Abstract
Several post-translational modifications of lysine residues of lens proteins have been implicated in cataractogenesis. In the present study, the molecular weight of an α-crystallin isolated from the water-soluble portion of a cataractous human eye lens indicated that it was a modified αB-crystallin. Further analysis by mass spectrometry of tryptic digests of this modified protein showed that Lys 92 was modified and that the sample was structurally heterogeneous. Lys 92 was acetylated in one population and carbamylated in another. Although carbamylation of lens crystallins has been predicted, this is the first documentation of in vivo carbamylation of a specific site. These results are also the first documentation of in vivo lysine acetylation of αB-crystallin. Both modifications alter the net charge on αB-crystallin, a feature that may have significance to cataractogenesis.
Keywords: Lens crystallins, post-translational modifications, acetylation, carbamylation, mass spectrometry
Transparency of the eye lens is maintained by a highly organized arrangement of structural proteins called crystallins. Crystallins of mammalian eye lenses are classified into three groups based on the molecular weight of their aggregates and on homology in their amino acid sequences. The α-crystallins, which form high molecular mass aggregates of ∼800 kD in solution, include two proteins, αA- and αB-crystallin and comprise ∼30% of the proteins in the human lens. The α-crystallins are essential not only for the structural integrity required for lens transparency, but also for their chaperone-like role in maintaining the solubility of other lens proteins (Horwitz 1992; Groenen et al. 1994; Derham and Harding 1999). During aging of the lens, α-crystallins undergo numerous post-translational modifications that may cause substantial change in protein conformation (Voorter et al. 1988; Ma et al. 1998; Sun et al. 1998). In vivo post-translational modifications of human αB-crystallin include phosphorylation at several serine residues (Miesbauer et al. 1994), C-terminal truncation (He et al. 1995; Smith et al. 1995; Jimenez-Asensio et al. 1999), modification of the C-terminal lysine (Lin et al. 1997), deamidation of asparaginyl and glutamyl residues (Groenen et al. 1993; Lund et al. 1996), and racemization and isomerization of aspartic residues (Fujii et al. 1994). Although some of these modifications are present at levels of only 5–10% in the total pool of soluble αB-crystallin, in summary they may substantially interfere with the function of the active protein.
Analysis of human lens extracts by electrospray ionization mass spectrometry (ESIMS) has led to identification of two new in vivo modifications of αB-crystallins. A protein isolated from the soluble α-crystallin fraction of a cataractous lens with Mr 20,242 was identified as αB-crystallin with either carbamylation or acetylation at Lys 92. Neither in vivo modification has been reported previously. Although carbamylation of lens crystallins has been postulated to have a role in cataract (Harding and Rixon 1980), there are no reports of isolation and structural identification of products of in vivo carbamylation of lens crystallins. This report also notes fragmentation features in the tandem mass spectra (MS/MS) of carbamylated and acetylated peptides that may be generally useful for distinguishing between these modifications when the modified peptides are not separated by high pressure liquid chromatography (HPLC).
