural sensitivity. The weight loss was attempted initially through a reduction in dietary fat intake to <25% of calories. If weight loss did not occur with fat restriction alone, a calorie goal was added. Details of the intervention approach and materials are available at http://www.bsc.gwu.edu/dpp/index.htmlvdoc (37).
Measurements of Body Size and Shape
During the baseline screening visits, body size and shape measurements were obtained on all participants. All measurements were recorded twice and averaged. A third measurement was taken if the variability was greater than a predefined value. All staff members performing these measurements were certified annually. Height was measured on a stadiometer to the nearest 0.5 cm. Body weight was measured on a calibrated balance scale. Waist circumference and hip circumference were obtained, using a cloth tape, while the participant was standing. The waist was defined as the midpoint between the highest point of the iliac crest and the lowest part of the costal margin in the midaxillary line, and the hips were measured at the level of the greater femoral trochanters. The waist circumference divided by the hip circumference and the BMI (in kg/m2) were computed. Skinfolds were measured at five different sites (triceps, subscapular, suprailiac, abdomen, and medial calf) using calibrated Lange skinfold calipers and a standardized protocol (38). Measurements too large for the calipers were recorded as the upper limit on the caliper of 67 mm.
Primary Outcomes
After an average 3.2 years [i.e., 4 months longer than reported in the primary outcome paper (36)], diabetes incidence rates were 10.8, 7.7, and 5.0 per 100 person-years in the placebo, metformin, and lifestyle groups, respectively. Lifestyle reduced diabetes incidence by 55% (95% confidence interval, 45% to 63%) and metformin by 30% (95% confidence interval, 16% to 41%) vs. placebo, with lifestyle significantly more effective than metformin. Treatment effects were consistent across sex and self-reported race/ethnicity.
Statistical Analysis
Descriptive statistics of baseline body size and shape variables were computed by sex, race/ethnicity, and treatment group. Diabetes incidence was calculated by computing the number of cases per 100 person-years.
Cox proportional hazards models were used to assess the effect of baseline body size variables on the development of diabetes. Hazard ratios for the body size and shape measurements are reported per 1 standard deviation (SD) and are assumed to be constant over time. Models were run separately for each sex and body size measurement adjusted by treatment group, age, and self-reported race/ethnicity using a treatment-body fat interaction term. On the basis of sample size considerations in the multivariable analyses, self-described racial/ethnic categories were collapsed into four groups: white, African American, Hispanic, and other (American Indian and Asian/Pacific Islanders combined). Madalla’s likelihood ratio (39, p. 400) was used to describe the approximate variation explained by individual body fat measurements. The R2 allows comparison of the relative contribution of each body fat measurement added to a model adjusted for age and self-reported race/ethnicity within a treatment group. Model fit using residual analysis (deviance, Schoenfeld, likelihood displacement, and df betas) was used to determine whether the observations at the upper limit of the body fat calipers influenced model fit (40).
The methods described by Pencina and D’Agostino (41) were used to compute the overall c-index as a measure of discrimination in Cox regression models, and the methods of DeLong et al. (42) were used to compare receiver operating characteristic (ROC) curves. The c-index is a generalization of the area under the ROC curve. It is the probability of concordance between observed and predicted disease-free survival based on pairs of individuals. Similar to the area under the ROC curve, the c-index ranges from 0.5 (no predictive value) to 1.0 (perfect prediction). This analysis compared body fat measurements to each other separately by sex and treatment group. Each model was adjusted for age at randomization and self-reported race/ethnicity. Effects nominally significant at the 0.05 level are interpreted without adjustment for multiple tests. The SAS analysis system, version 8.2, was used for all analyses (SAS Institute, Inc., Cary, NC).
Results
Tables 1 and 2 show the baseline characteristics of the participants by sex and self-reported race/ethnicity. Eight men and 25 women with missing body size measurements were excluded. There were few skinfolds at the upper limit of caliper size except for abdominal skinfolds. Abdominal skinfolds at the upper limit were recorded for 2% of the men and 10% of the women. None of the baseline measures of body fat differed significantly among treatment groups, except for calf skinfold thickness in women (p < 0.05). On average, participants in the DPP were obese, with a mean BMI of 32.0 (±5.7 SD) kg/m2 in men and 34.9 (±6.9 SD) kg/m2 in women. The waist circumference was slightly larger in men than in women (108.0 ± 13.4 cm vs. 103.7 ± 14.8 cm), and the waist-to-hip ratio was also larger in men than in women (1.00 ± 0.06 vs. 0.89 ± 0.07). All skinfolds were larger in women than in men.
Table 1.
