Abstract
The heterotrimeric GTP binding proteins, G proteins, consist of three distinct subunits: α, β, and γ. There are 12 known mammalian γ subunit genes whose products are the smallest and most variable of the G protein subunits. Sequencing of the bovine brain γ10 protein by electrospray mass spectrometry revealed that it differs from the human protein by an Ala to Val substitution near the N-terminus. Comparison of γ isoform subunit sequences indicated that they vary substantially more at the N-terminus than at other parts of the protein. Thus, species variation of this region might reflect the lack of conservation of a functionally unimportant part of the protein. Analysis of 38 γ subunit sequences from four different species shows that the N-terminus of a given γ subunit isoform is as conserved between different species as any other part of the protein, including highly conserved regions. These data suggest that the N-terminus of γ is a functionally important part of the protein exhibiting substantial isoform-specific variation.
Keywords: G proteins, γ subunit, γ 10, mass spectrometry
The heterotrimeric G proteins that mediate signal transduction across the plasma membrane are a family of GTP binding proteins composed of three different subunits: α, β, and γ (Kuhn 1980; Bokoch et al. 1984; Hildebrandt et al. 1984). Seven transmembrane receptors activate G proteins by promoting the binding of GTP, which induces dissociation into an α subunit and a βγ dimer. These activated components independently regulate intracellular target proteins (Gilman 1987; Iniguez-Lluhi et al. 1993). There are multiple isoforms of each of the three G protein subunits. These isoforms allow a large number of possible heterotrimer combinations, which are likely to be important in the role of G proteins in signal integration in cells (Hildebrandt 1997).
G protein γ subunits are relatively small proteins of about 8 kD. Twelve subunit isoforms have been identified, all of which are predicted to be post-translationally modified by prenylation at the C-terminus of the mature proteins. This modification involves three sequential events: prenylation of the cysteine four residues from the C-terminus, proteolytic removal of the three C-terminal amino acids, and carboxymethylation of the new C-terminal prenylated cysteine (Clarke 1992). The type of prenyl group attached to the protein is determined by the C-terminal residue. If the C-terminal residue is Leu, the protein is geranylgeranylated (20-carbon group); however, if the C-terminal residue is Cys, Ser, Ala, Met, or Gln, the protein is farnesylated (15-carbon group) (Cox 1995). This modification is essential to the function of the G protein. Without prenylation, the resultant βγ dimer does not associate with membranes, fails to permit coupling to receptors, and does not interact with downstream effector proteins such as adenyl cyclase (Iniguez-Lluhi et al. 1992; Muntz et al. 1993).
Notwithstanding the important role of γ subunit prenylation in G protein function, the role of γ subunit heterogeneity in signaling by G proteins remains to be clarified. The γ subunits are the least similar of the G protein subunits, with <30% identity among some isoforms, in contrast to the β subunits, which have >90% identity for four of the five known isoforms (Hurowitz et al. 2000). Based upon these differences, it might have been predicted that the γ subunits would encode any specificity associated with βγ dimers. Nevertheless, the β subunit is best characterized as a determinant of downstream effector specificity (Yan and Gautam 1996, 1997 ;McIntire et al. 2001), and only the β subunit interacts with α in crystals of intact heterotrimers (Wall et al. 1995; Lambright et al. 1996). The γ subunit has been shown to be a determinant of receptor specificity (Kleuss et al. 1993; Kisselev and Gautam 1993), but this may in part be due to the C-terminal modifications of the γ subunits (Yasuda et al. 1996). One important strategy for determining the role of the specificity of G protein γ subunits would be to identify the structurally important elements of the γ subunits that might be required for their specific functions.
Here, we isolated and sequenced the bovine γ10 subunit isoform from the brain and show that its sequence differs near the N-terminus from that of the cloned human protein (Ray et al. 1995). This finding led us to determine how conserved regions of the γ subunit isoforms are between different species. These comparisons showed that the N-terminus of γ is hypervariable among different subunit isoforms, but that these differences are highly conserved between different species. This identifies the N-terminus of γ as a likely site for isoform-specific functions of the γ subunits.
