Abstract
Crystallographic studies of the intermediate states between unliganded and fully liganded hemoglobin (Hb) have revealed a large range of subtle but functionally important structural differences. Only one T state has been reported, whereas three other quaternary states (the R state, B state, and R2 or Y state) for liganded Hb have been characterized; other studies have defined liganded Hbs that are intermediate between the T and R states. The high-salt crystal structure of bovine carbonmonoxy (CO bovine) Hb has been determined at a resolution of 2.1 Å and is described here. A detailed comparison with other crystallographically solved Hb forms (T, R, R2 or Y) shows that the quaternary structure of CO bovine Hb closely resembles R state Hb. However, our analysis of these structures has identified several important differences between CO bovine Hb and R state Hb. Compared with the R state structures, the β-subunit N-terminal region has shifted closer to the central water cavity in CO bovine Hb. In addition, both the α- and β-subunits in CO bovine Hb have more constrained heme environments that appear to be intermediate between the T and R states. Moreover, the distal pocket of the β-subunit heme in CO bovine Hb shows significantly closer interaction between the bound CO ligand and the Hb distal residues Val 63(E11) and His 63(E7). The constrained heme groups and the increased steric contact involving the CO ligand and the distal heme residues relative to human Hb may explain in part the low intrinsic oxygen affinity of bovine Hb.
Keywords: X-ray crystal structure, bovine, hemoglobin, R state, oxygen affinity
The human adult hemoglobin (HbA) tetramer consists of two α- (141 amino acid residues) and two β- (146 amino acid residues) subunits. The α1β1 and α2β2 dimers are related by a molecular twofold axis of symmetry or molecular dyad that intersects the central water cavity. Perutz (1970, 1972) and Baldwin and Chothia (1979) elucidated at atomic resolution the structures of the T (tense) and R (relaxed) forms embodied in the two-state MWC model (Monod et al. 1965), which assumes that the T and the R states switch allosterically without intermediate states. A ligand-bound Hb known as R2 (Silva et al. 1992) or Y (Smith et al. 1991; Smith and Simmons 1994) has been proposed as an intermediate between the T and R structures. However, further analysis has shown that R2 (Y) is not an intermediate in the T to R transition, but rather is another relaxed end-state structure (Janin and Wodak 1993). Srinivasan and Rose (1994) have further suggested that R2 (Y) may be the authentic liganded conformation and that the classical R structure actually lies in the pathway from T to R2 (Y) transition. Another liganded Hb, termed B (Kroeger and Kundrot 1997), also has been discovered recently. This is a mutant Hb with βAsn108 mutated to a Lys, and the α-subunits covalently bound together by glycine. Schumacher et al. (1995) have reported liganded Hb with cross-linked β-subunits as possible transitional intermediates between the T and R states.
Only one classical T state conformation has been observed for unliganded human (Fermi et al. 1984), bovine (Perutz and Fermi 1993), and horse (Bolton and Perutz 1970) Hbs. Several partially and fully liganded T state structures of HbA (Brzozowski et al. 1984; Abraham et al. 1992; Liddington et al. 1992; Paoli et al. 1996) have also been reported, but they do not differ significantly in quaternary structure from the unliganded T state structure (Fermi et al. 1984).
This study reports the crystal structure of high-salt bovine carbonmonoxy (CO bovine) Hb at a resolution of 2.1 Å. Bovine Hb has been of interest in part because of its ability to function without the aid of 2,3-diphosphoglycerate (DPG), the principal allosteric effector of HbA. This usually conserved allosteric effector binding site in mammals is altered by the deletion of βHis 2(NA2) in bovine Hb, an important residue that binds the in vivo allosteric effector DPG. As noted by Perutz and Imai (1980), mammals (such as human) with hydrophilic residues at the second position of the β-subunit N terminus have high intrinsic oxygen affinity, whereas those like bovine Hb, which lack this residue through deletion and replacement by an N-terminal Met or substitution by a hydrophobic residue (as in cat and lemur Hbs), have low oxygen affinity. The investigators predicted that the deleted or substituted hydrophobic residues would lead to hydrophobic interactions between the β-subunit N-terminal residues and the hydrophobic core of the β-subunits, resulting in the shifting of the N-terminal residues toward the center of the molecular dyad. The crystal structure of bovine deoxygenated (dxy bovine) Hb solved by Perutz and Fermi (1993) indeed showed a 2.1-Å shift of the N termini and A-helices of the β-subunits toward the molecular dyad. The investigators associated this movement with the low intrinsic oxygen affinity of bovine Hb. In fact, the contraction of the N terminus and A-helix in bovine and other mammals mimics the effect of DPG in HbA, in which the binding of DPG leads to the contraction of the secondary structure toward the molecular dyad (Arnone 1972). The CO bovine Hb reported here shows a similar displacement (2.1 Å) of the N termini and A-helices of the β-subunits toward the molecular dyad relative to the human oxygenated (oxy human) Hb. In addition, the CO bovine Hb structure displays a number of tertiary and quaternary structural features that are intermediate between T and R state Hb, including constrained α- and β-subunit heme groups and environments. Moreover, the distal pockets at the β-subunit heme groups show close nonbonded contacts between the CO ligand and Hb residues Val 67(E11) and His 63(E7). It therefore appears that the more constrained heme groups in CO bovine Hb, in addition to the increased steric hindrance to ligand binding at the β-subunit hemes, might partially explain its low oxygen affinity. There are also other significant differences between the two. The rigid body rotation angle relating the α2β2 dimers of CO bovine Hb and Rstate Hb, after superposition of the α1β1 dimers, is relatively higher than values observed between classical R state hemoglobins. Also, the α1β2 (α2β1) interface of the so-called "switch region" (Baldwin and Chothia 1979) of CO bovine Hb is more open than that of R state Hb.
Results and Discussion
Crystallographic refinement and overall structural comparison
The asymmetric unit of CO bovine Hb contains one α1β1α2β2 tetramer, with 572 Hb amino acid residues, 4 heme groups, 4 carbon monoxide, and 87 water molecules. The 2.1-Å crystallographic R-factor and R-free are 20.5% and 25.4%, respectively. The electron density map (2Fo-Fc) at 1.0σ shows continuous density for the entire polypeptide main-chain atoms except for diffuse density at the N- and C-terminal residues (αVal 1, βMet 2, αArg141, and βHis 146). The main subunit dihedral angles for most of the residues in the final model (91.1%) lie within the most-favored regions of the Ramachandran plot, as calculated by PROCHECK (Laskowski et al. 1993), with only one residue (β1Leu 3) found in the disallowed region. The error in the atomic coordinates is 0.26 Å by Sigmaa (Read 1986). Other statistics are listed in Table 1.