Baseline characteristics in men by self-reported race/ethnicity
| Baseline characteristics | White (n = 602) | African-American (n = 165) | Hispanic (n = 167) | American Indian (n = 20) | Asian/Pacific Islander (n = 83) | Total (n = 1035 |
|---|---|---|---|---|---|---|
| Age (years) | 55.3 ± 11.2 | 54.3 ± 10.3 | 50.4 ± 10.9 | 44.2 ± 10.5 | 50.7 ± 10.7 | 53.8 ± 11.2 |
| Family history of diabetes | 390 (64.3) | 117 (70.9) | 112 (67.1) | 13 (65) | 58 (69.9) | 690 (66.2) |
| Body fat measurements | ||||||
| Weight (kg) | 101.3 ± 19.2 | 101.8 ± 22.1 | 93.6 ± 19.6 | 95.9 ± 20.9 | 84.0 ± 19.8 | 98.7 ± 20.5 |
| BMI (kg/m2) | 32.5 ± 5.8 | 32.5 ± 6.0 | 31.7 ± 5.0 | 31.2 ± 4.1 | 28.3 ± 3.7 | 32.0 ± 5.7 |
| Waist circumference (cm) | 110.5 ± 13.2 | 107.2 ± 13.4 | 104.9 ± 12.8 | 107.2 ± 11.2 | 97.1 ± 9.3 | 108.0 ± 13.4 |
| Hip circumference (cm) | 110.1 ± 11.2 | 108.4 ± 11.3 | 105.3 ± 10.4 | 105.5 ± 10.7 | 99.3 ± 7.6 | 108.1 ± 11.2 |
| Waist-to-hip ratio | 1.00 ± 0.06 | 0.99 ± 0.06 | 1.00 ± 0.06 | 1.02 ± 0.04 | 0.98 ± 0.04 | 1.00 ± 0.06 |
| Waist-to-height ratio | 0.63 ± 0.07 | 0.61 ± 0.08 | 0.61 ± 0.07 | 0.63 ± 0.05 | 0.57 ± 0.05 | 0.62 ± 0.07 |
| Skin fold measurements | ||||||
| Subscapular (mm) | 27.9 ± 9.6 | 32.0 ± 10.4 | 26.7 ± 9.2 | 27.0 ± 8.9 | 24.5 ± 6.8 | 28.1 ± 9.6 |
| Triceps (mm) | 21.8 ± 8.9 | 23.2 ± 10.1 | 19.8 ± 9.7 | 20.0 ± 7.7 | 17.3 ± 6.8 | 21.3 ± 9.2 |
| Suprailiac (mm) | 29.3 ± 11.4 | 31.6 ± 11.6 | 28.0 ± 11.5 | 30.5 ± 9.4 | 26.7 ± 8.5 | 29.2 ± 11.3 |
| Abdominal (mm) | 38.1 ± 13.2 | 41.4 ± 13.0 | 35.3 ± 13.2 | 36.2 ± 12.7 | 29.7 ± 8.5 | 37.5 ± 13.2 |
| Calf (mm) | 17.8 ± 8.9 | 16.9 ± 9.0 | 15.6 ± 9.7 | 14.3 ± 5.5 | 13.4 ± 5.3 | 16.9 ± 8.8 |
| Sum of measurements (mm) | 134.5 ± 42.5 | 145.1 ± 44.6 | 125.4 ± 45.2 | 128.0 ± 39.1 | 110.9 ± 26.7 | 132.7 ± 43.0 |
| Sum of measurements excluding calf (mm) | 116.7 ± 36.4 | 128.2 ± 38.0 | 109.7 ± 37.5 | 113.7 ± 34.9 | 97.7 ± 23.8 | 115.9 ± 36.7 |
| Triceps + subscapular (cm) | 49.6 ± 16.8 | 55.2 ± 18.9 | 46.5 ± 17.8 | 47.0 ± 15.7 | 41.6 ± 11.9 | 49.3 ± 17.3 |
Plus-minus values are means ± standard deviation; other measures are n (%).
Table 2.
Baseline characteristics in women by self-reported race/ethnicity
| Baseline characteristics | White (n = 1139) | African-American (n = 477) | Hispanic (n = 340) | American Indian (n = 151) | Asian/Pacific Islander (n = 59) | Total (n = 2166) |
|---|---|---|---|---|---|---|
| Age (years) | 50.3 ± 10.2 | 48.8 ± 9.8 | 47.3 ± 9.6 | 44.5 ± 9.7 | 48.9 ± 8.5 | 49.1 ± 10.1 |
| Family history of diabetes | 799 (68.9) | 360 (75) | 243 (71.3) | 116 (76.8) | 35 (59.3) | 1553 (70.9) |
| Body fat measurements | ||||||
| Weight (kg) | 93.7 ± 20.9 | 99.0 ± 22.2 | 84.7 ± 17.7 | 86.7 ± 18.3 | 77.7 ± 18.6 | 92.6 ± 21.1 |
| BMI (kg/m2) | 34.9 ± 7.1 | 36.4 ± 7.1 | 34.0 ± 6.0 | 33.8 ± 6.39 | 30.8 ± 6.5 | 34.9 ± 6.9 |
| Waist circumference (cm) | 104.1 ± 15.0 | 106.4 ± 15.3 | 99.7 ± 12.7 | 105.0 ± 13.1 | 93.3 ± 13.7 | 103.7 ± 14.8 |
| Hip circumference (cm) | 117.7 ± 14.0 | 118.6 ± 13.6 | 112.4 ± 12.0 | 115.8 ± 13.9 | 106.6 ± 12.6 | 116.7 ± 13.8 |
| Waist-to-hip ratio | 0.88 ± 0.07 | 0.90 ± 0.08 | 0.89 ± 0.07 | 0.91 ± 0.08 | 0.88 ± 0.07 | 0.89 ± 0.07 |
| Waist-to-height ratio | 0.64 ± 0.09 | 0.65 ± 0.09 | 0.63 ± 0.08 | 0.66 ± 0.08 | 0.59 ± 0.08 | 0.64 ± 0.09 |
| Skin fold measurements | ||||||
| Subscapular (mm) | 32.4 ± 10.1 | 38.5 ± 11.1 | 30.9 ± 9.5 | 30.4 ± 8.0 | 31.2 ± 8.6 | 33.3 ± 10.4 |
| Triceps (mm) | 32.8 ± 9.3 | 35.9 ± 9.8 | 29.5 ± 8.7 | 30.9 ± 8.2 | 27.7 ± 7.3 | 32.7 ± 9.4 |
| Suprailiac (mm) | 33.5 ± 10.2 | 37.5 ± 10.2 | 32.1 ± 10.4 | 32.1 ± 7.2 | 32.4 ± 9.4 | 34.0 ± 10.2 |
| Abdominal (mm) | 44.7 ± 13.0 | 49.7 ± 12.6 | 41.9 ± 13.5 | 39.4 ± 11.8 | 38.0 ± 11.1 | 44.8 ± 13.2 |
| Calf (mm) | 27.7 ± 9.9 | 28.5 ± 10.1 | 25.5 ± 9.4 | 23.8 ± 8.8 | 22.5 ± 7.5 | 27.1 ± 9.8 |
| Sum of measurements (mm) | 170.8 ± 42.4 | 190.1 ± 43.0 | 159.8 ± 41.9 | 156.5 ± 36.5 | 151.8 ± 37.6 | 171.9 ± 43.4 |
| Sum of measurements excluding calf (mm) | 143.3 ± 36.0 | 161.6 ± 36.2 | 134.3 ± 35.8 | 132.7 ± 30.1 | 129.4 ± 32.1 | 144.8 ± 36.8 |
| Triceps + subscapular (cm) | 65.2 ± 17.7 | 74.3 ± 19.2 | 60.4 ± 16.9 | 61.3 ± 15.1 | 58.9 ± 14.7 | 66.0 ± 18.3 |
Plus-minus values are means ± standard deviation; other measures are n (%).