Results
Isolation, identification, and sequencing of the bovine γ10 subunit
Previously, we isolated and completely sequenced γ2 from bovine brain (Wilcox et al. 1995), as well as two different variants of γ5, which differ in their pattern of modification at the C-terminus (Cook et al. 1998). One technique used to characterize these proteins, acid hydrolysis, took advantage of the presence of a single acid-sensitive Asp–Pro bond in all known γ subunit isoforms except γ10 (Cook et al. in press). Acid hydrolysis of this bond produces N-terminal (∼5 kD) and C-terminal (∼2.5 kD) fragments characteristic of each γ subunit isoform. During further analysis of the potential γ subunits found in bovine brain extracts, many of them could be shown to produce Asp–Pro fragments typical of γ subunit isoforms (data not shown). In fact, when HPLC fractions from the separation of γ subunits in purified bovine brain G proteins were analyzed by MALDI MS after acid treatment, only one prominent protein in the γ subunit range, at m/z 7134.9 ± 0.7, n = 11 (average [M+H]+) was insensitive to acid treatment (data not shown). This protein was a likely candidate for the bovine brain γ10 subunit, which lacks an Asp–Pro bond, but the predicted mass of the [M+H]+ ion of the human γ10 protein is 7106.3 Daltons (Ray et al. 1995) and not 7134.9 Daltons.
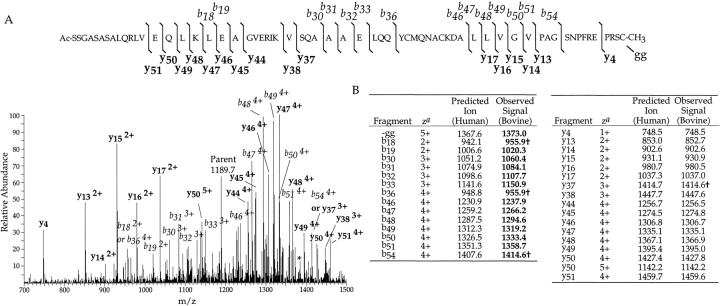
The above results could be explained by species differences in the protein sequence of the γ10 subunit isoforms found in cows versus humans. To test this possibility, HPLC fractions containing the suspected γ10 isoform, as well as γ2 and γ3 (Fig. 1 ▶), were analyzed on a Finnigan LCQ (ESI-ion trap) mass spectrometer (Fig 2 ▶). This instrument provides more accurate protein molecular weight estimates than MALDI-MS, and can be used to determine the sequence of the protein. MS/MS data of intact γ10 ([M+6H]6+ selected at m/z 1190.0) contained two series of y ions (y13, y14, y15, y16, y17 and y44, y45, y46, y47, y48, y49, y50, y51) compatible with the known sequence of γ10 with the predicted C-terminal prenylation pattern. However, the series of b ions in the spectra (b30, b31, b32, b33 and b46, b47, b48, b49, b50, b51) indicated a mass increase ranging from 27 to 29.6 Daltons greater than that predicted from the human sequence (average increase = 27.9 ± 0.16 Daltons) compatible with the mass difference observed between the human and bovine γ10 proteins. These data localized the difference in mass to the first 13 residues of γ10.
Fig. 1.
MALDI mass spectrum of a representative HPLC fraction containing γ10, γ2, and γ3. This spectrum was internally calibrated with insulin and cytochrome c and is the average of 111 scans.
Fig. 2.
MS/MS of a possible γ10 subunit mass by nanospray on a Finnigan LCQ mass spectrometer. (A) MS/MS spectrum of the bovine γ10 [M+6H]6+ ion, m/z 1190.0 selected, average of 22 scans with labeled b (italics) and y (bold) ions. (B) Table of b and y ions predicted for the human γ10 protein and the ions observed by MS/MS from (A). Those masses in bold are 27–29.6 mass units higher than that predicted for bovine γ10. The † indicates ions with more than one assignment. Ions with no charge state indicated are in the 1+ charge state. az = charge state of ion. The asterisk in the spectrum is the parent ion minus the geranylgeranyl group. The bovine γ10 sequence was assumed to be identical to the human γ10 sequence, except for the substitution described here, for the purposes of our analysis. Predicted ions are determined from the Sherpa 3.3.1 program (Taylor et al. 1996) and monoisotopic masses are used below mass 1500.