Results and Discussion
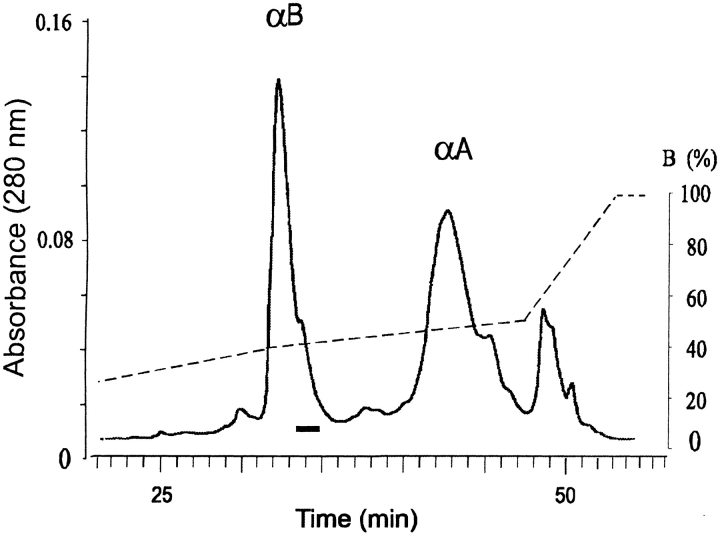
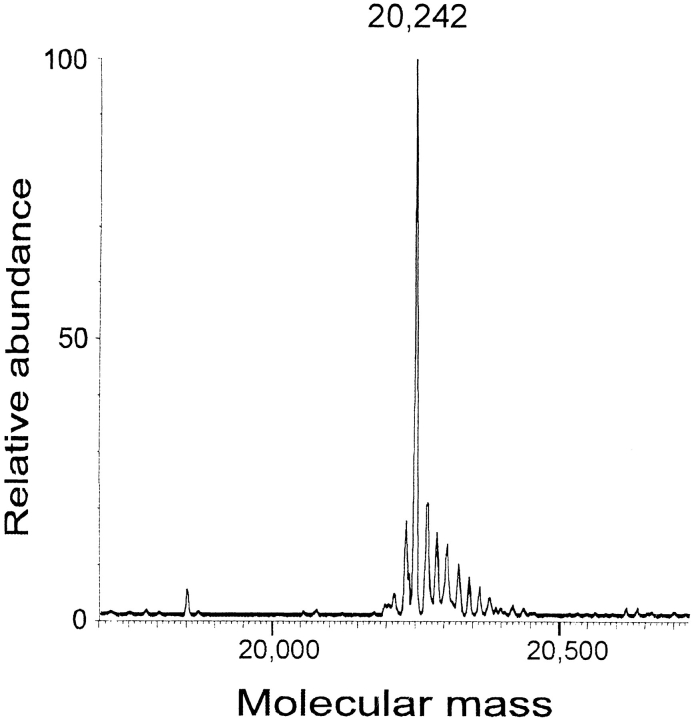
The α-crystallin fraction of human eye lens extracts consists of two proteins, αA and αB, which are easily separated by reversed-phase HPLC. Many age-related post-translational modifications of the crystallins cause broadening of the HPLC peaks. Analysis of the late-eluting shoulder of αB-crystallin (Fig. 1 ▶) by ESIMS showed the presence of unmodified αB-crystallin (Mr 20,200), an unidentified protein with Mr 20,242 and αB-crystallin with one (Mr 20,280) or two (Mr 20,360) phosphate groups (spectra not shown). The protein of interest in this study is the one with Mr 20,242. Because the uncertainty in the molecular mass determination is ±2 daltons, the apparent 42-dalton increase in the molecular mass of αB-crystallin could be due either to carbamylation (+43 daltons) or acetylation (+42 daltons). Either modification leads to a reduction in net charge, suggesting that the modified protein could be isolated by ion exchange chromatography. Desalting and analysis by ESIMS of the ion exchange fractions verified that the modified αB-crystallin was pure and that it had a molecular weight of 20,242 (Fig. 2 ▶).
Fig. 1.
Chromatogram of the reversed-phase HPLC separation of soluble α-crystallins from a cataractous human lens from a 77-year-old patient. The shoulder of αB-crystallin, marked by the bar, contained the 20,242-dalton protein.
Fig. 2.
Deconvoluted electrospray ionization mass spectrum of a modified αB-crystallin with Mr 20,242 after isolation by anion-exchange chromatography. Some minor additional peaks can be attributed to sodium adducts.
Analysis of a tryptic digest of the purified αB-crystallin by HPLC ESIMS showed that the molecular masses of most of the peptides in the digest matched those expected for tryptic cleavages of αB-crystallin. Two abundant peptides with molecular weights of 1434.2 and 1435.2 were not found in a tryptic digest of unmodified αB-crystallin. These molecular masses were 42 and 43 daltons larger than the molecular mass of an expected αB-crystallin tryptic peptide including residues 91–103. Present at much lower levels were two more peptides with unexpected molecular weights (1987 and 3057), which correspond to similar modifications of segments including residues 91–107 and 91–116. These results coupled with failure to find any other modified peptides indicated that the unidentified form of αB-crystallin actually had two forms of modification, one increasing the molecular mass by 42 daltons, the other by 43 daltons. In addition, these results showed that the modifications occurred within the segment including residues 91–103.