Table 1.
Summary of crystallographic analysis
| Data collection | |
| Space group | P212121 |
| Unit cell dimensions (Å) | 79.41 110.63 65.40 |
| Resolution (Å) | 2.1 (2.15–2.1) |
| No. of measurements | 122102 |
| No. of unique reflections | 32949 |
| Rmerge | 10.3 (42.0) |
| Completeness (%) | 96.2 (92.7) |
| I/sigma I | 5.7 |
| Structure Refinement | |
| Resolution limit | 64.5–2.1 |
| No. of reflections | 32949 |
| Rfactor | 20.5 |
| Rfree | 25.4 |
| Rmsd from standard geometry | |
| Bond-length (Å) | 0.013 |
| Bond-angles (°) | 1.73 |
| Average B-values | |
| All nonhydrogen atoms | 36.6 |
| Protein atoms | 36.6 |
| Heme atoms | 34.5 |
| CO atoms | 31.0 |
| Water atoms | 44.0 |
Numbers in parentheses refer to the outermost resolution bin.
The α- and β-subunits of bovine and human Hbs have 88% and 84% sequence identity, respectively. The two Hbs differ by the deletion of βHis 2 in bovine, which reduces the β-subunit to 145 amino acids. As in human Hb, the α-subunit of bovine Hb contains 141 amino acids. The α- and β-subunit sequences of bovine and horse Hbs are 90% and 83% identical, respectively, and bovine and pig Hbs are 87% and 84% identical, respectively. The CO bovine Hb dimer (α1β1) superimposes on the corresponding oxy (Shaanan 1983) and dxy (Fermi et al. 1984) human Hb dimers with rmsd of 0.65 and 0.80 Å, respectively, for all Cα atoms (minus the three residues at the N and C terminus of each subunit). Figure 1A ▶ shows the superposition of α1β1 of CO bovine and oxy human Hbs. Most regions of the structures are very similar; however, significant differences occur at the N terminus and A-helix of the β-subunit, which have moved in CO bovine Hb toward the central water cavity by approximately 2.1 Å relative to oxy human Hb. A similar contraction for the N-terminal region is also observed in horse methemoglobin (met horse) Hb (1.1 Å) and pig methemoglobin (met pig) Hb (1.2 Å) relative to oxy human Hb. In dxy bovine Hb there is also a contraction of the N-terminal region by 2.1 Å relative to dxy human Hb (Perutz and Fermi 1993). The observed contraction in dxy bovine Hb has been attributed to the single-residue deletion (βHis 2) and the dimer pair residues βPro 5→Ala and βTyr 130→Phe (Perutz and Fermi 1993). This contraction has been associated with the low intrinsic oxygen affinity of bovine Hb compared with human Hb (Perutz and Fermi 1993). The P50 values measured in stripped Hb solutions are 4.1 mmHg for human Hb, 5.6 mmHg for pig Hb, and 13.1 mmHg for bovine Hb (Bunn 1971).
Fig. 1.

Superposition of CO bovine Hb and other Hb structures. The structures were superimposed with the α1β1 dimer as described in the text. (A) Figure of the least-squares superposition of the α1β1 dimer of CO bovine and oxy human Hbs. The α-subunits are on top and colored magenta and cyan for CO bovine Hb and oxy human Hb, respectively. The β-subunits are colored red and yellow for CO bovine Hb and oxy human Hb, respectively. (B) Stereo figure of the switch region of T state dxy human Hb (cyan), CO bovine Hb (yellow), R state oxy human Hb (red), and R2 state CO human Hb (purple).
α1β1 (α2β2) interface
The α1β1 (α2β2) dimer interface of CO bovine Hb contains similar noncovalent interactions as the R state structures of met pig Hb (Katz et al. 1994), met horse Hb (Ladner et al. 1977), oxy human Hb (Shaanan 1983), and CO human Hb (Baldwin and Chothia 1979). All residues involved in hydrogen bonding or salt bridge interactions at this interface are conserved in these five Hb structures, except for βHis 116, which is arginine in pig, horse, and bovine Hbs. Similar interactions are also observed in the T state dxy human (Fermi et al. 1984), the R2 state CO human (Silva et al. 1992), and the Y state CO Ypsilanti (Smith et al. 1991) Hb structures.
α1β2 (α2β1) interface
As indicated by Baldwin and Chothia (1979), the α1β2 (α2β1) dimer interface of the T and R states is characterized by diagnostic switch and flexible joint regions. They noted that transition between the T and R quaternary forms results in significant changes in the relative positions of the residues in the switch region, whereas residue positions in the joint region remain relatively unchanged. An analysis of the contacts between the α1β2 interface of CO bovine Hb, and selected T state, R state, and R2 state Hbs, are listed in Table 2. The values in parentheses are the distances between the Cα-Cα of the listed residues. In T state Hb, the switch region has β2His 97(FG4) positioned between α1Pro 44(CD2) and α1Thr 41(C6). The transition to the R state repositions β2His 97(FG4) to lie between residues α1Thr 38(C3) and α1Thr 41(C6). This results in formation of a hydrogen bond between β2His 97(FG4) and α1Thr 38(C3) as observed in oxy human Hb (Shaanan 1983) and CO human Hb (Baldwin and Chothia 1979), or β2His 97(FG4) with α1Thr 41(C6) as found in met pig Hb (Katz et al. 1994). These contacts have been lengthened beyond 3.7 Å in met horse Hb (Ladner et al. 1977). Similarly, R2 or Y state has β2His 97(FG2) positioned between α1Thr 38(C3) and α1Thr 41(C6); however, β2His 97(FG2) no longer forms hydrogen bonds with either residue. According to Silva et al. (1992), the disengagement of the β2His 97(FG2) from α1Thr 38(C3) and α1Thr 41(C6) in the R2 state would facilitate a transition to the T state with less steric interference than is observed for the R to T transition.
Table 2.