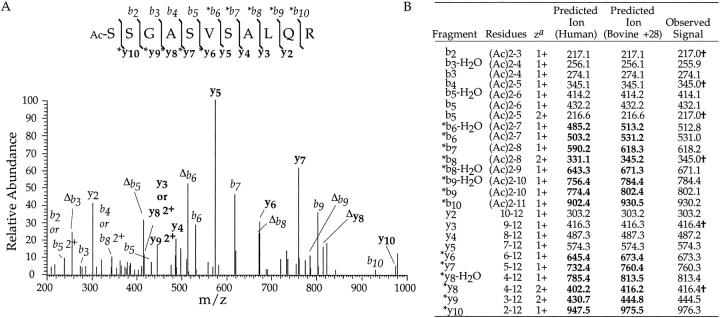
To determine what is responsible for this mass increase in the bovine protein, tryptic peptides of the putative bovine γ10 were analyzed. The HPLC fraction containing γ10 was digested with trypsin, and peptides generated from this digest were analyzed. The sample was separated by HPLC on a C-18 column in line with a Finnigan LCQ-Ion trap mass spectrometer, allowing collection of MS and MS/MS data for each eluting peptide. A mass compatible with an acetylated (minus methionine) N-terminal peptide (residues 2–12 at m/z 1104.5) 28 Daltons higher in mass than that predicted from the human sequence was identified. To determine the position of the increased mass in the N-terminal peptide, this fragment was sequenced by MS/MS analysis (Fig. 3 ▶). The b2–b5 ions and y2–y5 ions are identical to those predicted for the human γ10 isoform assuming the N-terminus is acetylated after removal of methionine, as would be predicted for this protein. However, the b6–b10 ions and y6–y10 ions are all 28 mass units higher than what is predicted from the human γ10 sequence. The change in mass occurred at the sixth (seventh in protein sequence) amino acid, which is Ala in the human sequence. The increase in 28 mass units at this position would be explained by a Val residue. Thus, the 7134.9 mass represents the bovine equivalent of γ10, which is acetylated at the amino terminus, as predicted, with a substitution of Val7 for Ala7.
Fig. 3.
Electrospray ionization MS/MS spectrum of a γ10 tryptic peptide. (A) MS/MS spectrum of bovine γ10 trypsin fragment containing amino acids 2–12, [M+2H]2+, m/z 552.84 selected, 1 scan, with labeled b (italics) and y (bold) ions indicating the sequence variation. (B) Table of predicted and observed ions for MS/MS spectrum of bovine γ10 trypsin fragment containing amino acids 2–12. Those marked by an asterisk indicate ions which are 28 mass units higher than that predicted for bovine γ10. The † indicates ions with more than one assignment. Those marked by Δ indicate ions that have lost 1 water molecule. Ions with no charge state indicated are in the 1+ charge state. Note: the ions labeled as 2+ are unlikely to be generated in this experiment. az = charge state of ion. Predicted ions are determined from the Sherpa 3.3.1 program (Taylor et al. 1996) and monoisotopic masses are used below mass 1500.
Sequencing of clones from an Expressed Sequence Tag database
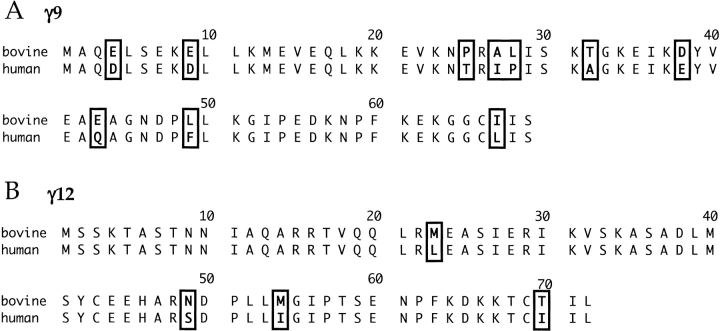
Because the human and bovine γ10 isoforms had a sequence difference at the N-terminus, we wanted to determine if this was also true of other γ subunit isoforms. To augment human data available to us at the time of these experiments, and to answer this question, we screened the human dbEST (Expressed Sequence Tag) database for previously unreported human sequences homologous to γ subunits cloned from other species. From these analyses we found two possible sequences: one homologous to bovine γ12 (dbEST clone ID no. 270914, accession number: AF365871) (Morishita et al. 1995), and another homologous to bovine γ9 (dbEST clone ID no. 190321, accession number: AF365870) (Fig. 4 ▶) (Ong et al. 1995). Clones for these cDNAs were obtained from ATCC (American Type Culture Collection) and resequenced to verify the dbEST sequences. The human γ9 sequence contained 10 amino acid differences from the published bovine sequence (Ong et al. 1995). Most importantly, these differences appeared to be randomly dispersed throughout the protein when γ subunits from different species were compared. The human γ12 sequence had four amino acid differences from the published bovine sequence (Morishita et al. 1995), and these, too, were found dispersed throughout the protein sequence. (These conclusions about the human γ subunit sequences have also been reached by others independently [Ong et al. 1997; Hurowitz et al. 2000] while our work was in progress.) These data suggest that the amino acid differences in a single γ subunit between different species are random, and are not localized to any particular region of the protein.