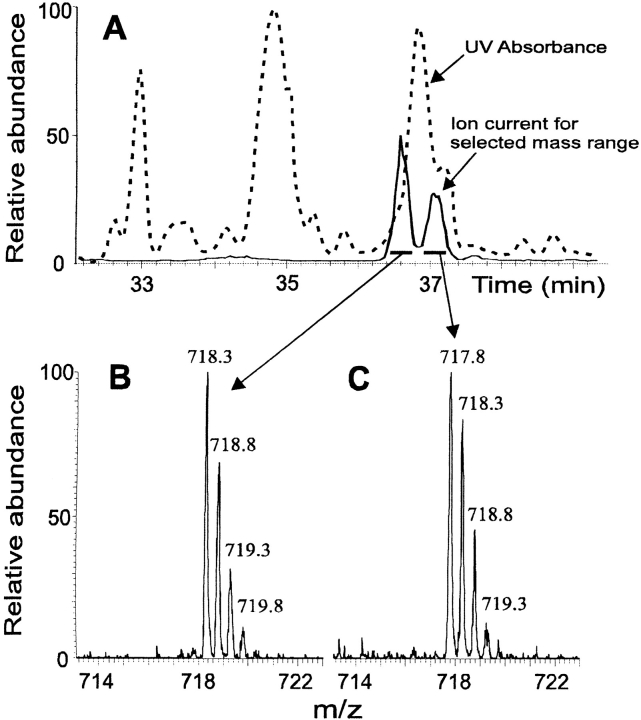
Carbamylation of proteins may be an artifact when urea containing solutions are used to isolate proteins because urea decomposes to isocyanate, a carbamylating agent. Although freshly deionized urea solutions had been used in preparing the buffers for ion-exchange chromatography of the αB-crystallin, there existed a possibility that the proteins were carbamylated during isolation. This potential source of carbamylation was investigated by omitting the ion-exchange step and analyzing a tryptic digest of the same late eluting shoulder by HPLC ESIMS. The sensitivity and resolution of the ion trap mass spectrometer was increased by operating in the zoom scan mode to detect the singly and doubly charged ions (mass-to-charge ratio [m/z] 1436 or 718 ± 5 daltons, respectively) of the modified tryptic fragments including residues 91–103. With these conditions, two separate peaks were evident in the total ion current chromatogram of the doubly charged ions (Fig. 3A ▶, solid line). Mass spectra of these peaks (Fig. 3B,C ▶) showed that the carbamylated form (calculated m/z 718.3) eluted before the acetylated form (calculated m/z 717.8), and that carbamylation was indeed an in vivo modification.
Fig. 3.
Detection of the modified tryptic fragment (residues 91–103) of αB-crystallin isolated using only reversed-phase HPLC (i.e., no exposure to urea). (A) Reversed-phase HPLC chromatogram of the tryptic digest. (dashed line) UV absorbance (214 nm); (solid line) selected ion current (m/z 718.6 ± 5). (B) Mass spectrum of the doubly charged ion eluting in the first peak of the selected ion current plot in A. The mass corresponds to carbamylated peptide 91–103, an addition of 43 dalton. (C) Mass spectrum of the doubly charged ion eluting in the second peak of the selected ion current plot in A. The mass corresponds to acetylated peptide 91–103, an addition of 42 daltons.
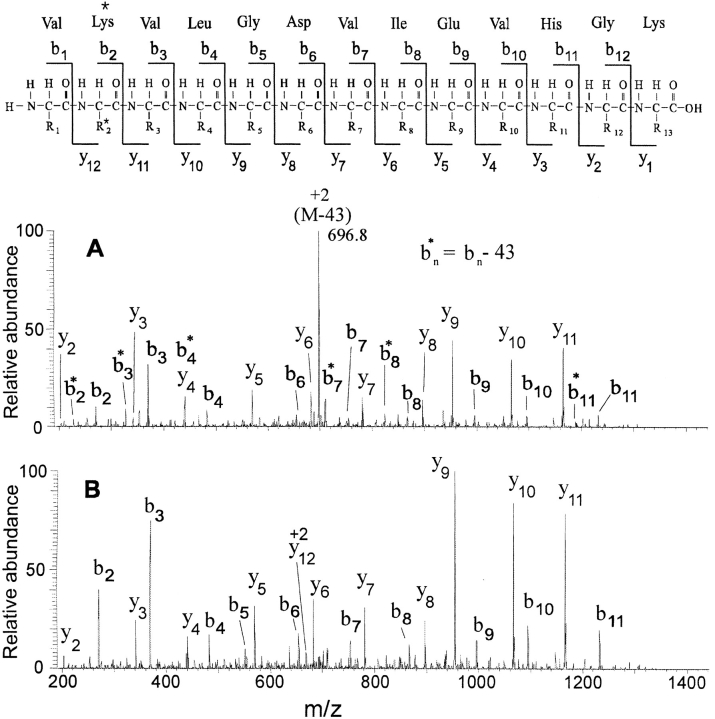
During the HPLC ESIMS analysis of the tryptic digest of the modified αB-crystallin, the mass spectrometer alternated between zoom scans and collision-induced dissociation (CID) MS/MS scans. The CID MS/MS spectra of the doubly charged forms of the carbamylated (Fig. 4A ▶) and acetylated (Fig. 4B ▶) peptides illustrate their fragmentation. A generic scheme illustrating fragmentation of this peptide and the nomenclature of the fragments is presented at the top of Fig. 4 ▶. As can be seen from Table 1, singly charged ions for nearly all b- and y-series ions were found for both the carbamylated and acetylated tryptic fragments. The difference between calculated and found mass-to-charge ratios of all b and y ions was not >0.2 daltons. The mass-to-charge ratios of the y2–11 ions of both the carbamylated and acetylated forms correspond to those expected for unmodified residues of this peptide, consistent with modification of Lys 92. The mass-to-charge ratios of the b2–12 ions, as well as y12 for the acetylated peptide, confirm modification of αB-crystallin at Lys 92.
