Abstract
Background
Obstructive sleep apnea (OSA) has been associated with increased systemic inflammatory responses that may contribute to an increased risk for end-organ morbidity. The changes in levels of pro-inflammatory cytokine IL-6 , and the anti-inflammatory cytokine IL-10, both of which play a major role in atherogenesis, a major consequence of OSA, have not specifically been assessed in pediatric patients.
Methods
Consecutive non-obese children (aged 4–9 years) who were polysomnographically diagnosed with OSA, and age-, gender-, ethnicity-, and BMI- matched control children underwent a blood draw the next morning after a sleep study and plasma samples were assayed for interleukins 6 (IL-6) and 10 (IL-10). These tests were repeated 4–6 months after adenotonsillectomy (T&A) in children with OSA.
Results
IL-6 levels were higher and IL-10 plasma levels were lower in children with OSA and returned to control levels after T&A.
Conclusions
Systemic inflammation is a constitutive component and consequence of OSA in many children, even in the absence of obesity, and is reversible upon treatment in most patients.
Keywords: obstructive sleep apnea, inflammation, cytokines, atherogenesis, interleukin 10, interleukin 6
Introduction
Obstructive sleep apnea (OSA) is a frequent condition characterized by repeated events of partial or complete upper airway obstruction during sleep, resulting in disruption of normal gas exchange and sleep integrity. OSA, which is particularly common in young children, has been identified in recent years as a major cause of cardiovascular morbidity in adults with this condition (1, 2). While the literature pertaining to the impact of OSA on the cardiovascular system in pediatric patients is still not as extensive, there is clear evidence for increased surges in sympathetic activity in children with OSA (3–5). Furthermore, dose-dependent decreases in left ventricular contractility and geometry as well as elevation of arterial blood pressure have become apparent in children with OSA (6–8), along with altered left ventricular geometry and contractility (9). The latter findings would suggest that similar to adults, children with OSA are at increased risk for developing a cluster of inflammatory responses that ultimately leads to endothelial dysfunction and atherogenesis. In support of this hypothesis, we and others have reported on the association between increases in circulating levels of interleukin 6 and high- high-sensitivity C-reactive protein (hsCRP) and sleep-related measures in snoring children sensitivity (10–12), and on the reversibility of such inflammatory responses upon treatment of the underlying OSA (13). Similarly, circulating adhesion molecules suggestive of platelet-endothelial cell activation are increased in children with OSA, even after correcting for endothelial ponderal indices (14). Taken together, these findings suggest that OSA may induce a systemic pro-inflammatory response, the magnitude of which may have implications for end-organ morbidity (15). However, it is possible that OSA may not only affect pro-inflammatory cytokines such as IL-6, but may also down-regulate the expression of anti-inflammatory cytokines such as interleukin 10 (IL-10).
IL-10 is a pleiotropic cytokine produced by Th2-type T cells, B cells, monocytes, and macrophages that inhibits a broad array of pro-inflammatory immune responses, including those of the vessel wall (16–18). Furthermore, endogenous IL-10 plays a major role in mouse models of atherosclerosis. For example, IL-10 deficiency in C57BL/6 mice fed an atherogenic diet promotes early atherosclerotic lesion formation, (19–21). Conversely, systemic or local overexpression of IL-10 by adenoviral gene transfer was highly efficacious in the prevention of atherosclerosis (22–24). As a corollary of these findings in murine models, patients with unstable coronary heart disease have lower IL-10 levels (25, 26). Thus, we examined whether IL-10 plasma levels are altered in pre-pubertal children with OSA and whether treatment is associated with reversal of such changes.
Patients and Methods
Consecutive pre-pubertal non-obese children aged 4–9 years, who were diagnosed with OSA at Kosair Children’s Hospital Sleep Medicine and Apnea Center in Louisville, KY, were invited to participate in the study, which was approved by the University of Louisville Human Research Committee. Informed consent was obtained from the legal caretaker of each participant. Assent was also obtained from children if they were >6 years of age.
Inclusion criteria were the presence of OSA according to polysomnographic criteria (see below) and age between 4 and 9 years. In addition, age-, gender-, and r race-matched children without snoring who underwent overnight polysomnography in the context of another ongoing research study were also invited. Exclusion criteria included the presence of obesity or being overweight, elevated blood pressure, diabetes or pre-diabetes, craniofacial, neuromuscular, syndromic or defined genetic abnormalities, diabetes, current or previous use of montelukast (in the preceding 6 months), current use of anti-inflammatory drugs such as aspirin or ibuprofen, acute upper respiratory tract infection, inflammatory use of any systemic, intranasal, or inhaled corticosteroids or antibiotics in the four weeks preceding the initial sleep st study. In addition, any children who already had undergone T&A in the past and children receiving anti- hypertensive medications or other medications were not considered eligible to participate.