Hispanic and Asian/Pacific Islander men weighed less than whites and African Americans, and Asian/Pacific Islanders had the lowest BMI values. Asian/Pacific Islanders also had consistently smaller skinfold measurements than whites, African Americans, or Hispanics. Among women, whites and African Americans generally had larger body size measurements than Hispanics, Asian/Pacific Islanders, and American Indians.
The interactions between treatment arm and body size measurement were significant in several models. The Cox regression models were then run separately by treatment arm. Neither age nor race/self-reported ethnicity was significant in any of the Cox regression models. The residual analyses of these models did not indicate a problem with model fit.
Tables 3 and 4 present the Cox regression models for baseline measures of body size and shape as predictors of diabetes. These tables also contain the c-statistic from the ROC curves analyzing the sensitivity and specificity for this prediction and the explained variation, expressed as % R2. In men in the placebo and lifestyle groups, the Cox hazard ratio for developing diabetes was significant for waist circumference, waist-to-height ratio, BMI, waist-to-hip ratio, and body weight. In the placebo group, none of the skin-folds was a significant predictor, but in the lifestyle group, calf skinfold was a significant predictor. In contrast to the data for placebo and lifestyle groups, there were no measures of body size that predicted diabetes in the metformin-treated subgroup.
Table 3.
Cox regression models estimating hazard ratios for baseline body fat composition variables predicting diabetes by treatment group in men
| Placebo (n = 332)
|
Metformin (n = 363)
|
Lifestyle (n = 340)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline variable | % R2 | c-index* | HR | 95% CI | p | % R2 | c-index* | HR | 95% CI | p | % R2 | c-index* | HR | 95% CI | p |
| Waist circumference (cm) | 3.68 | 0.610 | 1.42 | (1.14–1.77) | <0.01 | 0.86 | 0.553 | 1.14 | (0.89–1.45) | 0.30 | 4.10 | 0.623a | 1.48 | (1.18–1.87) | <0.01 |
| Waist-to-height ratio | 2.81 | 0.597 | 1.32 | (1.08–1.63) | <0.01 | 1.27 | 0.577 | 1.22 | (0.96–1.54) | 0.11 | 3.94 | 0.634b | 1.44 | (1.16–1.79) | <0.01 |
| BMI (kg/m2) | 2.59 | 0.591 | 1.30 | (1.07–1.57) | <0.01 | 1.17 | 0.567 | 1.20 | (0.95–1.53) | 0.13 | 3.46 | 0.616 | 1.40 | (1.13–1.73) | <0.01 |
| Hip circumference (cm) | 2.54 | 0.580 | 1.32 | (1.06–1.65) | 0.01 | 0.60 | 0.546 | 1.04 | (0.81–1.32) | 0.78 | 2.45 | 0.589a,b | 1.30 | (1.03–1.64) | 0.03 |
| Waist-to-hip ratio | 2.39 | 0.597 | 1.29 | (1.04–1.59) | 0.02 | 1.28 | 0.562 | 1.20 | (0.96–1.50) | 0.10 | 4.69 | 0.669 | 1.68 | (1.26–2.24) | <0.01 |
| Weight (kg) | 2.30 | 0.612 | 1.23 | (1.04–1.45) | 0.02 | 0.68 | 0.547 | 1.09 | (0.84–1.41) | 0.53 | 3.62 | 0.597 | 1.49 | (1.15–1.91) | <0.01 |
| Triceps skinfold (cm) | 0.84 | 0.562 | 0.95 | (0.76–1.19) | 0.67 | 0.58 | 0.548 | 1.00 | (0.78–1.29) | 0.97 | 1.46 | 0.581 | 1.14 | (0.88–1.49) | 0.33 |
| Calf skinfold† (cm) | 0.81 | 0.557 | 0.97 | (0.77–1.21) | 0.76 | 0.63 | 0.556 | 0.94 | (0.72–1.23) | 0.66 | 2.30 | 0.594 | 1.26 | (1.01–1.57) | 0.04 |
| Abdominal skinfold (cm) | 0.80 | 0.558 | 1.02 | (0.83–1.25) | 0.85 | 0.58 | 0.549 | 1.01 | (0.79–1.29) | 0.96 | 1.25 | 0.589 | 1.07 | (0.80–1.41) | 0.66 |
| Subscapular skinfold (cm) | 0.79 | 0.560 | 1.01 | (0.82–1.24) | 0.92 | 1.57 | 0.596 | 1.25 | (1.00–1.57) | 0.05 | 2.17 | 0.593 | 1.30 | (0.99–1.69) | 0.06 |
| Suprailiac skinfold (cm) | 0.79 | 0.560 | 0.99 | (0.81–1.23) | 0.96 | 0.59 | 0.548 | 1.02 | (0.81–1.28) | 0.88 | 1.34 | 0.603 | 0.89 | (0.65–1.23) | 0.48 |
| Sum of skinfolds (cm) | 0.79 | 0.559 | 0.99 | (0.80–1.23) | 0.94 | 0.63 | 0.550 | 1.05 | (0.83–1.35) | 0.67 | 1.50 | 0.581 | 1.16 | (0.88–1.51) | 0.29 |