Fig. 4.
Tandem mass spectrometry of modified tryptic peptides of αB-crystallin including residues 91–103. (A) The MS/MS spectrum of the carbamylated form (Fig. 3B ▶); (B) The MS/MS spectrum of the acetylated form (Fig. 3C ▶). (top) The amino acid sequence of this peptide, as well as definition of b and y ions. Both CID MS/MS spectra have nearly complete series of b- and y-fragment ions, which indicate that both carbamylation and acetylation occur on Lys 92. In addition, the MS/MS spectrum of the carbamylated peptide has a large peak at m/z 696.8, corresponding to loss of the carbamyl functionality (CONH), as well as a nearly complete series of corresponding b-fragment ions showing this loss (indicated as b*).
Table 1.
Masses of fragment ions of the b and y series for αB-crystallin peptide 91-103 carbamylated and acetylated at Lys 92
| Carbamylated peptide | Acetylated peptide | ||||||
| bn series | observed m/z | yn series | observed m/z | bn series | observed m/z | yn series | observed m/z |
| b1 | — | y1 | — | b1 | — | y1 | — |
| b2 | 271.1 | y2 | 204.1 | b2 | 270.1 | y2 | 204.1 |
| b2−43 | 228.2 | ||||||
| b3 | 370.2 | y3 | 341.2 | b3 | 369.2 | y3 | 341.2 |
| b3−43 | 327.2 | ||||||
| b4 | 483.2 | y4 | 440.2 | b4 | 482.3 | y4 | 440.2 |
| b4−43 | 440.2 | ||||||
| b5 | — | y5 | 569.2 | b5 | — | y5 | 569.2 |
| b6 | 655.4 | y6 | 682.2 | b6 | 654.3 | y6 | 682.3 |
| b6−43 | 612.3 | ||||||
| b7 | 754.4 | y7 | 781.3 | b7 | 753.4 | y7 | 781.3 |
| b7−43 | 711.4 | ||||||
| b8 | 867.5 | y8 | 896.5 | b8 | 866.5 | y8 | 896.5 |
| b8−43 | 824.5 | ||||||
| b9 | 996.4 | y9 | 953.3 | b9 | 995.6 | y9 | 953.3 |
| b10 | 1095.5 | y10 | 1066.6 | b10 | 1094.6 | y10 | 1066.6 |
| b11 | 1232.5 | y11 | 1165.7 | b11 | 1231.6 | y11 | 1165.7 |
| b11−43 | 1189.7 | ||||||
| b12 | — | y12 | — | b12 | — | y12 | 1335.6a |
a Observed as a doubly charged ion at m/z 668.1+2.The experimental masses differed by <0.2 daltons from the expected masses.(m/z) mass-to-charge ratio.
The amount of αB modified at Lys 92 was estimated to be ∼3% of the total water-soluble αB. This estimate was based on comparisons of the ultraviolet (UV) absorbances of the main peak and the shoulder in the reversed-phase HPLC chromatogram (Fig. 1 ▶), and the size of the subsequent ion exchange peak (not shown) from which αB modified at Lys 92 was isolated. The shoulder was ∼20% of the main αB peak, whereas the 20,242-dalton protein was ∼15% of the protein in the shoulder. Because the carbamylated and acetylated peptides have the same charge and similar structures, it is likely that they have similar ionization efficiencies. Assuming this to be true, the relative abundance of the carbamylated and acetylated peptides, estimated from the total ion current of the zoom scans is ∼2 : 1 (see Fig. 3A ▶). Although only one lens was analyzed in detail as described here, a modified intact αB-crystallin with the same molecular mass has been detected, but not quantified, in several other cataractous lenses as well as an old clear lens, suggesting that carbamylation/acetylation may be a general phenomenon (unpublished data).
These spectra also show distinct differences in the fragmentation of carbamylated and acetylated side chains in peptides. In addition to the backbone cleavages expected for peptides (b- and y-series ions), CID of the carbamylated peptide gave an intense peak at m/z 696.8. These ions are attributed to doubly charged ions that have lost CONH (43 daltons), the carbamylation group. Subsequent cleavage of various peptide amide linkages with charge retention on the N terminus leads to a second b series of ions, indicated by b* in Fig. 4A ▶ (b−43 in Table 1). A similar loss from the side chains of acetylated peptides is not evident from the spectrum in Fig. 4B ▶. The loss of CONH from carbamylated peptides may be particularly useful for analyses of mixtures of carbamylated and acetylated peptides when the two forms are not separated by HPLC, as is often the case (Qin et al. 1993). Whether the facile loss of CONH is a general phenomenon of carbamylated peptides or is unique to this segment of αB-crystallin, which has two widely spaced Lys residues, awaits analysis of peptides with different sequences.