Height and weight were obtained from each child. Body mass index (BMI) was calculated and also expressed as relative BMI (relBMI), using the following formula: (BMI/BMI of the 50th percentile for age and gender) × 100, based on standardized percentile curves (27). Overweight and obesity were defined as BMI greater than the 85th percentile and 95th percentile for gender and age, respectively, and all children fulfilling such criteria were excluded from this study. Similarly, if mean blood pressure measurements obtained during the initial clinic visit as well as those obtained before and after the overnight sleep study were >95% predicted for age and gender (28), children were considered to have hypertension and were excluded.
Polysomnographic Assessment
Children were studied for up to 12 hours in a quiet, darkened room with an ambient temperature of 24°C in the company of one of their parents. No drugs were used to induce sleep. The following parameters were measured during the overnight sleep recordings: chest and abdominal wall movement by respiratory impedance or inductance plethysmography, heart rate by ECG, air flow was triply monitored with a sidestream end-tidal capnograph which also provided breath-by-breath assessment of end-tidal carbon dioxide levels (PETCO2; BCI SC-300, Menomonee Falls, WI), a nasal pressure ; cannula, and an oronasal thermistor. Arterial oxygen saturation (SpO2) was assessed by ) pulse oximetry (Nellcor N 100; Nellcor Inc., Hayward, CA), with simultaneous recording of the pulse waveform. The bilateral electro-oculogram (EOG), 8 channels of electroencephalogram (EEG), chin and anterior tibial electromyograms (EMG), and analog output from a body position sensor (Braebon Medical Corporation, Ogsdenburg, NY) were also monitored. All measures were digitized using a commercially available polysomnography system (Rembrandt, MedCare Diagnostics, Amsterdam). Tracheal sound was monitored with a microphone sensor (Sleepmate, Midlothian, VA), and a digital time-synchronized video recording was performed.
Sleep architecture was assessed by standard techniques (29). The proportion of time spent in each sleep stage was expressed as percentage of total sleep time (%TST). Central, obstructive and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for duration of at least two breaths (30, 31). Hypopneas were defined as a decrease in oronasal flow of ≥50% with a corresponding decrease in SpO2 of ≥4% and/or arousal (30). The obstructive apnea/hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of TST. The obstructive apnea index (AI) was defined as the number of apneas per hour of TST. The diagnostic criteria for OSA included an obstructive apnea index greater than 1/hr TST and/or an obstructive AHI greater than 5/hr TST with a nadir oxygen saturation value of at least <92%. Control children were defined as non-snoring children with an obstructive AHI≤1/hrTST.
Children with OSA were referred for surgical removal of enlarged tonsils and adenoids and 4–6 months later underwent a second overnight polysomnographic evaluation and morning blood draw.
Plasma IL-6 and IL-10 ELISA Assays
Fasting blood samples were drawn by venipuncture in the morning immediately after the initial diagnostic sleep study into tubes containing ethylenediaminetetraacetic acid EDTA. Blood samples were immediately centrifuged and frozen at −80°C until assay. Plasma IL-6 and IL-10 levels were measured using commercially available ELISA kits (R&D systems, Minneapolis, MN for IL-6 (Q6000B) and BD Biosciences, San Diego, CA for IL-10; OptEIA Kit 555157). For IL-6 the assay has a sensitivity of 0.15 pg/ml, and with a linearity range of 93–97%. The intra-assay and inter-assay coefficients of variability were 5.8% and 8.2%, respectively. For IL-10, the assay has a sensitivity of 7.8 pg/ml, a linearity range of 96–99%, an intra-assay coefficient of variability of 2.8%, and an inter-assay coefficient of variability of 5.8%.
Data Analysis
Data are presented as means ± standard error unless otherwise indicated. Paired Student t-tests were used to compare pre- and post-surgical findings. Analyses of variance and tests independent t-tests were used for comparisons of polysomnography, and IL-10 concentrations, and were followed by post-hoc tests as appropriate. All p-values reported are two-tailed with statistical significance set at <0.05.