Contact residues of the α1β2 interface of various hemoglobin states
| Contact | CO bovine (R) | Met horse (R) | CO human (R) | Oxy human (R) | Met pig (R) | CO human (R2) | Dxy human (T) | |
| Switch region | ||||||||
| α1Thr38(OH) | − β2His97(O) | 3.8 (6.3) | 4.3 (5.4) | 2.8 (5.2) | 3.2 (5.2) | 4.9 (5.3) | 5.7 (7.0) | 9.9 (8.9) |
| α1Thr41(OH) | −β2His97(NH) | 3.7 (7.8) | 3.8 (7.4) | 3.7 (7.2) | 4.0 (7.3) | 3.1 (7.1) | 5.3 (9.5) | 7.0 (4.7) |
| α1Pro44 | −β2His97 | (13.1) | (13.0) | (12.8) | (12.6) | (12.3) | (14.8) | (7.2) |
| α1Thr38(OH) | −β2Asp99(N) | 5.6 (8.3) | 5.0 (7.0) | 4.2 (6.7) | 4.3 (6.9) | 4.7 (6.5) | 7.5 (9.5) | 5.6 (6.1) |
| α1Tyr42(OH) | −β2Asp99(ODI) | 7.6 (12.8) | 8.6 (12.7) | 8.5 (12.7) | 8.5 (12.7) | 7.9 (12.2) | 9.4 (13.9) | 2.5 (7.8) |
| Intermediate region | ||||||||
| α1Val96 | −β2Asp99 | (7.8) | (8.0) | (8.3) | (8.0) | (8.0) | (7.6) | (10.1) |
| α1Asn97(ND2) | −β2Asp99(ODI) | 5.8 (9.1) | 5.0 (8.9) | 4.7 (9.0) | 4.7 (9.0) | 4.2 (8.7) | 7.2 (9.5) | 2.8 (9.4) |
| α1Asp94(OD2) | −β2Asn102(ND2) | 2.8 (9.2) | 2.9 (9.2) | 3.0 (9.0) | 2.8 (9.2) | 2.8 (9.4) | 2.8 (9.2) | 5.7 (8.1) |
| α1Thr41(O) | −β2Arg40(NH2) | 3.1 (11.4) | 3.4 (11.0) | 2.9 (10.2) | 3.4 (10.4) | 5.1 (11.3) | 3.5 (11.5) | 4.8 (12.0) |
| Joint region | ||||||||
| α1Arg92(NH2) | −β2Gln39(OEI) | 3.0 (7.5) | 4.2 (7.9) | 2.9 (8.0) | 3.2 (7.8) | 4.9 (8.4) | 4.4 (7.5) | 4.5 (8.5) |
| α1Arg92 | −β2Glu43a | (10.6) | (11.5) | (11.9) | (11.3) | (11.0) | (10.9) | (12.5) |
| α1Asp94(ODI) | −β2Trp37(NE1) | 3.8 (5.5) | 3.7 (5.6) | 3.5 (5.6) | 3.5 (5.7) | 3.6 (5.4) | 3.7 (5.4) | 3.0 (7.1) |
Values in parentheses are the Ca–Ca distances. In the structures with a tetramer in the asymmetric unit, the contact distances shown are those between the α1β2 interface. Similar contact distances are also observed at the α2β1 contact region. Distances are in Å.
aIn horse hemoglobin, Glu43 is Asp43.
Like the other R state Hb, the CO bovine Hb structure has β2His 97(FG4) positioned between α1Thr 38(C3) and α1Thr 41(C6). Similar to met horse Hb, the hydrogen-bond contacts between β2His 97(FG4) and α1Thr 38(C3) or α1Thr 41(C6) in CO bovine Hb have also become longer and broken (Table 2). Comparison of the Cα–Cα distances also reveals significant separation of the switch region in CO bovine Hb, compared with the other R state structures. The Cα–Cα distance between β2His 97(FG4) and the two residues α1Thr 38(C3) and α1Thr 41(C6) in CO bovine Hb are lengthened (6.3 and 7.8 Å, respectively). The corresponding distances in the other R state structures are 5.2–5.4 Å and 7.1–7.4 Å, respectively. Similarly, the Cα–Cα distance between α1Thr 38(C3) and β2Asp99(FG6) is lengthened (8.3 Å), compared with 6.5–7.0 Å found in the other R state structures. Although a similar disengagement is found in the switch region of the R2 state, the magnitude and direction of displacement is different. In the R2 state, the Cα–Cα contact of α1Thr 38 (C3) and β2Asp99(FG6) move even further apart to 9.5 Å. The β2His 97(FG4) in the R2 state also moves 7.0 and 10.9 Å away from α1Thr 38(C3) and α1Thr 41(C6), respectively. Figure 1B ▶ shows the α1β2 switch region interface of CO bovine Hb, R state oxy human Hb (Shaanan 1983), R2 state CO human Hb (Silva et al. 1992), and T state dxy human Hb (Fermi et al. 1984) with the α1β1 of all four structures superimposed. We used the α1β1 dimer reference frame, which has been found to remain structurally unchanged during the T to R transition (Baldwin and Chothia 1979), to superimpose the various Hb structures. It is quite obvious that in the R2 state the FG corner of the β2-subunit, including the β2His 97(FG4), rotates and shifts downward away from the C-helix of the α1-subunit and also moves sideways in the direction of the C-terminal direction of the α-subunit chain. A similar downward movement of the FG corner is also observed in CO bovine Hb, but it is smaller in magnitude and the displacement is toward the N-terminal end, in the direction of the T state position. Although not shown in Figure 1B ▶, the position of β2His 97(FG4), and for that matter the whole switch interface in the Y state CO Ypsilanti (Smith et al. 1991), is similar to that of R2 state CO human Hb. The result is an α1β2 switch interface in both CO bovine Hb and R2 state (or Y state) structures that is significantly more open than the R state structures. This could explain why the quaternary difference (see Table 3 and later discussion) between CO bovine and R2 or Y state Hbs is slightly smaller than that between the classical R state and the R2 or Y states.
Table 3.