Fig. 4.
Sequence of human γ9 (A) and human γ12 (B) derived from an EST database. (A) Sequence of human γ9 obtained from dbEST clone ID no. 190321 (accession no. AF365870) sequenced with T7 primer. The coding region for the published sequence of γ9 (accession no. U20085) was used to search The Institute for Genomic Research (TIGR) Human cDNA Database (HCD). (B) Sequence of human γ12 obtained from dbEST clone ID no. 270914 (accession no. AF365871). The coding region for the published sequence of γ12 (accession no. U37561) was used to search The Institute for Genomic Research (TIGR) Human cDNA Database (HCD).
Analysis of the conservation of G protein γ subunit sequences
Because we were interested in determining which regions of the γ subunits vary between isoforms, or between species, we sought to compare all available γ subunit sequences. We used the sequence for the bovine γ10 protein described here, and retrieved 37 partial or full G protein γ subunit sequences from Genbank and the mouse genome database. The relationship of the nucleotide coding region sequences of these γ subunits is shown in Figure 5 ▶, indicating five major subfamilies.
Fig. 5.
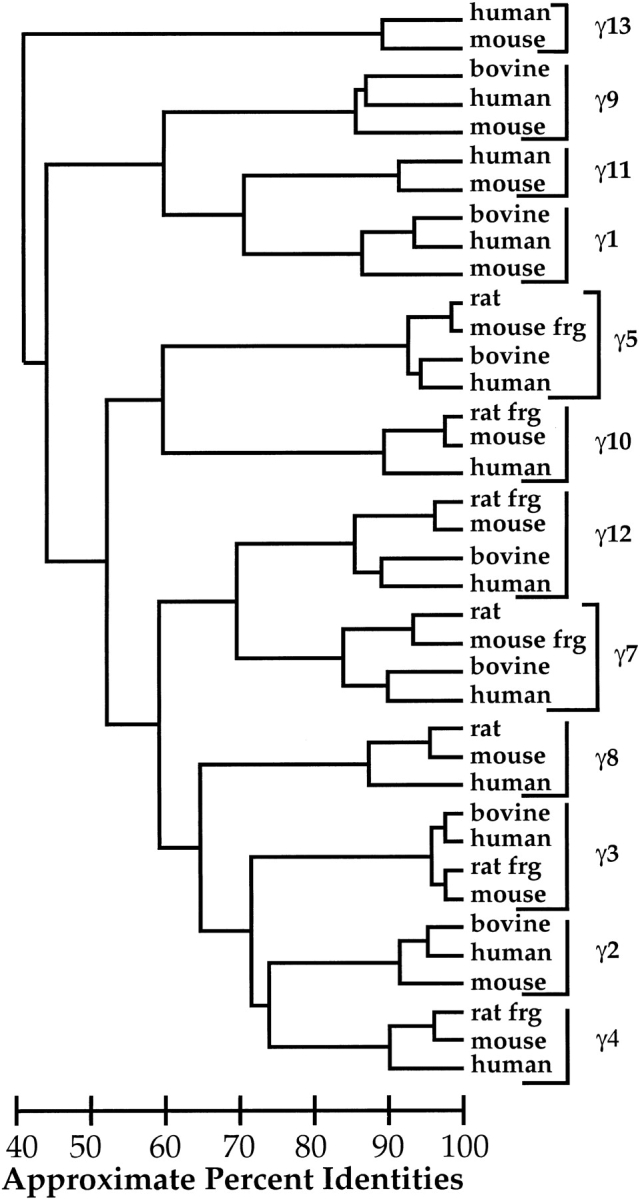
G Protein γ subunit nucleotide coding region alignment. Accession numbers for nucleotide sequences are as follows: γ1 bovine K02436, γ1 human S62027, γ1 mouse AK020863, γ2 bovine M37183, γ2 human AA868346, γ2 mouse NM_010315, γ3 bovine M58349, γ3 human AF092129, γ3 rat frg AF022088, γ3 mouse NM_010316, γ4 rat frg AF022089, γ4 mouse NM_010317, γ4 human U31382, γ5 rat M95780, γ5 mouse BC002316, γ5 bovine M95779, γ5 human AF038955, γ7 rat L23219, γ7 mouse frg U38499, γ7 bovine M99393, γ7 human AB010414, γ8 rat L35921, γ8 mouse AF188180, γ8 human AF188179, γ9(8cone) bovine U20085, γ9(8cone) human AF365870, γ9(8cone) mouse AK010554, γ10 rat frg AF022090, γ10 mouse NM_025277, γ10 human HSU31383, γ11 human HSU31384, γ11 mouse NM_025331, γ12 rat frg AF022091, γ12 mouse NM_025278, γ12 bovine U37561, γ12 human AF365871, γ13 human AB030207 and γ13 mouse AB030194. The coding regions for human γ9 and human γ12 are those for the EST clones we resequenced. (frg) Partial sequences.