Carbamylation of the ɛ-amino group of lysine, determined as homocitrulline, has been detected in a variety of proteins, particularly from patients with renal impairment (Harding and Rixon 1980; Kraus et al. 1994; Trepanier et al. 1996; Kraus and Kraus 1998). The present results appear to be the first published structural evidence of in vivo carbamylation at the molecular level. Several investigators have suggested that carbamylation of lens crystallins might be an initiator of cataract (Harding and Rixon 1980; Crompton et al. 1985; Plater et al. 1997). In vitro carbamylation of lens crystallins has been shown to affect protein conformation (Beswick and Harding 1984) and to lead to high molecular weight aggregates (Beswick and Harding 1987), leading to a proposal that cataract in renal failure patients may be due to carbamylated crystallins (Harding and Crabbe 1984; van Heyningen and Harding 1988). According to this interesting hypothesis, the high levels of urea common to impaired kidney function lead to high levels of isocyanate, which may carbamylate the crystallins. However, examination of crystallins from renal failure patients using procedures that permitted detection of modifications present at 5% or higher, yielded no evidence of carbamylated lysines. Glutathione adducts, not carbamylated lysines, were the distinguishing feature of these lens crystallins (Smith et al. 1995). The present results indicate that ∼2% of αB-crystallin in this cataractous lens is carbamylated. The lower detection limit in this study was obtained by using additional chromatographic steps and improved mass spectrometric methods.
In vivo acetylation has been studied much more extensively than carbamylation. There is now considerable evidence that acetylation may have a role in signaling, similar to that of phosphorylation (Kouzarides 2000; Magnaghi-Jaulin et al. 2000; Sterner and Berger 2000). Lysyl residues of many proteins, most notably histones, have been found acetylated, and several acetylases and deacetylases have been reported. Within the family of human lens crystallins, Lin et al. reported acetylation of ∼5% of Lys 70 in αA-crystallin (Lin et al. 1998). Results from more recent studies indicate that the level of acetylation at this position may be as high as 20% in some cataractous lenses (unpublished data). The ∼1% acetylation found at Lys 92 in the present study is the first report of in vivo acetylation of a lysine residue in αB-crystallin.
The potential importance of carbamylation and acetylation of lens crystallins to cataractogenesis has been discussed extensively (Harding and Crabbe 1984; Crompton et al. 1985; Rao et al. 1985; Qin et al. 1993; Derham and Harding 1999). For proteins at a physiological pH, both modifications of lysyl residues result in neutralization of the positive charge at an amino group, thus altering the net charge of the protein. Such changes remove the potential for favorable ion-pair interactions that might enhance close packing of crystallins required for lens transparency. Even when present only at very low concentrations, modified α-crystallins may contribute to conformational changes that substantially affect protein–protein interactions in crystallin aggregates. Ultimately, these modifications may alter the chaperone-like properties widely attributed to α-crystallins (Groenen et al. 1994; Cherian and Abraham 1995; van Boekel et al. 1996; Derham and Harding 1999).
In vitro carbamylation and acetylation of crystallins have been studied extensively (Beswick and Harding 1984, 1987; Crompton et al. 1985; Rao et al. 1985; Rao and Cotlier 1988; Sen and Chakrabarti 1990; Qin et al. 1992 Qin et al. 1993; Hasan et al. 1993). Some of these studies have led to identification of the modified products and specific sites of modification. Chemical modification of bovine αA-crystallin with ascorbic acid (Ortwerth et al. 1992), aspirin (Hasan et al. 1993), and isocyanate (Qin et al. 1992) showed that all Lys residues were modified to various extents. In vitro studies of αB-crystallin showed that Lys 92 as well as three other Lys residues were modified substantially by ascorbic acid (Ortwerth et al. 1992). Although the order of reactivity of the lysyl residues in α-crystallins depends on the reagent, these in vitro studies showed that several lysyl residues in both αA- and αB-crystallins are highly reactive, and that Lys 70 in αA and Lys 92 in αB are among the more reactive residues.
Finding only Lys 70 in αA-crystallin and Lys 92 in αB-crystallin modified in vivo is consistent with previous in vitro studies, showing these residues among the most active of the lysine residues. However, finding no evidence for carbamylation or acetylation of other lysyl residues was unexpected, based on results of in vitro studies. Given the many reports of acetylases, this high level of specificity suggests that one or more acetylases may be involved with acetylation of the α-crystallins. However, we are not aware of specific enzymes that lead to carbamylation of lysyl residues. Yet in vivo carbamylation of αB-crystallin appears to be restricted to Lys 92. Although this result suggests the possibility of a carbamylase, it also may point to significant structural differences between α-crystallins in the lens tissue and α-crystallins in solution. Perhaps both modifications occur through nonenzymatic reactions that are specific only because the close packing of crystallins in the lens limits access of reagents to lysyl residues other than Lys 70 of αA and Lys 92 of αB. Discrepancies between in vivo and in vitro phosphorylation of lens crystallins support this interpretation (Kleiman et al. 1988; Miesbauer et al. 1994; Schey et al. 1997).