Tertiary and quaternary structure differences of selected hemoglobins
| CO bovine (R) | Met horse (R) | CO human (R) | Oxy human (R) | Met pig (R) | CO human (Y) | CO human (R2) | Dxy human (T) | |
| CO bovine (R) | — | 0.44 | 0.61 | 0.65 | 0.63 | 0.64 | 0.67 | 0.80 |
| — | 1.56 | 1.93 | 1.90 | 2.49 | 2.73 | 3.27 | 5.78 | |
| — | 2.4 | 4.5 | 4.9 | 5.6 | 7.9 | 9.2 | 13.2 | |
| Met horse (R) | 0.44 | — | 0.54 | 0.57 | 0.57 | 0.56 | 0.62 | 0.78 |
| 1.56 | — | 0.99 | 0.79 | 1.16 | 3.62 | 4.35 | 5.41 | |
| 2.4 | — | 2.1 | 2.7 | 3.6 | 8.8 | 10.5 | 13.0 | |
| CO human (R) | 0.61 | 0.54 | — | 0.34 | 0.64 | 0.39 | 0.49 | 0.83 |
| 1.93 | 0.99 | — | 0.57 | 1.26 | 3.82 | 4.55 | 5.19 | |
| 4.5 | 2.1 | — | 1.6 | 3.0 | 9.7 | 11.7 | 13.6 | |
| Oxy human (R) | 0.65 | 0.57 | 0.34 | — | 0.65 | 0.23 | 0.45 | 0.82 |
| 1.90 | 0.79 | 0.57 | — | 1.03 | 3.89 | 4.62 | 5.06 | |
| 4.9 | 2.7 | 1.6 | — | 1.4 | 11.1 | 13.0 | 12.2 | |
| Met pig (R) | 0.63 | 0.57 | 0.64 | 0.65 | — | 0.66 | 0.76 | 0.78 |
| 2.49 | 1.16 | 1.26 | 1.03 | — | 4.61 | 5.36 | 4.88 | |
| 5.6 | 3.6 | 3.0 | 1.4 | — | 12.3 | 14.1 | 10.8 | |
| CO human (Y) | 0.64 | 0.56 | 0.39 | 0.23 | 0.66 | — | 0.40 | 0.85 |
| 2.73 | 3.62 | 3.82 | 3.89 | 4.61 | — | 0.98 | 8.19 | |
| 7.9 | 8.8 | 9.7 | 11.1 | 12.3 | — | 2.3 | 21.1 | |
| CO human (R2) | 0.67 | 0.62 | 0.49 | 0.45 | 0.76 | 0.40 | — | 0.92 |
| 3.27 | 4.35 | 4.55 | 4.62 | 5.36 | 0.98 | — | 8.60 | |
| 9.2 | 10.5 | 11.7 | 13.0 | 14.1 | 2.3 | — | 22.1 | |
| Dxy human (T) | 0.80 | 0.78 | 0.83 | 0.82 | 0.78 | 0.85 | 0.92 | — |
| 5.78 | 5.41 | 5.19 | 5.06 | 4.88 | 8.19 | 8.60 | — | |
| 13.2 | 13.0 | 13.6 | 12.2 | 10.8 | 21.1 | 22.1 | — |
The Ca atoms (minus three residues at the terminal ends) were used for the superposition with the program LSQMAN (Kleywegt and Jones 1996). For each pair of hemoglobin tetramers compared, the top entry corresponds to the rmsd of the superimposed α1β1 dimers. The middle entry corresponds to the rmsd between the nonsuperimposed α2β2. The bottom entry corresponds to the rigid body rotation relating the nonsuperimposed α2β2 dimers. The rmsds and rigid body rotation differences are in Å and degrees, respectively.
For all three Hb states, only a slight movement of the residues in the flexible joint region occurs during any of the transitions as indicated by both hydrogen bonding and Cα–Cα contacts (Table 2). Unlike the switch region, the joint region of the T state seems slightly more open than in the liganded Hb as indicated by the Cα–Cα distances, although the hydrogen bond between α1Asp94(FG4) and β2Trp 37(C3) is relatively shorter in the T state than in the liganded Hb. CO bovine Hb, and to some extent R2 state Hb, show a slight compression in this region compared with the R state Hb. In the region between the switch and joint (termed as intermediate), the changes observed between the T and R states are mostly differences in hydrogen bonds. Compared with the switch region, changes in Cα–Cα distances between the residues in the intermediate region are relatively small during the T to R transition. The intermediate regions of CO bovine Hb and R2 state CO human Hb are also characterized by the classical R state hydrogen bond contacts.
Quaternary structure
For a measure of quaternary variation among the structures, we used the α1β1 dimer reference frame to superimpose the various Hb structures, and the rmsd of the superimposed α1β1 dimers is reported in the top entry of Table 3. The rmsd of the nonsuperimposed α2β2 dimers, and the rigid body rotation that relates the nonsuperimposed α2β2 dimers of the structures, were then determined to illustrate quaternary differences between the hemoglobins. The two quaternary indices are reported in the middle and bottom entries of Table 3, respectively.
The largest rmsd values between the superimposed α1β1 dimers are between the liganded and the T state Hb, which are likely due to changes in tertiary structures that result from ligand binding. The rmsd between the nonsuperimposed α2β2 dimers ranges from 0.57 to 8.60 Å, with the largest deviations of about 8.60 Å observed between the T to R2 (Y) transition. The rmsd between the T and R state structures varies between 4.88 and 5.78 Å, whereas values for the R to R2 (Y) transitions range from 2.73 to 5.36 Å. The corresponding deviations among the R state structures, including CO bovine Hb, are relatively small, with values from 0.57 to 2.49 Å. The rigid body rotation also shows a transition from T to R2 (Y) to be the largest, involving a rotation of about 21.5°. This value is almost twice as much as that observed in the T to R transition (10.8°–13.7°). The R to R2 (Y) transition involves a rotation of 7.9°–14.1°.
Both analyzed quaternary indices indicate that the conformational changes in the T to R2 (Y) transition are the largest, followed by the T to R transition, and lastly the R to R2 (Y) transition. Thus, it would appear that the quaternary differences argue against the R2 state or Y state as an intermediate on the T to R pathway. As indicated by Janin and Wodak (1993) and Srinivasan and Rose (1994), the R2 or Y state may represent another relaxed end state, with the classical R state lying on the pathway between the T to R2 (Y) transition.