| Sum of skinfolds excluding calf (cm) | 0.79 | 0.560 | 1.00 | (0.80–1.24) | 0.98 | 0.67 | 0.555 | 1.07 | (0.85–1.37) | 0.55 | 1.34 | 0.585 | 1.11 | (0.83–1.47) | 0.48 |
| Triceps + subscapular (cm) | 0.79 | 0.560 | 0.98 | (0.79–1.22) | 0.88 | 0.92 | 0.567 | 1.15 | (0.90–1.45) | 0.26 | 1.85 | 0.581 | 1.23 | (0.95–1.59) | 0.12 |
Ordered by magnitude of R2 in placebo group; HRs are 1 unit per standard deviation. All models are adjusted for age and self-reported race/ethnicity. HR, hazard ratio; CI, confidence interval.
Equal superscripts (a, b) within treatment group on the c-index indicate measurements that are significantly different from each other at p = 0.05. For example, the c-index for waist circumference is significantly more than that of hip circumference in the lifestyle group.
Significant interaction between body fat measurement and treatment group (p ≤ 0.05).
Table 4.
Cox regression models estimating hazard ratios for baseline body fat composition variables predicting diabetes by treatment group in women
| Placebo (n = 738)
|
Metformin (n = 701)
|
Lifestyle (n = 727)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline variable | % R2 | c-index* | HR | 95% CI | p | % R2 | c-index* | HR | 95% CI | p | % R2 | c-index* | HR | 95% CI | p |
| Waist circumference (cm) | 2.23 | 0.597a | 1.29 | (1.11–1.50) | <0.01 | 0.62 | 0.561 | 1.07 | (0.90–1.29) | 0.43 | 2.87 | 0.637a | 1.53 | (1.27–1.85) | <0.01 |
| Waist-to-height ratio | 2.05 | 0.594 | 1.27 | (1.09–1.47) | <0.01 | 0.78 | 0.574 | 1.12 | (0.95–1.34) | 0.18 | 2.05 | 0.628b | 1.41 | (1.17–1.70) | <0.01 |
| Waist-to-hip ratio | 1.92 | 0.591 | 1.27 | (1.08–1.48) | <0.01 | 0.71 | 0.567 | 1.10 | (0.93–1.29) | 0.26 | 2.28 | 0.621 | 1.44 | (1.20–1.74) | <0.01 |
| Subscapular skinfold† (cm) | 1.67 | 0.581 | 1.22 | (1.05–1.42) | <0.01 | 0.53 | 0.553 | 0.99 | (0.83–1.19) | 0.93 | 1.30 | 0.592 | 1.30 | (1.08–1.57) | <0.01 |
| Suprailiac skinfold (cm) | 1.58 | 0.583 | 1.20 | (1.04–1.38) | 0.01 | 0.54 | 0.553 | 1.02 | (0.85–1.22) | 0.86 | 0.54 | 0.565 | 1.14 | (0.94–1.38) | 0.19 |
| BMI† (kg/m2) | 1.56 | 0.583 | 1.20 | (1.04–1.39) | 0.01 | 0.56 | 0.554 | 0.96 | (0.80–1.15) | 0.67 | 2.12 | 0.631c | 1.42 | (1.18–1.70) | <0.01 |
| Weight† (kg) | 1.54 | 0.584 | 1.18 | (1.04–1.35) | 0.01 | 0.80 | 0.567 | 0.87 | (0.71–1.07) | 0.18 | 3.18 | 0.639d | 1.59 | (1.32–1.93) | <0.01 |
| Sum of skinfolds excluding calf† (cm) | 1.51 | 0.578 | 1.19 | (1.03–1.38) | 0.02 | 0.54 | 0.555 | 0.97 | (0.81–1.16) | 0.76 | 0.79 | 0.573 | 1.20 | (0.99–1.46) | 0.06 |
| Sum of skinfolds† (cm) | 1.38 | 0.577 | 1.18 | (1.01–1.36) | 0.03 | 0.55 | 0.556 | 0.97 | (0.81–1.16) | 0.71 | 0.74 | 0.573 | 1.19 | (0.98–1.45) | 0.07 |
| Hip circumference (cm) | 1.29 | 0.568a | 1.16 | (1.00–1.35) | 0.05 | 0.53 | 0.554 | 0.99 | (0.83–1.19) | 0.94 | 1.13 | 0.583a–d | 1.27 | (1.05–1.54) | 0.01 |
| Triceps + subscapular (cm) | 1.28 | 0.574 | 1.16 | (1.00–1.34) | 0.05 | 0.53 | 0.553 | 0.99 | (0.82–1.18) | 0.87 | 1.01 | 0.585 | 1.25 | (1.03–1.51) | 0.02 |
| Abdominal skinfold (cm) | 1.23 | 0.567 | 1.15 | (0.99–1.33) | 0.06 | 0.61 | 0.564 | 0.93 | (0.78–1.11) | 0.45 | 0.48 | 0.558 | 1.12 | (0.92–1.36) | 0.27 |
| Triceps skinfold (cm) | 0.90 | 0.558 | 1.08 | (0.93–1.25) | 0.33 | 0.54 | 0.553 | 0.98 | (0.82–1.17) | 0.83 | 0.56 | 0.566 | 1.15 | (0.94–1.39) | 0.17 |
| Calf skinfold (cm) | 0.81 | 0.553 | 1.05 | (0.90–1.20) | 0.55 | 0.56 | 0.555 | 0.96 | (0.81–1.14) | 0.63 | 0.40 | 0.553 | 1.08 | (0.89–1.31) | 0.42 |
Ordered by magnitude of R2 ratio in placebo group; HRs are 1 unit per standard deviation. All models are adjusted for age and self-reported race/ethnicity. HR, hazard ratio; CI, confidence interval.