Results
A total of 40 children were recruited and completed the experimental protocol. These included 20 control children who did not snore and who also exhibited normal polysomnographic studies, and 20 children with polygraphically demonstrated OSA. As shown in Table 1, these children were similar with respect to age, gender, ethnicity, and BMI. Table 2 shows the polysomnographic characteristics pre- and post-surgery in children with OSA as well as those in control subjects.
Table 1.
Demographic characteristics of 20 children with OSA before and after surgical adenotonsillectomy and of 20 matched control children.
| (1) Controls (n=20) | OSA Children (n=20) | P value | ||||
|---|---|---|---|---|---|---|
| (2) Pre-T&A | (3) Post T&A | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||
| Age (years) | 6.4±0.7 | 6.5±0.6 | 7.2±0.6 | NS | NS | NS |
| Gender (F:M) | 8:12 | 8:12 | 8:12 | NS | NS | NS |
| African American N (%) | 7 (38%) | 7 (30%) | 7 (30%) | NS | NS | NS |
| BMI (kg/m2) | 17.1±0.5 | 17.3±0.6 | 17.5±0.5 | NS | NS | NS |
| IL-6 (pg/mL) | 1.67±0.07 | 2.98±0.27 | 1.96±0.14 | <0.01 | NS | <0.01 |
| IL-10 (pg/L L) | 458.5±102.2 | 195.2±33.6 | 476.3±86.1 | <0.001 | NS | <0.001 |
Table 2.
Polysomnographic characteristics in 20 children with OSA, before and after tonsillectomy and adenoidectomy, and in 20 matched control children.
| (1) Controls (n=20) | OSA Children (n=20) | P value | ||||
|---|---|---|---|---|---|---|
| (2) Pre-T&A | (3) Post T&A | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||
| Sleep Latency (min) | 29.2±12.1 | 16.1±10.6 | 24.5±12.2 | <0.01 | NS | <0.01 |
| REM Latency (min) | 123.1±23.6 | 110.5±25.6 | 118.5±26.6 | NS | NS | NS |
| Total Sleep Time (hours) | 8.5±0.4 | 8.6±0.4 | 8.5±0.7 | NS | NS | NS |
| Sleep Efficiency (%) | 90.6±5.9 | 89.5±6.4 | 90.2.7±6.1 | NS | NS | NS |
| Stage 1 (%) | 7.2±4.1 | 8.4±5.5 | 8.0±5.2 | NS | NS | NS |
| Stage 2 (%) | 40.6±6.7 | 52.1±9.0 | 43.8±6.9 | <0.05 | NS | NS |
| Slow Wave Sleep (%) | 25.8±6.3 | 17.8±6.9 | 23.1±6.8 | <0.01 | NS | <0.01 |
| REM Sleep (%) | 25.8±5.7 | 16.9±7.8 | 27.7±8.1 | <0.01 | NS | <0.01 |
| Spontaneous arousal index (/hr TST) | 8.7±2.4 | 4.6±4.3 | 6.7±3.9 | <0.01 | NS | <0.05 |
| Respiratory arousal index (/hr TST) | 0.0±0.0 | 2.7±0.9 | 1.8±1.8 | <0.001 | <0.001 | <0.001 |
| AHI (/hr TST) | 0.0±0.0 | 13.3±2.8 | 1.4 ±0.8 | <0.0001 | <0.05 | <0.001 |
| AI (/hr TST) | 0.0±0.0 | 4.4±0.9 | 0.4±0.6 | <0.0001 | NS | <0.001 |
| Mean SpO SpO2 | 97.9±0.7 | 95.3 ±1.6 | 96.7±1.0 | NS | NS | NS |
| SpO SpO2 nadir | 94.1±0.3 | 77.9±1.9 | 91.8±2.3 | <0.00001 | <0.01 | <0.00001 |
| % TST SpO SpO2<90% | <0.0±0.0 | 2.5±0.2 | 0.1±0.2 | <0.00001 | NS | <0.00001 |
| Mean PETCO PETCO2 | 42.3±0.9 | 49.9±1.9 | 48.1±1.9 | <0.001 | <0.01 | NS |
| %TST PETCO PETCO2>50mmHg | >3.3±0.5 | 37.4±3.7 | 23.7±4.1 | <0.0001 | <0.00001 | <0.01 |
IL-6 plasma levels were significantly higher in patients with OSA compared to controls (Table 1; Figure 1) and decreased after tonsillectomy and adenoidectomy (T&A) to values that were similar to controls.