The rmsd of the nonsuperimposed α2β2 dimers of CO bovine Hb and the other R state Hb is 1.56–2.49 Å. The corresponding values between the R state structures without CO bovine Hb are 0.57–1.26 Å. Similarly, the rigid-body rotation relating the nonsuperimposed α2β2 dimers of CO bovine Hb to the R state structures is between 2.4° and 5.6°, compared with values of 1.3°–3.6° among the R state structures without the CO bovine structure. It appears that the quaternary differences between CO bovine Hb and the R state structures are unusually high, if we assume that CO bovine Hb is in the R state conformation. We also note that the quaternary indices between CO bovine and met horse Hbs are the smallest. It is also interesting to note that, in all cases, the quaternary differences between R2 (Y) state and CO bovine Hbs are slightly smaller than those observed between R2 (Y) state and the other R state structures. This is consistent with an α1β2 switch interface in CO bovine and R2 state (Y state) Hbs that is significantly more open than the R state structures.
Heme environment
In CO bovine Hb, all four heme groups, as well as the CO ligands, are well-defined by electron density in both omit and 2Fo-Fc maps. Figure 2 ▶ shows a final 2Fo-Fc electron density map of the heme groups and their environments. The temperature factors for the CO ligand atoms are comparable to the rest of the heme atoms. Table 4 compares the stereochemistry of the Fe, bound CO (O2 in oxy human Hb), and their immediate environment for CO bovine Hb, R state CO human Hb (Baldwin and Chothia 1979), R state oxy human Hb (Shaanan 1983), and R2 state CO human Hb (Silva et al. 1992). It appears that the noncovalent contact between the CO ligand and the distal residues βHis 63(E7) and βVal 67(E11) at the two β-hemes of CO bovine Hb are consistently shorter than the corresponding distances in the other structures. As pointed out by Perutz (1970), steric hindrance to ligand binding by these distal residues is dominant in β-subunit hemes and is a contributing factor to the low oxygen affinity of the T state. Thus, the low oxygen affinity of the bovine Hb could be explained partly by the increased steric hindrance to ligand binding by the distal residues in the β-hemes. In contrast, we do not observe any significant and consistent differences in these contacts at the α-hemes for all four liganded structures.
Fig. 2.
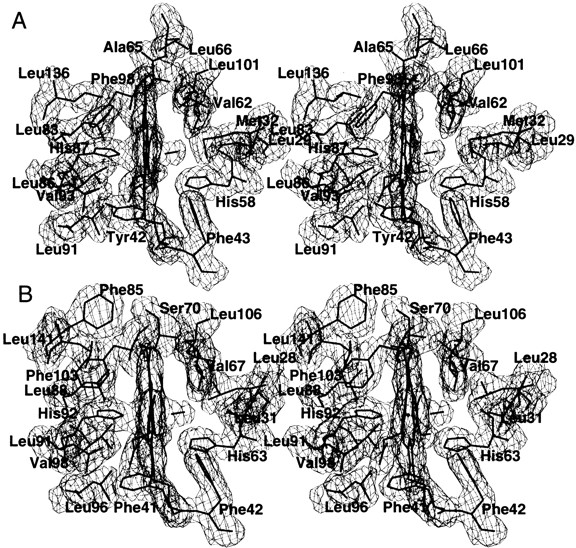
Stereo figure of the final 2Fo-Fc electron density map of the heme and associated protein residues of CO bovine Hb contoured at 1 σ for α1-subunit (A) and β1-subunit (B). Similar electron densities are observed at the α2 and β2 subunit hemes and environments.
Table 4.
Geometry of heme groups and environments in liganded hemoglobins
| CO bovine (R) | CO human (R2) | CO human (R) | Oxy human (R) | |||||||||
| α1 | α2 | β1 | β2 | α1 | α2 | β1 | β2 | α1 | β1 | α1 | β1 | |
| Fe-His(F8)NE2 | 2.05 | 2.13 | 2.13 | 2.05 | 2.08 | 2.13 | 2.10 | 2.10 | 2.09 | 2.08 | 1.94 | 2.07 |
| Fe-Val(E11)CG2 | 4.95 | 5.08 | 4.84 | 4.89 | 4.80 | 4.96 | 4.87 | 4.97 | 5.02 | 4.87 | 4.79 | 4.66 |
| ≤Fe-Nporph>a | 2.02 | 2.03 | 2.00 | 2.02 | 2.00 | 2.03 | 2.00 | 2.00 | 2.00 | 2.00 | 1.99 | 1.96 |
| Fe-Val(E11)CG2 | 4.95 | 5.08 | 4.84 | 4.89 | 4.80 | 4.96 | 4.87 | 4.97 | 5.02 | 4.87 | 4.79 | 4.66 |
| Fe-Ctb | −0.01 | −0.01 | −0.07 | −0.06 | 0.00 | 0.00 | −0.01 | −0.02 | 0.00 | −0.02 | 0.12 | −0.11 |
| Fe-Cc | 1.78 | 1.80 | 1.77 | 1.77 | 1.77 | 1.78 | 1.77 | 1.75 | 1.81 | 1.81 | 1.66 | 1.87 |
| C-His(E7)NE2a | 3.26 | 3.17 | 3.36 | 3.25 | 3.47 | 3.49 | 3.40 | 3.56 | 4.11 | 3.56 | 2.99 | 3.21 |
| O-His(E7)NE2d | 3.06 | 2.88 | 3.24 | 2.96 | 3.23 | 3.30 | 3.19 | 3.27 | 4.02 | 3.42 | 2.57 | 3.49 |
| C-Val(E11)CG2c | 3.76 | 3.78 | 3.58 | 3.51 | 3.70 | 3.57 | 3.71 | 3.58 | 3.81 | 3.84 | 3.48 | 3.57 |
| O-Val(E11)CG2d | 3.35 | 3.21 | 3.09 | 3.08 | 3.20 | 3.38 | 3.19 | 3.20 | 3.31 | 3.52 | 3.25 | 3.36 |
| Fe-C-Oc,d | 169 | 178 | 172 | 169 | 173 | 156 | 172 | 163 | 179 | 180 | 153 | 159 |
| Fe-C-His(F8)NE2c | 173 | 178 | 174 | 178 | 174 | 178 | 175 | 176 | 170 | 170 | 177 | 175 |
Distances and angles are in Å and degrees, respectively. aAverage distance between Fe and the four porphyrin nitrogens. bDistance of the Fe to the least-square fit plane of the porphyrin nitrogens. cC is O1 in oxy human. dO is O2 in oxy human.