Equal superscripts (a– d) within treatment group on the c-index indicate measurements that are significantly different from each other at p = 0.05. For example, in the lifestyle group, the c-index for waist circumference is significantly greater than the c-index for hip circumference.
Significant interaction between body fat measurement and treatment group (p ≤ 0.05).
In women in both the placebo and lifestyle groups, a larger group of body size measures were significant in predicting diabetes. In the placebo and lifestyle groups, all except three skinfolds (abdominal, calf, and triceps) were significant. In the lifestyle group, also, most measures predicted diabetes except a few skinfold measurements (suprailiac, sum of skinfolds, abdominal, triceps, and calf). As in the men, no measure of body size predicted diabetes in women in the metformin-treated subgroup.
The ROCs of the Cox regression models were examined using the c-statistic (41). In men in the lifestyle group, the waist circumference and the waist-to-height ratio were significantly better predictors of diabetes than hip circumference (Table 3, upper half). In men in the placebo group, waist circumference was not significantly different from the other measures that predicted diabetes. In women in the lifestyle group, waist circumference, waist-to-height ratio, BMI, and weight were all significantly better predictors than hip circumference. In women in the placebo group, waist circumference was also a significantly better predictor than hip circumference. In the metformin group, there were no significant predictors (Table 4, lower half).
The % R2 reported in Tables 3 and 4 was also used to evaluate the importance of individual measures. The R2 is the amount of variation explained by each variable. Among the men in the placebo group, waist circumference explained more of the variation than any other variable, with waist-to-height ratio, BMI, and hip circumference being clustered together (Table 3). In the lifestyle group of men, waist-to-hip ratio and waist circumference explained more of the variation than other variables, but the top four variables in the lifestyle group explained more variance than the best variable (waist circumference) in the placebo group. Explained variation in the metformin group was small (<1) for all variables. In women (Table 4), R2 explained less variation than the corresponding variables in the male group. Waist circumference explained more of the variation in the placebo group than in any other group, just as in the male group. In the lifestyle group, body weight explained the most variation, with waist circumference second. Again, no variable in the metformin group explained much of the variation.
Figures 1 and 2 show the relationship among tertiles of waist circumference (Figure 1) and tertiles of BMI (Figure 2) for each of the three treatment groups. There are graded increases in the risk for developing diabetes as the tertile of waist circumference increases in the placebo and lifestyle groups and a smaller positive gradient in the metformin group, but no relationship in women in the metformin group (Figure 1, A and B). As the tertile of BMI increases, there is also a clear increase in the incidence of diabetes in the placebo and lifestyle groups, particularly in the BMI >35 kg/m2 group, but no relationship in men (Figure 2A) and a negative relationship in women (Figure 2B) of BMI to the incidence of diabetes in the metformin-treated group.
Figure 1.