Materials and methods
The water-soluble proteins from a lens with mild cataract from a 77-year-old patient were extracted by 50 mM 2–[N-morpholino]ethane sulfonic acid (MES) buffer, pH 6.0, containing 500 mM NaCl, 1 mM EDTA and fractionated by gel-filtration chromatography (Toyo-Pearl HW-55sf column, 2.5 × 95 cm) using the same MES buffer as described previously (Lapko et al. 2000). The medical history of the donor included cancer, hypertension, and arthritis, but there was no indication of renal insufficiency or diabetes. The α-crystallin fraction was fractionated by reversed-phase HPLC using a column (Vydac C4, 4.6 × 150 mm) equilibrated with a solution of 15% acetonitrile in H2O with 0.1% trifluoroacetic acid (TFA). The flow rate was 1 mL/min with 0.1% TFA in water as solvent A and 0.1% TFA in 95% acetonitrile as solvent B. The proteins were eluted using a gradient of 15–40% solvent B in 25 min, followed by 40–48% solvent B in 16 min. The main αB-crystallin peak and its back shoulder containing a protein with Mr 20,242 were collected separately and concentrated to dryness (SpeedVac vacuum concentrator, Savant). Pure protein with Mr 20,242 was obtained by anion exchange chromatography (Mono-Q anion-exchange column; Pharmacia). The dry HPLC fraction (∼300 μg protein) was dissolved in 0.8 mL of 20 mM Tris-HCl buffer, 7.5 M urea, pH 8.3 and applied to the column equilibrated with the same buffer. The proteins were eluted at a flow rate 0.6 mL/min by a gradient of 0–80 mM NaCl in the same buffer developed over 20 min, followed by 80–110 mM NaCl in 10 min, and finally to 200 mM NaCl in 5 min. The 8-M urea solution used in preparation of the buffers was deionized immediately before use. Collected protein fractions were stored at −80°C until further analysis. Stored proteins were desalted by reversed-phase HPLC (Vydac C4 column, 2 × 50 mm) before analysis by mass spectrometry. Proteins collected from HPLC were dried, dissolved in 50% acetonitrile/water (0.2% formic acid), and injected directly into an ESIMS (Platform II; Micromass) by using a solvent flow of 7 μL/min of 50% acetonitrile.
To prepare tryptic digests, we dissolved the proteins (100 pmole) in 30 μL of 100 mM ammonium bicarbonate buffer (pH 8.3) and incubated them with trypsin at an enzyme : protein ratio of 1 : 30 (w/w) for 12 h at 37°C. The resulting peptides were fractionated by capillary reversed-phase HPLC (C18-PM LC-Packings column, 0.3 × 250 mm, 5 μm, 300 Å, Shimadzu LC-10AT HPLC system). Both solvents (A water; B 66% acetonitrile) contained 0.01% TFA. Peptides were eluted with a gradient of 0–50% solvent B over 55 min. The flow rate (5 μL/min) was maintained by a microflow processor (AcuRate; LC-Packings). The eluate from the column was directed to an electrospray ionization mass spectrometer (Ion Trap; Finnigan MAT LCQ). To analyze the digest of the protein with Mr 20,242 isolated by ion-exchange chromatography, we operated the instrument in the full-scan/MS mode with the most abundant ion of each scan analyzed by MS/MS. To analyze the digest of the mixture of αB-crystallins present in the shoulder of the HPLC peak (i.e., no ion-exchange chromatography), we operated the mass spectrometer in a zoom scan mode for singly (m/z 1436.2) and doubly (m/z 718.6) charged ions of the segment including residues 91–103. The m/z window for both measurements was ±5 daltons. In the third step of each cycle, the CID fragmentation spectrum was recorded for ions at m/z 718 ± 3 (collision energy 35%).
Acknowledgments
This research was supported by a grant (EY RO1 07609) from the National Institutes of Health and the Nebraska Center for Mass Spectrometry. Lenses were obtained from the Lions Eye Bank, Omaha, NE or National Disease Research Interchange, Philadelphia, PA.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.40901.