Perutz (1972) has also attributed the low oxygen affinity in T state Hb to tension from the proximal histidine that restrains the movement of the Fe atom from its out-of-plane position in the T state to its in-plane position in the R state. Figure 3 ▶, A and B, illustrates the structural differences at the α-subunit heme, part of the C-helix (residue 42), CD corner (residues 43–46), E-helix (residues 58–62), F-helix (residues 84–90), and FG corner (residues 91–93) for CO bovine, oxy human (R state), CO Ypsilanti (Y state), and dxy human (T state) Hbs. The α1β1 reference frame was used for superposition of the structures, as already defined in the text. In CO bovine Hb, the F-helix (showing the proximal histidine) and the FG corner in both α1 and α2 subunits are not in the fully relaxed position, but rather show a small but significant shift of 0.36 Å in the α1-subunit and 0.38 Å in the α2-subunit (averaged for 11 Cα atoms of the F-helix and FG corner) toward the corresponding T state positions. In addition, the CO bovine heme, unlike the R state Hb, is essentially in the T state position. However, in the T state the heme shows a slight tilt toward the FG corner and also shows a doming made by the out-of-plane Fe atom. The E-helix, C-helix, and CD corner show very little variation among the structures; however, the side chain of the T state distal histidine drops slightly toward the heme position. In contrast to CO bovine Hb, the F-helix and FG corner of the Y state CO Ypsilanti are slightly more displaced from the T state than is the R state.
Fig. 3.

Stereo figure of the α-subunit heme group and environment showing the heme, bound ligand, C-helix, CD corner, E-helix, F-helix, and FG corner of the hemoglobin structures of T state dxy human Hb (cyan), CO bovine Hb (yellow), R state oxy human Hb (red), and Y state CO Ypsilanti Hb (purple). The structures were superimposed with the α1β1 dimer as described in the text. (A) α1-Subunit heme group and environment, (B) α2-subunit heme group and environment.
Like the α-subunits, the F-helix (residues 88–95) and FG corner (residues 96–98) in both β1- and β2-subunits of CO bovine Hb are displaced toward the T state direction (Fig. 4A,B) by 0.32 Å and 0.55 Å, respectively. Again, the F-helix and FG corner of the Y state CO Ypsilanti Hb are removed further from the T state than is the R state. In contrast to the heme positions in the α-subunits, the positions of the β-subunit hemes in all the liganded structures, including CO bovine Hb, are quite identical. In the T state, there is a large tilt and translation of the heme toward the FG corner. Although there is little variation at the C-helix (residues 41–42) and CD corner (residues 43–45) of all examined Hb states, that of CO bovine Hb is much closer to the T state, whereas the R and Y states occupy similar position.
Baldwin and Chothia (1979) found significant differences between the hemes, F- and E-helices, and the corner region FG in the T to R transition. Also, Perutz (1970, 1972) and Perutz et al. (1987) have shown that the F-helices and FG corners are essential for the quaternary allosteric transition from the T to R state. The location of the α- and β-subunit F-helices and FG corners in CO bovine Hb could result in a strain on the heme, forcing it to move to maintain proper coordination and geometry with the proximal histidine. Thus, we hypothesize that the strain on the hemes, in addition to the apparent increased steric contact between the CO and the distal Hb residues, is probably another reason why bovine Hb has a lower oxygen affinity. Perutz and Fermi (1993) previously associated the contraction of the β-subunit N-terminal region in the dxy bovine HB structure with the low intrinsic oxygen affinity of bovine Hb. It should be noted that the heme groups in CO bovine Hb are planar and fully ligated with the CO ligand.
Schumacher et al. (1995) have reported the structures of two cross-linked CO-ligated hemoglobins (α2β1STM82β and α2β1STM1,82β) as possible transitional intermediates between the T and R states. Similar to our findings, the two structures also indicate that both the α- and β-subunit hemes have not quite reached the R state position. Interestingly, both cross-linked hemoglobins have reduced oxygen affinities, which the investigators attributed to the strain on the heme groups and environment. In contrast, the heme, F-helix, and the FG corner in the Y state are shifted further from the T state position (compared with the transition from the R state to the T state). This indicates that the Y state is even more relaxed than the R state, which is in accord with Ypsilanti Hb having an increased oxygen affinity (Ackers et al. 1992; Doyle et al. 1992). Similar results are obtained when comparing the R2 state CO human Hb structure (instead of Y state) with the CO bovine, oxy human (R state), and dxy human (T state) Hb structures.
Interactions of the C- and N-terminal residues
In the CO bovine Hb structure, no clear densities for the N- and C-terminal residues (αVal 1, βMet 2, αArg141, and βHis 146) were observed. As a result, interactions made by these residues are uncertain. The hydroxyl group of the α-subunit penultimate α1Tyr 140 of CO bovine Hb hydrogen bonds to the carbonyl oxygen of α1Val 93, which is typical of other R and T state structures. Such hydrogen bonding is also observed in the Y state structure of CO Ypsilanti Hb (Smith et al. 1991). However, in the R2 structure of CO human Hb (Silva et al. 1992), this hydroxyl group makes a hydrogen bond with α1Pro 77, α1Ser 81, and α1Ser 84. In the β-subunits of CO bovine Hb, the hydroxyl group of the penultimate β1Tyr 145 makes hydrogen-bond contacts with both the carbonyl oxygen of β1Val 98 and the hydroxyl group of α2Thr 38. The interaction between β1Tyr 145 and β1Val 98 is also present in the R state (Ladner et al. 1977; Baldwin and Chothia 1979; Shaanan 1983; Katz et al. 1994), the R2 state (Silva et al. 1992), and the T state (Fermi et al. 1984) structures, but not in the Y state structure (Smith et al. 1991), in which β1Tyr 145 is hydrogen bonded to β2Asn139. The contact between β1Tyr 145 and α2Thr 38 in CO bovine Hb is absent in the T, R, R2, and Y structures.