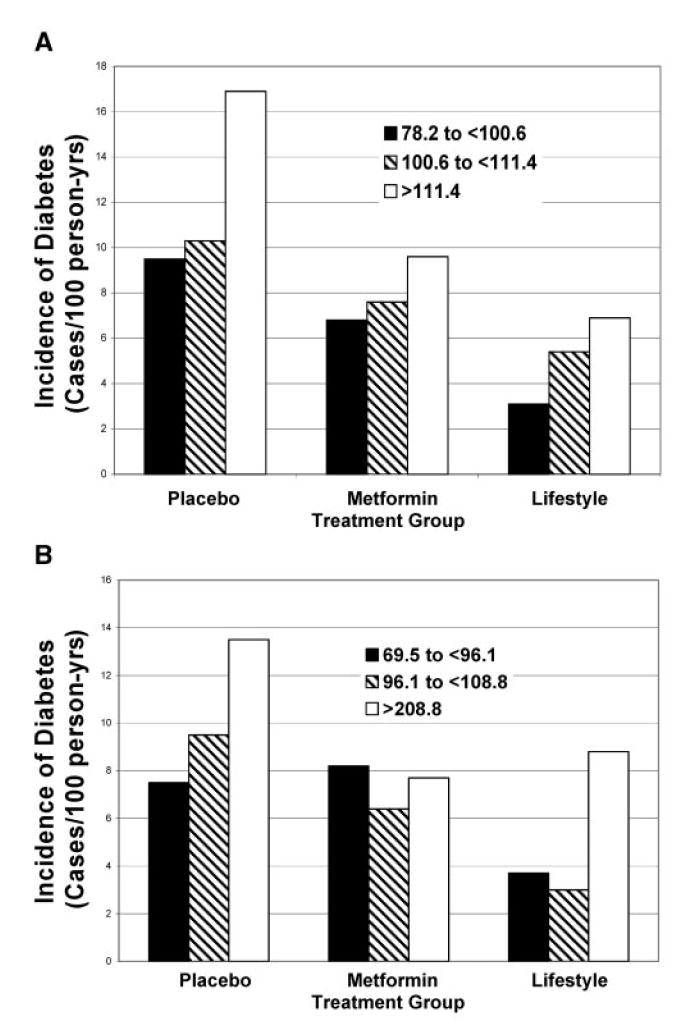
Incidence of diabetes by waist circumference and treatment group in men (A) and women (B). Lifestyle lowered the incidence of diabetes similarly in all three tertiles of waist circumference. The incidence of diabetes was similar with metformin at all three levels of waist circumference. In men, the largest effect, relative to placebo, was seen in the group with the largest waist circumferences. In women, in those with the largest waist circumference, the effect of metformin was similar to that of lifestyle on the incidence of diabetes.
Figure 2.
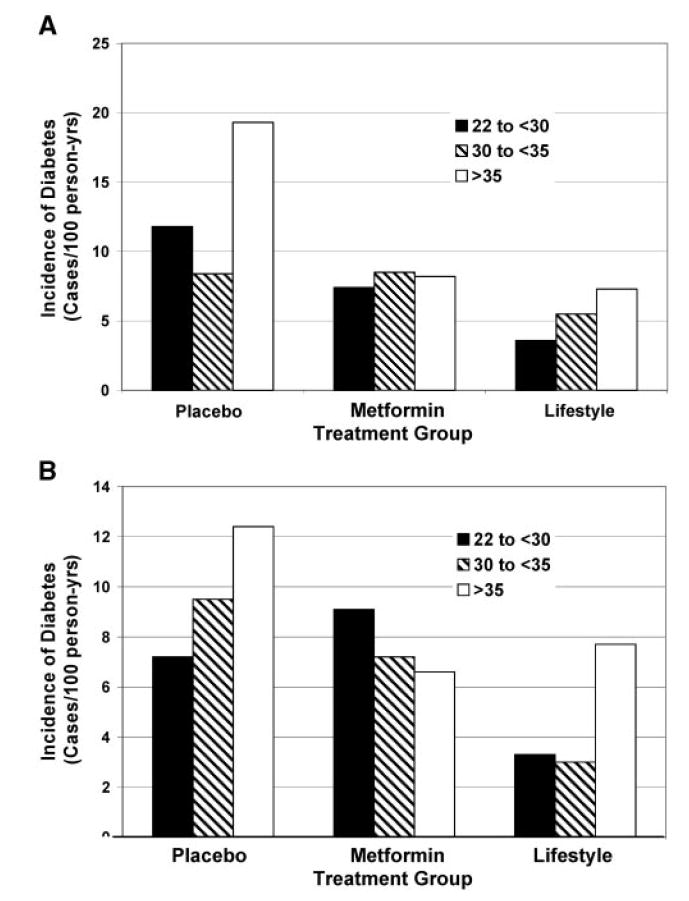
Incidence of diabetes by BMI and treatment group in men (A) and women (B). Lifestyle lowered the incidence rate at all three levels of BMI relative to placebo. In men, metformin was most effective in the highest BMI group, where the effect was similar to that of lifestyle. In women, metformin was effective only in the highest BMI group, where the effect was similar to that of lifestyle.
Discussion
The DPP, a large, randomized, multicenter clinical trial among adults in the United States, provided an opportunity to examine measures of body size and shape as predictors for developing diabetes. In Cox proportional hazards models, we found that in both men and women, weight, BMI, waist circumference, hip circumference, and waist-to-height and waist-to-hip ratios significantly predicted the development of diabetes after correction for age and self-reported race/ethnicity in the placebo group. In women, but not men, the subscapular, suprailiac, triceps plus subscapular, and sum of skinfolds were also predictive. This may reflect the higher amount of subcutaneous fat in women, as compared with men.
This was an obese population, as indicated by the average BMI of 33 kg/m2, which is similar to the mean BMI of diabetics in the National Center for Health Statistics National Health and Nutrition Examination Survey 1999–2000 (43). The DPP population also had central adiposity, as indicated by waist circumferences that, on average, were larger in all groups of women than the National Heart, Lung, and Blood Institute criteria of 88 cm and larger in all groups of men than the 102 cm upper limit (44), except in Asian/Pacific Islanders.
A pattern of central body fat distribution has been associated with both insulin resistance and risk for diabetes independently of BMI in most studies (10-25), but not all of them (45). In the latter study, BMI was the best predictor of developing diabetes in the Pima Indians, and its predictive power was not increased by adding other anthropometric measures (45). Using the c-statistic from the ROC curves for measures of body size, we found that in the lifestyle groups, waist circumference and waist-to-height ratio were significantly better predictors in both men and women than hip circumference and that BMI and weight were also significantly better predictors than hip circumference. Among women, but not men, in the placebo group, waist circumference was a significantly better predictor than hip circumference. The % R2 measure of variation indicated that, in the placebo group, waist circumference explained more of the variation, and, in the lifestyle group, it was second, behind waist-to-hip ratio in men and body weight in women. Thus, if a single measure is used, waist circumference would seem to be a better predictor of diabetes than other measures.