Figure 1.
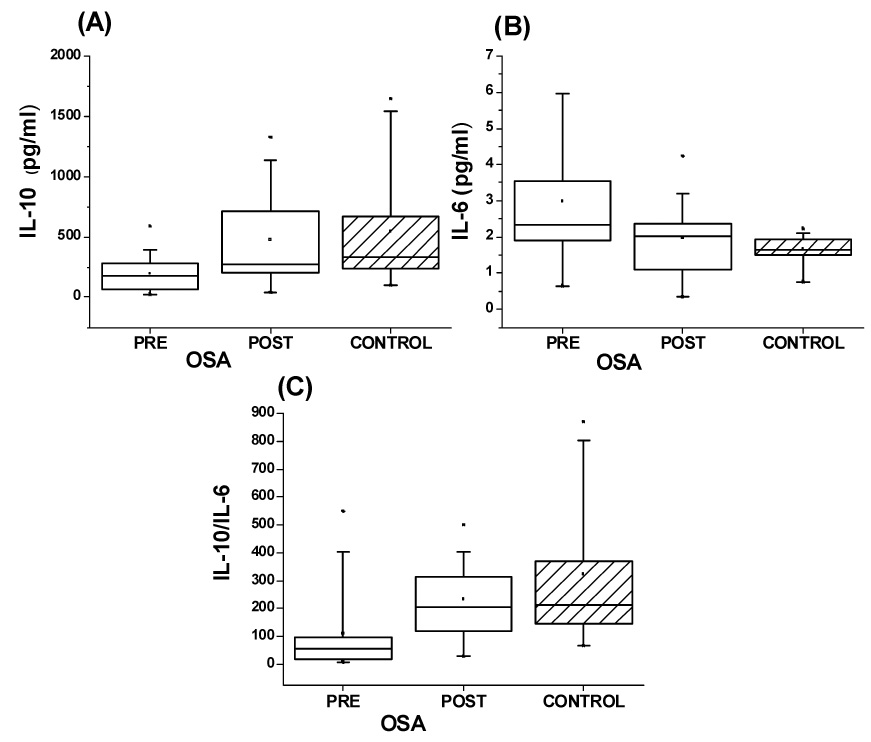
Box plots of IL-6, IL-10, and IL-10:IL-6 ratios in 20 children with OSA before and after T&A and in 20 matched controls.
Il-10 circulating plasma levels were markedly lower in patients with OSA compared to controls and returned to within normal levels after T&A (Table 1; Figure 1).
The individual ratios of the anti-inflammatory cytokine IL-10 and of the pro-inflammatory cytokine IL-6 were calculated and revealed markedly reduced ratios in OSA patients compared to controls. These ratios exhibited recovery to normal values after T&A (Figure 1; p>0.05 for post T&A vs. controls).
Discussion
In this study we have confirmed that IL-6 plasma levels are increased and IL-10 plasma levels are decreased in children with OSA when compared to healthy children. Furthermore, we have shown that in a substantial proportion of OSA children treatment with T&A will lead to normalization of both IL-6 and IL-10 circulating levels, the latter suggesting reduced global pro-inflammatory burden, as evidenced from their individual IL-10:IL-6 ratios. Our current findings further reinforce the concept that systemic inflammation is a constitutive component and consequence of OSA in children, even in the absence of obesity.
In a previous study conducted in a large cohort of snoring children, we found that those with moderate to severe OSA had elevated plasma IL-6 and CRP levels compared to children with mild OSA and controls, and that both plasma IL-6 and CRP levels were significantly correlated with OSA severity, independently of obesity (11). Our current findings in 20 non-obese children with moderate to severe OSA further confirm these observations and demonstrate that upon effective treatment, there is a significant return of IL-6 levels to near normal or normal values. Thus, OSA induces a reversible systemic low-grade inflammatory response, which in turn may further activate a variety of downstream pathways, all of which may ultimately lead to the typical end-organ morbidities thus far identified for OSA, and particularly those associated with atherogenesis and endothelial dysfunction as well as neurobehavioral deficits (15). While the present study was not designed to address these issues, we have r recently reported on the association between CRP (a downstream product of IL-6 activity) and neurocognitive morbidity in children with OSA (32), whereby the probability for such deficits was greatly increased if CRP was elevated in these patients (32). Thus, close monitoring of IL-6 and CRP levels in the context of pediatric OSA could allow for more accurate identification of those children with increased susceptibility to adverse consequences from their underlying sleep-disordered breathing.