References
- Beswick, H.T. and Harding, J.J. 1984. Conformational changes induced in bovine lens α-crystallin by carbamylation. Biochem. J. 223 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick, H.T. and Harding, J.J. 1987. High-molecular-weight crystallin aggregate formation resulting from non-enzymic carbamylation of lens crystallins: Relevance to cataract formation. Exp. Eye Res. 45 569–578. [DOI] [PubMed] [Google Scholar]
- Cherian, M. and Abraham, E.C. 1995. Decreased molecular chaperone property of α-crystallins due to posttranslational modifications. Biochem. Biophys. Res. Commun. 208 675–679. [DOI] [PubMed] [Google Scholar]
- Crompton, M., Rixon, K.C., and Harding, J.J. 1985. Aspirin prevents carbamylation of soluble lens proteins and prevents cyanate-induced phase separation opacities in vitro: A possible mechanism by which aspirin could prevent cataract. Exp. Eye Res. 40 297–311. [DOI] [PubMed] [Google Scholar]
- Derham, B.K. and Harding, J.J. 1999. α-Crystallin as a molecular chaperone. Prog. Retin. Eye Res. 18 463–509. [DOI] [PubMed] [Google Scholar]
- Fujii, N., Ishibashi, Y., Satoh, K., Fujino, M., and Harada, K. 1994. Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta 1204 157–163. [DOI] [PubMed] [Google Scholar]
- Groenen, P.J.T.A., van Dongen, M.J.P., Voorter, C.E.M., Bloemendal, H., and de Jong, W.W. 1993. Age-dependent deamidation of αB-crystallin. FEBS Lett. 322 69–72. [DOI] [PubMed] [Google Scholar]
- Groenen, P.J.T.A., Merck, K.B., de Jong, W.W., and Bloemendal, H. 1994. Structure and modifications of the junior chaperone α-crystallin: From lens transparency to molecular pathology. Eur. J. Biochem. 225 1–19. [DOI] [PubMed] [Google Scholar]
- Harding, J.J. and Crabbe, M.J.C. 1984. The lens: Development, proteins, metabolism and cataract. In The eye. (ed. H. Davson), pp. 207–492. Academic Press, Orlando, FL.
- Harding, J.J. and Rixon, K.C. 1980. Carbamylation of lens proteins: A possible factor in cataractogenesis in some tropical countries. Exp. Eye Res. 31 567–571. [DOI] [PubMed] [Google Scholar]
- Hasan, A., Smith, J.B., Qin, W., and Smith, D.L. 1993. The reaction of bovine lens αA-crystallin with aspirin. Exp. Eye Res. 57 29–35. [DOI] [PubMed] [Google Scholar]
- He, S., Pan, S., Wu, K., Amster, J., and Orlando, R. 1995. Analysis of normal human fetal eye lens crystallins by high-performance liquid chromatography/mass spectrometry. J. Mass Spectrom. 30 424–431. [Google Scholar]
- Horwitz, J. 1992. α-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 89 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Asensio, J., Colvis, C.M., Kowalak, J.A., Duglas-Tabor, Y., Datiles, M.B., Moroni, M., Mura, U., Rao, C.M., Balasubramanian, D., Janjani, A., et al. 1999. An atypical form of αB-crystallin is present in high concentration in some human cataractous lenses. J. Biol. Chem. 274 32287–32294. [DOI] [PubMed] [Google Scholar]
- Kleiman, N.J., Chiesa, R., Gawinowicz-Kolks, M.A., and Spector, A. 1988. Phosphorylation of β-crystallin B2 (βBp) in the bovine lens. J. Biol. Chem. 263 14978–14983. [PubMed] [Google Scholar]
- Kouzarides, T. 2000. Acetylation: A regulatory modification to rival phosphorylation? EMBO J. 19 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, L.M. and Kraus, A.P. 1998. The search for the uremic toxin: The case for carbamoylation of amino acids and proteins. Wien. Klin. Wochenschr. 110 521–530. [PubMed] [Google Scholar]
- Kraus, L.M., Elberger, A.J., Handorf, C.R., Pabst, M.J., and Kraus, A.P. 1994. Urea-derived cyanate forms ɛ-amino-carbamoyl-lysine (homocitrulline) in leukocyte proteins in patients with end-stage renal disease on peritoneal dialysis. J. Lab. Clin. Med. 123 882–891. [PubMed] [Google Scholar]
- Lapko, V.N., Smith, D.L., and Smith, J.B. 2000. Identification of an artifact in the mass spectrometry of proteins derivatized with iodoacetamide. J. Mass Spectrom. 35 572–575. [DOI] [PubMed] [Google Scholar]
- Lin, P., Smith, D.L., and Smith, J.B. 1997. In vivo modification of the C-terminal lysine of human lens αB-crystallin. Exp. Eye Res. 65 673–680. [DOI] [PubMed] [Google Scholar]
