Abstract
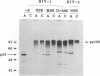
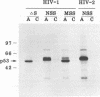
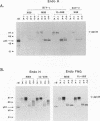
Conflicting results have been reported regarding the role of carbohydrate on human immunodeficiency virus (HIV) envelope glycoprotein gp120 in CD4 receptor binding. Glycosylated, deglycosylated, and nonglycosylated forms of HIV type 1 (HIV-1) and HIV-2 gp120s were used to examine CD4 receptor-binding activity. Nonglycosylated forms of gp120 generated either by deletion of the signal sequence of HIV-1 gp120 or by synthesis in the presence of tunicamycin failed to bind to CD4. In contrast, highly mannosylated gp120 bound to soluble CD4 molecules well. Enzymatic removal of carbohydrate chains from glycosylated gp120 by endoglycosidase H or an endoglycosidase F/N glycanase mixture had no effect on the ability of gp120 to bind CD4. An experiment which measured the ability of gp120 to bind to CD4 as an assay of the proper conformation of gp120 showed that carbohydrate chains on gp120 are not required for the interaction between gp120 and CD4 but that N-linked glycosylation is essential for generation of the proper conformation of gp120 to provide a CD4-binding site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthos J., Deen K. C., Chaikin M. A., Fornwald J. A., Sathe G., Sattentau Q. J., Clapham P. R., Weiss R. A., McDougal J. S., Pietropaolo C. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989 May 5;57(3):469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- Camerini D., Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the virus binding site. Cell. 1990 Mar 9;60(5):747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- Cordonnier A., Rivière Y., Montagnier L., Emerman M. Effects of mutations in hyperconserved regions of the extracellular glycoprotein of human immunodeficiency virus type 1 on receptor binding. J Virol. 1989 Oct;63(10):4464–4468. doi: 10.1128/jvi.63.10.4464-4468.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dirckx L., Lindemann D., Ette R., Manzoni C., Moritz D., Mous J. Mutation of conserved N-glycosylation sites around the CD4-binding site of human immunodeficiency virus type 1 GP120 affects viral infectivity. Virus Res. 1990 Dec;18(1):9–20. doi: 10.1016/0168-1702(90)90085-p. [DOI] [PubMed] [Google Scholar]
- Fennie C., Lasky L. A. Model for intracellular folding of the human immunodeficiency virus type 1 gp120. J Virol. 1989 Feb;63(2):639–646. doi: 10.1128/jvi.63.2.639-646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Clerget-Raslain B., Gluckman J. C., Guétard D., Montagnier L., Bahraoui E. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J Exp Med. 1989 Mar 1;169(3):807–822. doi: 10.1084/jem.169.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C., Bahraoui E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Grigera P. R., Mathieu M. E., Wagner R. R. Effect of glycosylation on the conformational epitopes of the glycoprotein of vesicular stomatitis virus (New Jersey serotype). Virology. 1991 Jan;180(1):1–9. doi: 10.1016/0042-6822(91)90002-s. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Jarvis D. L., Summers M. D. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989 Jan;9(1):214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- Morikawa Y., Moore J. P., Jones I. M. HIV-1 envelope protein gp120 expression by secretion in E. coli: assessment of CD4 binding and use in epitope mapping. J Virol Methods. 1990 Jul;29(1):105–113. doi: 10.1016/0166-0934(90)90013-6. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Moore J. P., Wilkinson A. J., Jones I. M. Reduction in CD4 binding affinity associated with removal of a single glycosylation site in the external glycoprotein of HIV-2. Virology. 1991 Feb;180(2):853–856. doi: 10.1016/0042-6822(91)90106-l. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Overton H. A., Moore J. P., Wilkinson A. J., Brady R. L., Lewis S. J., Jones I. M. Expression of HIV-1 gp120 and human soluble CD4 by recombinant baculoviruses and their interaction in vitro. AIDS Res Hum Retroviruses. 1990 Jun;6(6):765–773. doi: 10.1089/aid.1990.6.765. [DOI] [PubMed] [Google Scholar]
- Peterson A., Seed B. Genetic analysis of monoclonal antibody and HIV binding sites on the human lymphocyte antigen CD4. Cell. 1988 Jul 1;54(1):65–72. doi: 10.1016/0092-8674(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Datema R. Inhibition of glycosylation of viral glycoproteins. Biochem Soc Trans. 1979 Apr;7(2):322–326. doi: 10.1042/bst0070322. [DOI] [PubMed] [Google Scholar]
- Sodora D. L., Cohen G. H., Eisenberg R. J. Influence of asparagine-linked oligosaccharides on antigenicity, processing, and cell surface expression of herpes simplex virus type 1 glycoprotein D. J Virol. 1989 Dec;63(12):5184–5193. doi: 10.1128/jvi.63.12.5184-5193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Plummer T. H., Jr Enzymatic approaches for studying the structure, synthesis, and processing of glycoproteins. Methods Cell Biol. 1989;32:111–139. doi: 10.1016/s0091-679x(08)61169-3. [DOI] [PubMed] [Google Scholar]
- Tessier D. C., Thomas D. Y., Khouri H. E., Laliberté F., Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991 Feb 15;98(2):177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- Vidal S., Mottet G., Kolakofsky D., Roux L. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol. 1989 Feb;63(2):892–900. doi: 10.1128/jvi.63.2.892-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Compans R. W. Expression and characterization of a functional human immunodeficiency virus envelope glycoprotein in insect cells. Virology. 1990 Jun;176(2):575–586. doi: 10.1016/0042-6822(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Wright K. E., Salvato M. S., Buchmeier M. J. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology. 1989 Aug;171(2):417–426. doi: 10.1016/0042-6822(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]