We now also report for the first time on the marked decreases in the circulating levels of the anti-inflammatory cytokine, IL-10. We are aware of only one other similar study in the literature in which pediatric patients with OSA were examined for a large array of inflammatory proteins (33). However, these investigators failed to demonstrate any significant changes in either IL-6 or IL-10, probably b because their patients were milder, or, alternatively, because the sensitivity of their assays was insufficient for accurate detection of the low levels of these cytokines or to reflect the large variability in IL-10 responses. In adults with OSA, substantial decreases in IL-10 have been noted (34) and further confirmed in a subsequent study from another laboratory (35). However, in a more recent study by Ryan and colleagues, no differences in IL-10 emerged in a cohort of well-matched adult patients with OSA (36). Notwithstanding such considerations, the reversibility of IL-10 decreases upon surgical removal of enlarged tonsils and adenoids and lends further support to the concept that the major alterations associated with OSA, namely intermittent hypoxia and hypercapnia and sleep fragmentation, lead to activation of pro-inflammatory pathways and reciprocal down-regulation of anti-inflammatory proteins, as exemplified by the decreased levels of IL-10 in this study. Based on the known roles of cytokines in the pathophysiology of sleepiness (37, 38) and atherogenesis (20, 39), we postulate that the occurrence of such events may increase the risk for OSA-associated morbidity.
Some methodological considerations deserve comment. First and foremost, we excluded overweight and obese children, a major confounder in most studies dealing with OSA. Indeed, obesity is now viewed as a systemic inflammatory disorder (40, 41), and is also associated with an increased risk for OSA-induced morbidity (42, 43). We, therefore, anticipate that the presence of concurrent obesity in children with OSA will enhance the magnitude of the inflammatory response. Second, our study involved patients with relatively severe OSA, a fact that could have exacerbated the magnitude of the findings. Thirdly, we cannot infer on the relative contribution of upper airway tissues such as tonsils and adenoids to the systemic cytokine levels (44), an issue that clearly merits further investigation. Finally, the levels of IL-10 found herein are somewhat lower than those previously reported (45, 46). These differences may simply reflect slightly different methodologies in sample processing or in the assay itself. However, since all of our samples were assayed using the same technique, we believe that notwithstanding the lower values reported here the findings are still valid and indeed represent the increased pro-inflammatory load induced by OSA in children.
In summary, this study supports the hypothesis that OSA in childhood imposes an independent risk for the development of sub-clinical inflammation, though reciprocal induction of pro-inflammatory responses and suppression of anti-inflammatory substances, all of which in turn could promote the onset and progression of atherogenesis, particularly in children with a genetic predisposition for such condition.
Acknowledgments
This study was supported by NIH grant HL-65270, The Children’s Foundation Endowment for Sleep Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund.
Funding Sources: This study was supported by NIH grant HL-65270, The Children’s Foundation Endowment for Sleep Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lafranchi P, Somers VK. Obstructive sleep apnea and vascular disease. Respir Res. 2001;2:315–319. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–1437. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 3.Aljadeff G, Gozal D, Schechtman VL, Burrell B, Harper RM, Ward SL. Heart rate variability in children with obstructive sleep apnea. Sleep. 1997;20:151–157. doi: 10.1093/sleep/20.2.151. [DOI] [PubMed] [Google Scholar]
- 4.Baharav A, Kotagal S, Rubin BK, Pratt J, Akselrod S. Autonomic cardiovascular control in children with obstructive sleep apnea. Clin Auton Res. 1999;9:345–351. doi: 10.1007/BF02318382. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Sleep. 2005;28:747–752. doi: 10.1093/sleep/28.6.747. [DOI] [PubMed] [Google Scholar]
- 6.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 7.Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, Bokulic R, Daniels SR. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–956. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 8.Enright PL, Goodwin JL, Sherrill DL, Quan JR, Quan SF. Tucson Children's Assessment of Sleep Apnea study. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea study. Arch Pediatr Adolesc Med. 2003;157:901–904. doi: 10.1001/archpedi.157.9.901. [DOI] [PubMed] [Google Scholar]
- 9.Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, Witt SA, Glascock BJ, Daniels SR. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395–1399. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 10.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–e569. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 11.Tauman R, O'brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2006 Dec 15; doi: 10.1007/s11325-006-0085-7. [Epub ahead of print] PMID: 17171553. [DOI] [PubMed] [Google Scholar]