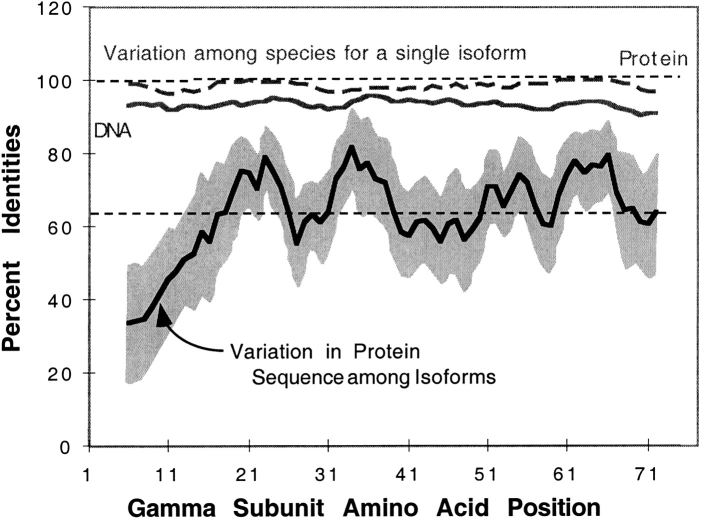
The observed species variation in the sequence of γ10 focused our attention on the differences at the N-termini for these proteins. Comparison of all 38 γ subunit sequences demonstrated that overall the γ subunits have 62% identities, but that the N-terminus of the γ subunit isoforms differs substantially more than other parts of the protein (Fig.6 ▶, solid black line). The N-terminus of γ is the most variable part of the protein. The degree of variability is striking compared to the rest of the protein, as indicated in Figure 6 ▶.
Fig. 6.
Percent identity of a given G protein γ subunit isoform among species (amino acids and DNA among species) and among all γ subunit isoforms at the protein level (amino acids among isoforms). See Materials and Methods section for details on how this figure was generated. The shaded area about the line for variation in protein sequence among isoforms represents the 95% confidence interval for the average percent identies when data for each species are analyzed separately and then averaged (n = 3 or 4).
We also addressed the question of whether the differences in the N-terminus of the protein might be functionally significant by evaluating how conserved these differences are between species. Lack of sequence conservation at the N-terminus of γ isoforms from different species could imply that this part of the protein is relatively unimportant to its function. Conversely, conservation of isoform differences between species could indicate that this is a primary site in the protein for determining the specific functional role for the γ subunit isoforms. To evaluate these ideas we analyzed all 12 different γ subunit isoforms cloned from as many as four mammalian species. We examined the conservation of different regions of the protein for a single γ isoform in several species. The results, shown in Figure 6 ▶ (protein indicated by broken gray lines, and nucleotide coding region indicated by solid gray lines), indicate that the protein and DNA sequences are highly conserved for a single isoform across species. This is just as true of the N-terminus of the protein even though it varies greatly among γ isoforms; and to a much greater extent than other regions of the protein. For example, the sequence of a given γ subunit (e.g., γ2) will be very conserved over the entire length of the protein when γ2 subunits from several species are compared. However, when the γ2 subunit is compared to other γ subunits, the variation in sequence will be much greater at the N-terminus of the protein. Thus, this variation of sequence at the N-terminus for each isoform suggests that this region of the protein might be important for the specialized function of that isoform. The N-terminus is as important as other parts of the protein that are more conserved, such as regions that are involved in interactions with the β subunit.
Discussion
The data presented here describe the variation in the N-terminus of the bovine and human γ10 proteins. The bovine brain protein has a single amino acid difference of Ala to Val at position 7, compared to that of the human cloned γ10 isoform (Ray et al. 1995). This single amino acid change can account for the increase in mass seen for bovine γ10, compared to the human protein. In the DNA sequence of the genes for these proteins, this is a single base difference of C to T, indicating that the sequence of this isoform is relatively conserved. Although this conclusion is compatible with our data, we cannot be certain that the Val-to-Ala substitution at position 7 is the only difference between the human and bovine proteins. We were also able to obtain MS/MS sequencing data on the sixth trypsin fragment corresponding to residues 46–60 of the protein. No differences were evident from the analysis of this peptide. We cannot rule out the possibility that there may be other substitutions in the proteins involving Ile and Leu, or Lys and Gln, because these amino acid pairs have equivalent nominal masses and would not be differentiated by MS/MS sequencing.