- Lin, P.P., Barry, R.C., Smith, D.L., and Smith, J.B. 1998. In vivo acetylation identified at Lysine 70 of human lens αA-crystallin. Protein Sci. 7 1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, A.L., Smith, J.B., and Smith, D.L. 1996. Modifications of the water-insoluble human lens α-crystallins. Exp. Eye Res. 63 661–672. [DOI] [PubMed] [Google Scholar]
- Ma, Z., Hanson, S.R., Lampi, K., David, L., Smith, D.L., and Smith, J.B. 1998. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp. Eye Res. 67 21–30. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin, L., Ait-Si-Ali, S., and Harel-Bellan, A. 2000. Histone acetylation and the control of the cell cycle. Prog. Cell Cycle Res. 4 41–47. [DOI] [PubMed] [Google Scholar]
- Miesbauer, L.R., Zhou, X., Yang, Z., Yang, Z., Sun, Y., Smith, D.L., and Smith, J.B. 1994. Post-translational modifications of the water soluble human lens crystallins from young adults. J. Biol. Chem. 269 12494–12502. [PubMed] [Google Scholar]
- Ortwerth, B.J., Slight, S.H., Prabhakaram, M., Sun, Y., and Smith, J.B. 1992. Site-specific glycation of lens crystallins by ascorbic acid. Biochim. Biophys. Acta 1117 207–215. [DOI] [PubMed] [Google Scholar]
- Plater, M.L., Boode, D., and Crabbe, M.J. 1997. Ibuprofen protects α-crystallin against posttranslational modification by preventing protein cross-linking. Ophthalmic Res. 29 421–428. [DOI] [PubMed] [Google Scholar]
- Qin, W., Smith, J.B., and Smith, D.L. 1992. Rates of carbamylation of specific lysyl residues in bovine α-crystallins. J. Biol. Chem. 267 26128–26133. [PubMed] [Google Scholar]
- ———. 1993. Reaction of aspirin with cysteinyl residues of lens γ-crystallins: A mechanism for the proposed anti-cataract effect of aspirin. Biochim. Biophys. Acta 1181 103–110. [DOI] [PubMed] [Google Scholar]
- Rao, G.N. and Cotlier, E. 1988. Aspirin prevents the nonenzymatic glycosylation and carbamylation of the human eye lens crystallins in vitro. Biochem. Biophys. Res. Commun. 151 991–996. [DOI] [PubMed] [Google Scholar]
- Rao, G.N., Lardis, M.P., and Cotlier, E. 1985. Acetylation of lens crystallins: A possible mechanism by which aspirin could prevent cataract formation. Biochem. Biophys. Res. Commun. 128 1125–1132. [DOI] [PubMed] [Google Scholar]
- Schey, K.L., Fowler, J.G., Schwartz, J.C., Busman, M., Dillon, J., and Crouch, R.K. 1997. Complete map and identification of the phosphorylation site of bovine lens major intrinsic protein. Invest. Ophthalmol. Vis. Sci. 38 2508–2515. [PubMed] [Google Scholar]
- Sen, A.C. and Chakrabarti, B. 1990. Effect of acetylation by aspirin on the thermodynamic stability of lens crystallins. Exp. Eye Res. 51 701–709. [DOI] [PubMed] [Google Scholar]
- Smith, J.B., Shun-Shin, G.A., Sun, Y., Miesbauer, L.R., Yang, Z., Yang, Z., Zhou, X., Schwedler, J., and Smith, D.L. 1995. Glutathione adducts, not carbamylated lysines are the major modification of lens α-crystallins from renal failure patients. J. Protein Chem. 14 179–188. [DOI] [PubMed] [Google Scholar]
- Sterner, D.E. and Berger, S.L. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64 435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T.X., Akhtar, N., and Liang, J.J. 1998. Conformational change of human lens insoluble α-crystallin. J. Protein Chem. 17 679–684. [DOI] [PubMed] [Google Scholar]
- Trepanier, D.J., Thibert, R.J., Draisey, T.F., and Caines, P.S. 1996. Carbamylation of erythrocyte membrane proteins: An in vitro and in vivo study. Clin. Biochem. 29 347–355. [DOI] [PubMed] [Google Scholar]
- van Boekel, M.A.M., Hoogakker, S.E.A., Harding, J.J., and de Jong, W.W. 1996. The influence of some post-translational modifications on the chaperone-like activity of α-crystallin. Ophthalmic Res. (Suppl. 1) 28 32–38. [DOI] [PubMed] [Google Scholar]
- van Heyningen, R. and Harding, J.J. 1988. A case-control study of cataract in Oxfordshire: Some risk factors. Br. J. Ophthalmol. 72 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorter, C.E.M., de Haard-Hoekman, W.A., van den Oetelaar, P.J.M., Bloemendal, H., and de Jong, W.W. 1988. Spontaneous peptide bond cleavage in aging α-crystallin through a succinimide intermediate. J. Biol. Chem. 263 19020–19023. [PubMed] [Google Scholar]