- 12.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, Zambito AM, Tracy RP, Jenny NS, Redline S. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 13.Kheirandish-Gozal L, Sans Capdevila O, Tauman R, Gozal D. Plasma C-reactive protein in reactive non-obese children with obstructive sleep apnea before and after adenotonsillectomy. J. Clin. Sleep Med. 2006;2:301–304. [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest. 2006;129:947–953. doi: 10.1378/chest.129.4.947. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Kheirandish L. Oxidant stress and inflammation in the snoring child: confluent pathways to upper airway pathogenesis and end-organ morbidity. Sleep Med Rev. 2006;10:83–96. doi: 10.1016/j.smrv.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Wakkach A, Cottrez F, Groux H. Can interleukin-10 be used as a true immunoregulatory cytokine? Eur Cytokine Netw. 2000;11:153–160. [PubMed] [Google Scholar]
- 17.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin-10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88:877–887. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- 19.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 20.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 21.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 22.Von Der Thusen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 23.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK, Berliner JA, Boisvert WA. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 24.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A, Mallat Z. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 25.Tziakas DN, Chalikias GK, Antonoglou CO, Veletza S, Tentes IK, Kortsaris AX, Hatseras DI, Kaski JC. Apolipoprotein E genotype and circulating interleukin-10 levels in patients with stable and unstable coronary artery disease. J Am Coll Cardiol. 2006;48:2471–2481. doi: 10.1016/j.jacc.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Kilic T, Ural D, Ural E, Yumuk Z, Agacdiken A, Sahin T, Kahraman G, Kozdag G, Vural A, Komsuoglu B. Relation between proinflammatory to anti-inflammatory cytokine ratios and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Heart. 2006;92:1041–1046. doi: 10.1136/hrt.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer LD, Kraemer HC, Wilson DM, et al. Standardized percentile curves of body mass index for children and adolescents. AJDC. 1991;145:259–263. doi: 10.1001/archpedi.1991.02160030027015. [DOI] [PubMed] [Google Scholar]
- 28.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 29.Rechtschaffen A, Kales A. Washington DC: National Institutes of Health; A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. 1968 Pul.No.204.
- 30.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-age children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society: Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 32.Gozal D, McLaughlin Crabtree V, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Resp Crit Care Med. 2007 doi: 10.1164/rccm.200610-1519OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam CS, Wong M, McBain R, Bailey S, Waters KA. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006;42:277–282. doi: 10.1111/j.1440-1754.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 34.Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, Gallai V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–311. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 35.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–350. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 36.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 37.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 38.Bravo MD, Serpero LD, Barcelo A, Barbe F, Agusti A, Gozal D. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath. 2007 Feb 6; doi: 10.1007/s11325-007-0100-7. [Epub ahead of print] PMID: 17279423. [DOI] [PubMed] [Google Scholar]
- 39.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 40.Ford ES, Galuska DA, Gillespie C, Will JC, Giles WH, Dietz WH. C-reactive protein and body mass index in children: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Pediatr. 2001;138:486–492. doi: 10.1067/mpd.2001.112898. [DOI] [PubMed] [Google Scholar]
- 41.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107(1):E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 42.Gozal D, Wang M, Pope DW., Jr Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108:693–697. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- 43.Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O’Brien LM, Ivanenko A, Gozal D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 44.Agren K, Andersson U, Nordlander B, Nord CE, Linde A, Ernberg I, Andersson J. Upregulated local cytokine production in recurrent tonsillitis compared with tonsillar hypertrophy. Acta Otolaryngol. 1995;115:689–696. doi: 10.3109/00016489509139388. [DOI] [PubMed] [Google Scholar]
- 45.Koopman LP, Savelkoul H, van Benten IJ, Gerritsen J, Brunekreef B, J Neijens H. Increased serum IL-10/IL-12 ratio in wheezing infants. Pediatr Allergy Immunol. 2003;14:112–119. doi: 10.1034/j.1399-3038.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 46.Malaponte G, Bevelacqua V, Li Volti G, Petrina M, Nicotra G, Sapuppo V, Li Volti S, Travali S, Mazzarino MC. Soluble adhesion molecules and cytokines in children affected by recurrent infections of the upper respiratory tract. Pediatr Res. 2004;55:666–673. doi: 10.1203/01.PDR.0000113770.22794.DF. [DOI] [PubMed] [Google Scholar]