This high degree of variability at the N-terminus, obvious when different isoforms were compared, was not evident when the sequences of a single γ isoform were compared from different species. In fact, when comparing the maximum percent identities for a single γ isoform from different species, the N-terminus of the protein is just as conserved as the rest of the protein at both the protein and the nucleotide (coding region) level (Fig. 6 ▶). The variation of the N-terminus for single γ isoforms could mean that this region is important for the specialized function of different γ subunits. From this, it can be hypothesized that the N-terminus of the γ subunits may have an important function in the cell. Some G protein γ subunits have known isoform-specific functions or localization. For example, γ5, which has been found in focal adhesions (Hansen et al. 1996), may be regulated differently than other γ isoforms, possibly by unique interactions of the N-terminus of γ5 with other proteins. The γ12 isoform has previously been shown to be phosphorylated at a serine residue in the N-terminus by protein kinase C. This phosphorylation increases the βγ dimer's affinity for α subunits, but not effectors, because the unphosphorylated and phosphorylated βγ12 dimer interacts with effectors to the same extent (Morishita et al. 1995). This implies that the γ subunit may play a role in interacting with α subunits.
The N-terminus of the γ subunit has not yet been shown to have a specific function. It has been implicated in interactions with α subunits and protects α from tryptic digestion (Rahmatullah et al. 1995). The γ subunit also lies near one of the regions of the β subunits that interact with the effectors (Wall et al. 1995; Lambright et al. 1996; Sondek et al. 1996), the α-helical N-terminal domain of the β subunit. Therefore, the γ subunit may influence interactions with those effectors binding at this region, but not with others that bind to a different site on the β subunit. In addition, the N-terminus may be involved in uncharacterized interactions of the heterotrimer or βγ dimer with other proteins. Variation of the N-terminal sequence among γ subunits may be an important determinant of their isoform-specific functions.
Materials and methods
Isolation of G proteins and separation of γ subunits
Bovine brain G proteins were isolated as previously described (Dingus et al. 1994). The γ subunit isoforms were separated from each other and from their associated α and β subunits in purified G protein heterotrimer by reverse-phase HPLC over a 220 × 4.6-mm Aquapore 7-μm phenyl column (Brownlee) eluted in line with a Finnigan LCQ (ESI-Ion Trap) mass spectrometer (Cook et al. 1998; Cook et al. in press). The flow was split postcolumn so that a small part of the eluent was sent to the ESI source while the remainder was collected as fractions and stored at −20°C until further analysis. Electrospray mass spectra (MS) and MS/MS mass spectra were obtained during the run.
MALDI and aspartate–proline bond cleavage
MALDI MS and acid hydrolysis of the D-P bond was performed as described previously on a PerSeptive Biosystems Voyager-DE MS instrument (Cook et al. 1998; Cook et al. in press). The flow was split postcolumn so that a small part of the eluent was sent to the ESI source while the remainder was collected as fractions and stored at −20°C until further analysis. Electrospray mass spectra (MS) and MS/MS mass spectra were obtained during the run.
MALDI and aspartate–proline bond cleavage
MALDI MS and acid hydrolysis of the D-P bond was performed as described previously on a PerSeptive Biosystems Voyager-DE MS instrument (Cook et al. 1998; Cook et al. in press).
Nanospray
Nanospray (ESI-MS/MS) was performed as described (Cook et al. in press).
Trypsin digestion of γ10 and HPLC separation of tryptic peptides
Approximately 425 μL of pooled γ10 fractions were dried under vacuum and resuspended in 8.4 μL 10 mM NH4HCO3 with 0.032 μg of sequencing grade trypsin (Pierce) (1:200 w/w) and incubated at 32°C for at least 12 h. The sample was again dried under vacuum and resuspended in 10 μL of 0.1 M acetic acid. Tryptic peptides were separated by HPLC as described previously in line with a Finnigan LCQ for electrospray analysis (Cook et al. in press).