Conclusion
Bovine Hb has a threefold decreased affinity for oxygen compared with human Hb. According to Perutz and Fermi (1993), the structural basis for this decrease in ligand affinity results from the β-subunits N termini and A-helices shifting closer to the molecular dyad. Significantly, the structure of CO bovine Hb reported here shows that both α- and β-subunit heme groups and their environments have not fully relaxed to the R state conformation, as the F-helices and FG corners lie between the T and R positions. In addition, it appears that in CO bovine Hb the CO ligand makes closer contacts with the distal Hb residues Val 67(E11) and His 63(E7) of the β-subunits. Therefore, we attribute the low oxygen affinity of bovine Hb at least in part to the observed steric strain at the hemes and closer contacts between the ligands and protein residues around the β-subunit heme groups. Our conclusion is consistent with results of the two cross-linked CO-liganded hemoglobins reported by Schumacher et al. (1995). Like CO bovine Hb, the heme structural features in these two mutant structures indicate that they have not fully reached the classical relaxed end-state location. Like bovine Hb, these mutants also have low oxygen affinity, which, as the investigators pointed out, is due to strain on the heme environment.
Materials and methods
Crystallization, X-ray data collection, and processing
Bovine Hb was purified from fresh cow blood according to the procedure of Perutz (1968), with slight modifications, to give a solution of 10.4 g% in 1.6 M sodium phosphate buffer (pH 6.5). The solution was reduced by the addition of 2.0 mM Na2S2O4, then saturated with CO to generate the fully reduced CO-bound Hb form. Crystallization was performed with a solution of 3 g% protein, 3.6 M phosphate (pH 7.3 to 7.4), using 10-mL test tubes. CO was again bubbled through each solution. One drop of pyridine or benzene was added to each tube, which was then sealed. Crystals grew in 5 d in several of the tubes containing 2 mL Hb, 3.3–3.6 mL phosphate, and 0.3 mL or 0.8 mL of water. X-ray diffraction data were collected using R-axis II image plate detector equipped with a Rigaku RU-200 generator operating at 50 KV and 180 mA. Diffraction data sets were collected at room temperature to 2.1 Å using one crystal. The data set was processed with the R-axis II software program and the CCP4 program suite (SCALA, AGROVATA, TRUNCATE) (Collaborative Computing Project No. 4 1994). The final structure factor file contains anomalous differences.
Solution of the structure
The crystal structure of CO bovine Hb was solved by the molecular replacement method using the program AMoRe (Navaza 1994). The α1β1 dimer of oxy human structure (Shaanan 1983), omitting all solvent molecules and oxygen ligands, was used as the search model. Based on the solvent content of the unit cell (∼45%), we expected two αβ dimers per asymmetric unit. The cross-rotation function was calculated using normalized structure factors with data in the resolution range of 8.0 to 4.0 Å. Two unique peaks with correlation coefficients of 0.162 and 0.144 were observed. Translation functions using data between 8.0 and 4.0 Å were calculated for the two possible space groups P212121 and P21212. The space group P212121 gave two distinct peaks for the two cross-rotation solutions, with correlation coefficients of 0.305 and 0.327 and Rfactors of 48.9% and 47.7%, respectively. The two solutions correspond to α1β1 and α2β2 dimers in the asymmetric unit; however, they are not related by a molecular twofold axis. The functional α1β1α2β2 Hb tetramer is generated by application of symmetry operations. The final tetrameric model has a correlation coefficient and Rfactor of 0.62 and 37.2%, respectively.
Refinement of the structure
All amino acids that were not present in the CO bovine Hb structure were mutated to alanine or to corresponding glycines (Perutz and Fermi 1993). Refinement was performed using X-plor (Brunger 1992a) with a bulk solvent correction. All data sets from 65.0 to 2.1 Å were used in the refinement. A statistically random selection of 5% of the total reflection data was excluded from the refinement and used to calculate the free Rfactor (Rfree) as a monitor of model bias (Brunger 1992b). The model was subjected to rigid body refinement with the four hemoglobin subunits treated as independent groups using data to 2.2 Å. The initial Rfactor dropped from 39.5% to 37.2% with an Rfree of 36.9%. The Fo-Fc map showed substantial positive densities near the four Fe sites corresponding to the four CO molecules, which were built into these densities. Also, most of the side-chain residues that were mutated to alanines became apparent in the density maps and were built into the model. At this stage, tight noncrystallographic symmetry restraint was applied to all nonhydrogen atoms, and the model was subjected to positional refinement. The rest of the mutated side-chain residues became apparent and were included in the model. The model was then subjected to several rounds of simulated annealing while the noncrystallographic symmetry restraint was slowly loosened, and finally at a resolution of 2.1 Å the restraint was removed completely. Water molecules having peaks above 3.0σ in the Fo-Fc map, and within acceptable hydrogen-bonding distance, were added to the model. Omit, 2Fo-Fc, and Fo-Fc electron density maps were used repeatedly to inspect the structure and make positional corrections to the model, especially at the heme groups and environments. Final refinement brought the Rfactor to 20.5% and the Rfree to 25.4%. All model building and model corrections were performed using the programs TOM (Cambillau and Horjales 1987) and O (Jones et al. 1991). Refinement statistics are summarized in Table 1. The atomic coordinate set and the structure factors have been deposited in the Protein data Bank, accession code 1FSX.
Fig. 4.

Stereo figure of the β-subunit heme group and environment showing the heme, bound ligand, C-helix, CD corner, E-helix, F-helix, and FG corner of the hemoglobin structures of T state dxy human Hb (cyan), CO bovine Hb (yellow), R state oxy human Hb (red), and Y state CO Ypsilanti Hb (purple). The structures were superimposed with the α1β1 dimer as described in the text. (A) β1-Subunit heme group and environment, (B) β2-subunit heme group and environment.
Acknowledgments
This work was supported by grants HL32793 to D.J.A. and HL04367–01 to M.K.S. from the National Institutes of Health.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.48301.
References
- Abraham, D.J., Peascoe, R.A., Randad, R.S., and Pannikker, J. 1992. X-ray diffraction study of di- and tetra-ligated T state hemoglobin from high salt crystals. J. Mol. Biol. 227 480–492. [DOI] [PubMed] [Google Scholar]
- Ackers, G.K., Doyle, M.L., Myers, D., and Daugherty, M.A. 1992. Molecular code for cooperativity in hemoglobin. Science 255 554–563. [DOI] [PubMed] [Google Scholar]
- Arnone, A. 1972. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237 146–149. [DOI] [PubMed] [Google Scholar]
- Baldwin, J. and Chothia, C. 1979. Haemoglobin: The structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 129 175–220. [DOI] [PubMed] [Google Scholar]
- Bolton, W. and Perutz, M.F. 1970. Three dimensional fourier synthesis of horse deoxy Hb at 2.8 Å. Nature 228 551–552. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T. 1992a. X-PLOR, Version 3.1: A system for X-ray crystallography and NMR. Yale Univ. Press, New Haven, CT.