Central adiposity, as measured by waist circumference, might reflect higher amounts of subcutaneous abdominal fat, higher amounts of visceral fat, or a combination of the two. Most of the research on the association between central obesity and type 2 diabetes has claimed that intra-abdominal visceral fat is largely responsible for central adiposity (46). In a study using computed tomography, greater amounts of visceral adiposity have been reported to precede the development of diabetes in Japanese Americans (47). On the other hand, in a study using magnetic resonance imaging scans, Abate et al. (23) have argued that subcutaneous fat has at least as important a role in insulin resistance as does intra-abdominal fat. The finding of a deep subcutaneous compartment of fat that behaves more like visceral fat (48,49) may provide an explanation for this difference. The current data are not able to distinguish the relative contributions of subcutaneous and visceral abdominal fat to the risk for diabetes associated with central obesity. Many, but not all, studies (45) have shown that central adiposity, as measured by the waist circumference, is a better predictor than BMI not only for diabetes (14,50-52) but also for the metabolic syndrome and heart disease (53). Waist circumference as a continuous variable has been reported to account for essentially all of the risk for metabolic syndrome and dyslipidemia that has been attributed to BMI (53). When waist circumference is used as a categorical variable using the National Heart, Lung, and Blood Institute or World Health Organization cut-off points (44), it is not as good (54), suggesting that using cut-off points is less informative than using measurements as continuous variables. Our data suggest that waist circumference is the preferred measurement; this is consistent with both older data from the United Kingdom (51) and Canada (52) and more recent data from the Nurses Health Study (46).
In the metformin group, in contrast to the lifestyle and placebo groups, no measure of body size and shape at baseline predicted future risk of diabetes. However, in the overall trial, metformin produced a 30% reduction in risk for developing diabetes during the 3.2-year follow-up period [which was longer than the 2.8 years reported previously (36)]. When the effects of metformin, lifestyle, and placebo were examined in relation to tertiles of waist circumference or BMI, it was evident that metformin, in contrast to lifestyle and placebo, was blunting the effect of increased weight on the development of diabetes. This may imply that metformin is differentially affecting either production of glucose by the liver or the sensitivity to glucose disposal in fatter individuals. Studies of the metformin subgroup from the DPP, compared with the placebo and lifestyle groups, suggest that the reduction in risk of developing diabetes (55) or the metabolic syndrome (56) may have been more closely related to improvements in insulin sensitivity and fasting glucose in the metformin group than body composition. This finding clearly merits further evaluation.
This study has several strengths. First, it is a large, randomized controlled trial in individuals who had documented impaired glucose tolerance. Individuals were followed sequentially for the development of diabetes by fasting glucose and glucose tolerance tests. A similar protocol was used in all centers, and the laboratory measurements were centralized and not provided to the investigators until the end of the trial. In contrast to these strengths, the trial suffers by not having directly measured visceral fat in all participants.
In conclusion, among the ethnically diverse DPP population of individuals with impaired glucose tolerance, larger waist circumference was the most significant predictor of diabetes in men and women in the lifestyle and placebo groups. Body weight and BMI were not as strong predictors as waist circumference. In contrast to waist circumference, skinfolds were predictive of diabetes only in women, and not in men. No measure of body fat was predictive of diabetes in the metformin-treated group.
Acknowledgments
We gratefully acknowledge the commitment and dedication of the participants of the DPP. Funding was provided by the NIH through the National Institute of Diabetes and Digestive and Kidney Diseases, National Heart, Lung, and Blood Institute, National Center on Minority and Health Disparities, National Institute of Child Health and Human Development, Office of Women’s Health, and National Institute on Aging. In addition, the Indian Health Service, the Centers for Disease Control and Prevention, the American Diabetes Association, and two pharmaceutical companies, Bristol-Myers Squibb and Parke-Davis, contributed support. The General Clinical Research Center Program, National Center for Research Resources, supported many of the centers. Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a cooperative agreement, except for the Southwestern American Indian centers, which were supported directly by the National Institute of Diabetes and Digestive and Kidney Diseases and the Indian Health Service. This research was also supported, in part, by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases. Members of the writing group were: George A. Bray (Chair), Kathleen A. Jablonski, Wilfred Y. Fujimoto, William C. Knowler, Elizabeth Barrett-Connor, Sharon L. Edelstein, Steven Haffner, James O. Hill, Van Hubbard, and F. Xavier Pi-Sunyer.
Footnotes
Nonstandard abbreviations: DPP, Diabetes Prevention Program; SD, standard deviation; ROC, receiver operating characteristic.