Analysis of nucleotide and amino acid sequences of γ subunits
Sequences of cloned γ subunit cDNAs were identified using the GCG computer programs to search the GenBank database. A total of 37 γ subunit sequences were identified in the database. A dendogram was constructed for these 37 nucleotide coding sequences using Pileup (GCG Wisconsin Package). The optimal alignment of protein sequences was used to calculate the percent homology at each position of the aligned proteins. The principle adjustment made involved aligning the prenylation sequence motif at the C-terminus of the protein. Alignment of the N-terminal sequences made little difference in the overall homology scores. The percent homology was taken to be the percent of time the most frequent amino acid or nucleotide was found in a group of sequences. To account for differences in the length of the proteins at the N-terminus, we considered the denominator for percent identities only the number of proteins with an amino acid at that position. To average variation across the protein, we averaged these identity scores across a window of seven residues. This, with the exception of a very few positions, normalized any effect of difference in length of the proteins at the N-terminus. To calculate homology between isoforms, we analyzed all protein sequences in our data set for a single species and then averaged the data for all species. To calculate homology within species, we analyzed all the sequences for a given γ subunit isoform from different species and then averaged the values for all the isoforms.
Acknowledgments
The authors thank Dr. Dan Knapp at MUSC for the use of the Mass Spectrometry Facility and for helpful comments. This project was supported by NIH Grant DK37219 (J.D.H.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.26401.
References
- Bokoch, G.M., Katada, T., Northup, J.K., Ui, M., and Gilman, A.G. 1984. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem. 259 3560–3567. [PubMed] [Google Scholar]
- Clarke, S. 1992. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 61 355–386. [DOI] [PubMed] [Google Scholar]
- Cook, L.A., Schey, K.L., Wilcox, M.D., Dingus, J., and Hildebrandt, J.D. 1998. Heterogeneous C-terminal processing of a G protein gamma subunit. Biochemistry 37 12280–12286. [DOI] [PubMed] [Google Scholar]
- Cook, L.A., Wilcox, M.D., Dingus, J., Schey, K.L., and Hildebrandt, J.D. Separation and analysis of G protein γ subunits. Methods Enzymol. (in press). [DOI] [PubMed]
- Cox, A.D. 1995. Mutation and analysis of prenylation signal sequences. Methods Enzymol. 250 105–121. [DOI] [PubMed] [Google Scholar]
- Dingus, J., Wilcox, M.D., Kohnken, R.E., and Hildebrandt, J.D. 1994. Biotinylated beta/gamma subunits. Methods Enzymol. 237 457–471. [DOI] [PubMed] [Google Scholar]
- Gilman, A.G. 1987. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 56 615–649. [DOI] [PubMed] [Google Scholar]
- Hansen, C.A., Schroering, A.G., Carey, D.J., and Robishaw, J.D. 1996. Localization of a heterotrimeric G protein gamma subunit to focal adhesions and associated stress fibers. J. Cell. Biol. 126 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt, J.D. 1997. Role of subunit diversity in signaling by heterotrimeric G proteins. Biochem. Pharmacol. 54 325–339. [DOI] [PubMed] [Google Scholar]
- Hildebrandt, J.D., Codina, J., Risinger, R., and Birnbaumer, L. 1984. Identification of a gamma subunit associated with the adenylyl cyclase regulatory proteins Ns and Ni. J. Biol. Chem. 259 2039–2042. [PubMed] [Google Scholar]
- Hurowitz, E.H., Melnyk, J.M., Chen, Y.-J., Kouros-Meir, H., Simon, M.I., and Shizuya, H. 2000. Genomic Characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 7 111–120. [DOI] [PubMed] [Google Scholar]
- Iniguez-Lluhi, J.A., Kleuss, C., and Gilman, A.G. 1993. The importance of G protein beta/gamma subunits. Trends. Cell. Biol. 3 230–236. [DOI] [PubMed] [Google Scholar]
- Iniguez-Lluhi, J.A., Simon, M.I., Robishaw, J.D., and Gilman, A.G. 1992. G protein beta/gamma subunits synthesized in sf9 cells. Functional characterization and the significance of prenylation of gamma. J. Biol. Chem. 267 23409–23417. [PubMed] [Google Scholar]