- ———. 1992b. Free R-value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 355 472–475. [DOI] [PubMed] [Google Scholar]
- Brzozowski, A., Derewenda, Z., Dodson, E., Dodson, G., Grabowoski, M.J., Liddington, R., Skarzynki, T., and Valley, D. 1984. Bonding of molecular oxygen to T state human haemoglobin. Nature 307 74–76. [DOI] [PubMed] [Google Scholar]
- Bunn, H.F. 1971. Differences in the interaction of 2,3-diphosphoglycerate with certain mammalian Hbs. Science 172 1049–1051. [DOI] [PubMed] [Google Scholar]
- Cambillau, C. and Horjales, E. 1987. TOM: A Frodo subpackage for protein-ligand fitting with interactive energy minimization. J. Mol. Graph 5 174–177. [Google Scholar]
- Collaborative Computing Project No. 4. 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50 760–763. [DOI] [PubMed] [Google Scholar]
- Doyle, M.L., Lew, G., Turner, G.J., Rucknagel, D., and Ackers, G.K. 1992. Regulation of oxygen affinity by quaternary enhancement: Does hemoglobin Ypsilanti represent an allosteric intermediate? Proteins: Struct. Funct. Genet. 14 351–362. [DOI] [PubMed] [Google Scholar]
- Fermi, G., Perutz, M.F., Shaanan, B., and Fourme, R. 1984. The crystal structure of human deoxyHb at 1.7 Å resolution. J. Mol. Biol. 175 159–174. [DOI] [PubMed] [Google Scholar]
- Janin, J. and Wodak, S.J. 1993. The quaternary structure of carbonmonoxy Hb Ypsilanti. Proteins 15 1–4. [DOI] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.-Y., Cowan, S.W., and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in models. Acta Crystallogr. A 47 392–400. [DOI] [PubMed] [Google Scholar]
- Katz, D.S., White, S.P., Huang, W., Kumar, R., and Christianson, D.W. 1994. Structure determination of aquamet porcine Hb at 2.8 Å resolution. J. Mol. Biol. 244 541–553. [DOI] [PubMed] [Google Scholar]
- Kleywegt, G.J. and Jones, T.A. 1996. LSQMAN: A superposition. ESF/CCP4 Newsletter 31 9–14. [Google Scholar]
- Kroeger, K.S. and Kundrot, C.E. 1997. Structures of Hb-based blood substitute: Insights into the function of allosteric proteins. Structure 5 227–237. [DOI] [PubMed] [Google Scholar]
- Ladner, R.C., Heidner, E.J., and Perutz, M.F. 1977. The structure of horse methaemoglobin at 2.0 Å resolution. J. Mol. Biol. 114 385–414. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A., Macarthur, M.W., Moss, D.S., and Thornton, J.M. 1993. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26 283–291. [Google Scholar]
- Liddington, R., Derewenda, Z., Dodson, E., Hubbard, R., and Dodson, G. 1992. High resolution crystal structures and comparison of T state deoxyhemoglobin and two ligated T state haemoglobins: T(α-oxy)haemoglobin and T(met) haemoglobin. J. Mol. Biol. 228 551–579. [DOI] [PubMed] [Google Scholar]
- Monod, J., Wyman, J., and Changeux, J.-P. 1965. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12 88–118. [DOI] [PubMed] [Google Scholar]
- Paoli, M., Liddington, R., Tame, J., Wilkinson, A., and Dodson, G. 1996. Crystal structure of T state haemoglobin with oxygen bound at all four haems. J. Mol. Biol. 256 775–792. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. 1968. Preparation of Hb crystals. J. Crystal Growth 2 54–56. [Google Scholar]
- ———. 1970. Stereochemistry of cooperative effects in haemoglobin. Nature 228 726–734. [DOI] [PubMed] [Google Scholar]
- ———. 1972. Haem-haem interaction. Nature 237 459–499. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. and Fermi, G. 1993. A novel allosteric mechanism in Hb. J. Mol. Biol. 233 536–545. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. and Imai, K. 1980. Regulation of oxygen affinity of mammalian Hbs. J. Mol. Biol. 136 183–191. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F., Fermi, G., Luisi, B., Shaanan, B., and Liddington, R.C. 1987. Stereochemistry of cooperative mechanisms in Hb. Acc. Chem. Res. 20 309–321. [DOI] [PubMed] [Google Scholar]
- Read, R.J. 1986. Improved fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42 140–149. [Google Scholar]
- Schumacher, M.A., Dixon, M.M., Kluger, R., Jones, R.T., and Brennan, R.G. 1995. Allosteric transition intermediates modelled by crosslinked haemoglobins. Nature 375 84–87. [DOI] [PubMed] [Google Scholar]
- Shaanan, B. 1983. Structure of oxyHb at 2.1 Å resolution. J. Mol. Biol. 171 31–59. [DOI] [PubMed] [Google Scholar]
- Silva, M.M., Rogers, P.H., and Arnone, A. 1992. A third quaternary structure of human Hb at 1.7 Å resolution. J. Biol. Chem. 267 17248–17256. [PubMed] [Google Scholar]
- Smith, F.R. and Simmons, K.C. 1994. Cyanomet human Hb crystallized under physiological conditions exhibits the Y quaternary structure. Proteins 18 295–300. [DOI] [PubMed] [Google Scholar]
- Smith, F.R., Lattman, E.E., and Carter, C.W. 1991. The mutation β99 Asp-Tyr stabilizes a new composite quaternary state of human Hb. Proteins 10 81–91. [DOI] [PubMed] [Google Scholar]
- Srinivasan, R. and Rose, G.D. 1994. The T-to-R transformation in Hb: A re-valuation. Proc. Natl. Acad. Sci. USA 91 11113–11117. [DOI] [PMC free article] [PubMed] [Google Scholar]