References
- 1.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima Indians: contributions of obesity and parental diabetes. Am J Epidemiol. 1981;113:144–56. doi: 10.1093/oxfordjournals.aje.a113079. [DOI] [PubMed] [Google Scholar]
- 2.Larsson B, Björntorp P, Tibblin G. The health consequences of moderate obesity. Int J Obes. 1981;5:97–116. [PubMed] [Google Scholar]
- 3.Holbrook TL, Barrett-Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989;13:723–9. [PubMed] [Google Scholar]
- 4.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132:501–13. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Willet WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–22. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 7.Wannamethee SB, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22:1266–72. doi: 10.2337/diacare.22.8.1266. [DOI] [PubMed] [Google Scholar]
- 8.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 9.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1580–6. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 10.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculus. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 11.Feldman R, Sender AJ, Siegelaub AB. Difference in diabetic and nondiabetic fat distribution patterns by skinfold measurements. Diabetes. 1969;18:478–86. doi: 10.2337/diab.18.7.478. [DOI] [PubMed] [Google Scholar]
- 12.Kissebah AH, Bydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–60. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 13.Hartz AJ, Rupley DK, Jr, Kalkhoff RD, Rimm AA. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med. 1983;12:351–7. doi: 10.1016/0091-7435(83)90244-x. [DOI] [PubMed] [Google Scholar]
- 14.Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–8. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 15.Sparrow D, Borkan GA, Gerzof SB, Wisniewski C, Silbert CK. Relationship of fat distribution to glucose tolerance. Diabetes. 1986;35:411–5. doi: 10.2337/diab.35.4.411. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–9. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 17.Despres JP, Nadeaur A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1988;38:304–9. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 18.Lundgren H, Bengtsson C, Blohme G, Lapidus L, Sjöstroöm L. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes. 1989;13:413–23. [PubMed] [Google Scholar]
- 19.Bergstrom RW, Newell-Morris LL, Leonetti DL, Shuman WP, Wahl PW, Fujimoto WY. Association of elevated fasting C-peptide level and increased intra-abdominal fat distribution with development of NIDDM in Japanese-American men. Diabetes. 1990;39:104–11. doi: 10.2337/diacare.39.1.104. [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, Stern MP, Braxton DM, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity and body fat distribution. Diabetes. 1990;39:283–8. doi: 10.2337/diab.39.3.283. [DOI] [PubMed] [Google Scholar]
- 21.Kaye SA, Folsom AR, Sprafka JM, Prineas RJ, Wallace RB. Increased incidence of diabetes mellitus in relation to abdominal adiposity in older women. J Clin Epidemiol. 1991;44:329–34. doi: 10.1016/0895-4356(91)90044-a. [DOI] [PubMed] [Google Scholar]
- 22.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–9. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 23.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross R, Fortier L, Hudson R. Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care. 1996;19:1404–11. doi: 10.2337/diacare.19.12.1404. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Thaete FL, Simoneau J-A, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 26.Bannerji MA, Chaiken RL, Gordon, et al. Does intra-abdominal adipose tissue in black men determine whether NIDDM is insulin-resistant or insulin-sensitive? Diabetes. 1995;44:141–6. doi: 10.2337/diab.44.2.141. [DOI] [PubMed] [Google Scholar]
- 27.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–62. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 28.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–71. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 29.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–24. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 30.Hill JO, Sidney S, Lewis CE, Tolan K, Scherziner AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) Study. Am J Clin Nutr. 1999;69:381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 31.Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat. Metabolism. 1999;48:194–9. doi: 10.1016/s0026-0495(99)90033-1. [DOI] [PubMed] [Google Scholar]
- 32.Perry AC, Applegate EB, Jackson ML, et al. Racial differences in visceral adipose tissue but not anthropometric markers of health-related variables. J Appl Physiol. 2000;89:636–43. doi: 10.1152/jappl.2000.89.2.636. [DOI] [PubMed] [Google Scholar]
- 33.Okosun IS, Tedders SH, Choi S, Dever GEA. Abdominal adiposity values associated with established body mass indexes in white, black and Hispanic Americans: a study from the Third National Health and Nutrition Examination Survey. Int J Obes Relat Disord. 2000;24:1279–85. doi: 10.1038/sj.ijo.0801414. [DOI] [PubMed] [Google Scholar]
- 34.Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–34. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program. Baseline characteristics of the randomized cohort: Diabetes Prevention Program Collaborative Group. Diabetes Care. 2000;23:1619–29. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wing RR, Hamman RF, Bray GA, et al. Diabetes Prevention Program Research Group: achieving weight and activity goals among Diabetes Prevention Program lifestyle participants. Obes Res. 2004;12:1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. abridged ed. [Google Scholar]
- 39.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. New York: John Wiley & Sons; 2000. [Google Scholar]
- 40.Hosmer DW, Jr, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: John Wiley & Sons; 1999. [Google Scholar]
- 41.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 42.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 43.Diabetes Prevention Program. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) Research Study. Diab Vasc Dis Res. 2006 doi: 10.3132/dvdr.2006.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 45.Tulloch-Reid MK, Williams DE, Looker HC, Hanson RL, Knowler WC. Do measures of body fat distribution provide information on the risk of type 2 diabetes in addition to measures of general obesity? Diabetes Care. 2003;26:2556–61. doi: 10.2337/diacare.26.9.2556. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–63. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 47.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 48.Kelley DE, Tyaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol. 2000;278:E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 49.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 50.Despres J-P, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids and lipoprotein lipase activity in men and women: the Health Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2000;20:1932–8. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 51.Lean ME, Han TS, Morrison CD. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres J. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–93. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 53.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 54.Bray GA. Don’t throw the baby out with the bath water. Am J Clin Nutr. 2004;79:347–9. doi: 10.1093/ajcn/79.3.347. [DOI] [PubMed] [Google Scholar]
- 55.Diabetes Prevention Program. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance: the Diabetes Prevention Program Research Group. Diabetes. 2005;54:1566–72. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diabetes Prevention Program. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program Randomized Trial. Ann Intern Med. 2005;142:611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