- Kisselev, O. and Gautam, N. 1993. Specific interaction with rhodopsin is dependent on the gamma subunit type in a G protein. J. Biol. Chem. 268 24519–24522. [PubMed] [Google Scholar]
- Kleuss, C., Scherubl, H., Hescheler, J., Schultz, G., and Wittig, B. 1993. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science 259 832–834. [DOI] [PubMed] [Google Scholar]
- Kuhn, H. 1980. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature 283 587–589. [DOI] [PubMed] [Google Scholar]
- Lambright, D.G., Sondek, J., Bohm, A., Skiba, N.P., Hamm, H.E., and Sigler, P.B. 1996. The 2.0 Angstrom crystal structure of a heterotrimeric G protein. Nature 379 311–319. [DOI] [PubMed] [Google Scholar]
- McIntire, W.E., MacCleery, G., and Garrison, J.C. 2001. The G protein beta subunit is a determinant in the coupling of Gs to the beta-1 adrenergic and A2a adenosine receptors. J. Biol. Chem. 276 15801–15809. [DOI] [PubMed] [Google Scholar]
- Morishita, R., Nakayama, H., Isobe, T., Matsuda, T., Hashimoto, Y., Okano, T., Fukada, Y., Mizuno, K., Ohno, S., Kozawa, O., Kato, K., and Asano, T. 1995. Primary structure of a gamma subunit of G protein, gamma-12, and its phosphorylation by protein kinase C. J. Biol. Chem. 270 29469–29475. [DOI] [PubMed] [Google Scholar]
- Muntz, K.H., Sternweis, P.C., Gilman, A.G., and Mumby, S.M. 1993. Influence of gamma subunit prenylation on association of guanine nucleotide binding regulatory proteins with membranes. Mol. Biol. Cell. 3 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, O.C., Hu, K., Rong, H., Lee, R.H., and Fung, B.K. 1997. Gene structure and chromosome localization of the G gamma c subunit of human cone G-protein (GNGT2). Genomics 44 101–109. [DOI] [PubMed] [Google Scholar]
- Ong, O., Yamane, H.K., Phan, K.B., Fong, H.K.W., Bok, D., Lee, R.H., and Fung, B.K. 1995. Molecular cloning and characterization of the G protein gamma subunit of cone photoreceptors. J. Biol. Chem. 270 8495–8500. [DOI] [PubMed] [Google Scholar]
- Rahmatullah, M., Ginnan, R., and Robishaw, J.D. 1995. Specificity of G protein alpha-gamma subunit interactions: N-terminal 15 amino acids of gamma subunit specifies interaction with alpha subunit. J. Biol. Chem. 270 2946–2951. [DOI] [PubMed] [Google Scholar]
- Ray, K., Kunsch, C., Bonner, L.M., and Robishaw, J.D. 1995. Isolation of cDNA clones encoding eight different human G protein gamma subunits, including three novel forms designated the gamma-4, gamma-10 and gamma-11 subunits. J. Biol. Chem. 270 21765–21771. [DOI] [PubMed] [Google Scholar]
- Sondek, J., Bohm, A., Lambright, D.G., Hamm, H.E., and Sigler, P.B. 1996. Crystal structure of a G protein beta/gamma dimer at 2.1 angstrom resolution. Nature 379 369–374. [DOI] [PubMed] [Google Scholar]
- Taylor, J.A., Walsh, K.A., and Johnson, R.S. 1996. Sherpa: A Macintosh-based expert system for the interpretation of ESI LC/MS and MS/MS of protein digests. Rapid. Commun. Mass. Spectrum. 10 679–687. [DOI] [PubMed] [Google Scholar]
- Wall, M.A., Coleman, D.E., Lee, E., Iniguez-Lluhi, J.A., Posner, B.A., Gilman, A.G., and Sprang, S.R. 1995. The structure of the G protein heterotrimer G alpha-i1 Beta-1 Gamma-2. Cell 83 1047–1058. [DOI] [PubMed] [Google Scholar]
- Wilcox, M.D., Schey, K.L., Busman, M., and Hildebrandt, J.D. 1995. Determination of the complete covalent structure of the gamma-2 subunit of bovine brain G proteins by mass spectrometry. Biochem. Biophys. Res. Commun. 212 367–374. [DOI] [PubMed] [Google Scholar]
- Yan, K. and Gautam, N. 1996. A domain on the G protein beta subunit interacts with both adenylyl cyclase 2 and the muscarinic atrial potassium channel. J. Biol. Chem. 271 17597–17600. [DOI] [PubMed] [Google Scholar]
- —. 1997. Structural determinants for the interaction with three different effectors on the G protein beta subunit. J. Biol. Chem. 272 2056–2059. [DOI] [PubMed] [Google Scholar]
- Yasuda, H., Lindorfer, M.A., Woodfork, K.A., Fletcher, J.E., and Garrison, J.C. 1996. Role of the prenyl group on the G protein gamma subunit in coupling trimeric G proteins to A1 adenosine receptors. J. Biol. Chem. 271 18588–18595. [DOI] [PubMed] [Google Scholar]