Abstract
To understand the interplay between tertiary and quaternary transitions associated with hemoglobin function and regulation, oxygen binding curves were obtained for hemoglobin A fixed in the T quaternary state by encapsulation in wet porous silica gels. At pH 7.0 and 15°C, the oxygen pressure at half saturation (p50) was measured to be 12.4 ± 0.2 and 139 ± 4 torr for hemoglobin gels prepared in the absence and presence of the strong allosteric effectors inositol hexaphosphate and bezafibrate, respectively. Both values are in excellent agreement with those found for the binding of the first oxygen to hemoglobin in solution under similar experimental conditions. The corresponding Hill coefficients of hemoglobin gels were 0.94 ± 0.02 and 0.93 ± 0.03, indicating, in the frame of the Monod, Wyman, and Changeux model, that high and low oxygen-affinity tertiary T-state conformations have been isolated in a pure form. The values, slightly lower than unity, reflect the different oxygen affinity of α- and β-hemes. Significantly, hemoglobin encapsulated in the presence of the weak effector phosphate led to gels that show intermediate oxygen affinity and Hill coefficients of 0.7 to 0.8. The heterogeneous oxygen binding results from the presence of a mixture of the high and low oxygen-affinity T states. The Bohr effect was measured for hemoglobin gels containing the pure conformations and found to be more pronounced for the high-affinity T state and almost absent for the low-affinity T state. These findings indicate that the functional properties of the T quaternary state result from the contribution of two distinct, interconverting conformations, characterized by a 10-fold difference in oxygen affinity and a different extent of tertiary Bohr effect. The very small degree of T-state cooperativity observed in solution and in the crystalline state might arise from a ligand-induced perturbation of the distribution between the high- and low-affinity T-state conformations.
Hemoglobin is an α2β2-tetramer that binds ligands cooperatively via the modulation of equilibria between tertiary and quaternary conformations. X-ray crystallographic studies have shown that hemoglobin exists in two quaternary states, T and R, characterized by the presence and absence of salt bridges, respectively, and by a different pattern of α1β2- and α2β1-interactions (Perutz 1970; Baldwin and Chothia 1979). Binding of ligands triggers a series of tertiary conformational changes that break salt bridges, destabilizing the T state and leading to the liganded R state (Perutz 1970; Antonini and Brunori 1971; Perutz et al. 1998). Oxygen binding curves have been extremely useful in evaluating the effect of allosteric effectors on the functional properties of quaternary states (Imai 1982). In particular, the oxygen affinity and the degree of cooperativity of T-state hemoglobin have been investigated on mixed metal hybrids, for which iron at either the α- or β-heme was substituted with metals that do not bind oxygen (Shibayama et al. 1986; Miyazaki et al. 1999), and on T-state hemoglobin crystals grown from polyethylene glycol solutions (Mozzarelli et al. 1991, 1997; Rivetti et al. 1993a,b; Bettati et al. 1997, 1998; Bruno et al. 2000). These new experiments were required to discriminate among the models proposed to explain cooperative ligand binding and functional modulation brought about by allosteric effectors. A critical difference among models concerns the predicted cooperative properties of the T state. The Monod, Wyman, and Changuex (MWC) model predicts noncooperative ligand binding (Monod et al. 1965), whereas the Koshland, Nemethy and Filmer model (Koshland et al. 1966), the "Monland" model of Perutz (1998), and the Symmetry Rule of Ackers (Ackers et al. 1992, 2000) predict cooperative ligand binding.
A particularly powerful approach to dissect tertiary and quaternary contributions to cooperativity is the encapsulation of hemoglobin in silica gels, which stabilizes the protein either in the T or in the R state (Shibayama and Saigo 1995Shibayama and Saigo 1999Shibayama and Saigo 2001; Bettati and Mozzarelli 1997; Das et al. 1999; Juszczak and Friedman 1999; Shibayama 1999; Khan et al. 2000). The quaternary transition is not prevented but is dramatically slowed down with respect to solution, the rate of the process being strongly dependent on temperature (Das et al. 1999; Shibayama and Saigo 1999). This behavior allows the detection of tertiary conformational properties de-coupled from the quaternary transition. Even the rates of tertiary transitions of hemoglobin in silica gels are decreased by several orders of magnitude (Juszczak and Friedman 1999; Shibayama 1999; Shibayama and Saigo 2001). Despite these restraints on dynamic properties, spectroscopic markers (Das et al. 1999; Juszczak and Friedman 1999; Khan et al. 2000), oxygen affinity (Shibayama and Saigo 1995Shibayama and Saigo 2001; Bettati and Mozzarelli 1997), and the Bohr effect (Bettati and Mozzarelli 1997) were found to be similar to those observed in solution, indicating that hemoglobin encapsulated in silica gels retains its functional and structural properties. One intriguing result was that the oxygen binding curves of hemoglobin gels showed Hill coefficients significantly lower than unity (Shibayama and Saigo 1995Shibayama and Saigo 2001; Bettati and Mozzarelli 1997), indicating conformational heterogeneity, possibly originated by the presence of noninterconverting states with different oxygen affinities (Bettati and Mozzarelli 1997). This heterogeneity was also present in the T-state hemoglobin gels recently investigated (Shibayama and Saigo 2001), the Hill coefficient varying between 0.5 and 0.7. Based on the extrapolation of the oxygen binding curves at very high and very low oxygen saturations, Shibayama and Saigo (2001) proposed the presence of a high oxygen-affinity state with p50 of 1 to 2 torr and a low oxygen-affinity state with p50 of 64 to 100 torr. These values are five- and twofold lower than those observed in solution for the binding of the first oxygen to T-state hemoglobin in the absence and presence of allosteric effectors, respectively (Poyart et al. 1978; Imai 1982).
In the present work, we have addressed the question whether the functional properties of the T-state hemoglobin in solution are generated by a continuous population of tertiary conformational states or by a distribution of two or more discrete interconverting states, modulated by allosteric effectors and characterized by distinct ligand affinities, as originally proposed by Rivetti et al. (1993a) and Bettati and Mozzarelli (1997). Deoxy-hemoglobin was encapsulated in silica gels in the absence and presence of different allosteric effectors with the aim to isolate distinct tertiary T-state conformations and to accurately characterize oxygen binding and allosteric modulation. Results add further information to the ongoing investigation on the distribution of tertiary and quaternary conformations as a function of ligand saturation and on the degree of cooperativity within the T-state hemoglobin (Perutz et al. 1998; Eaton et al. 1999; Perrella 1999; Ackers et al. 2000; Russo et al. 2001).
Results
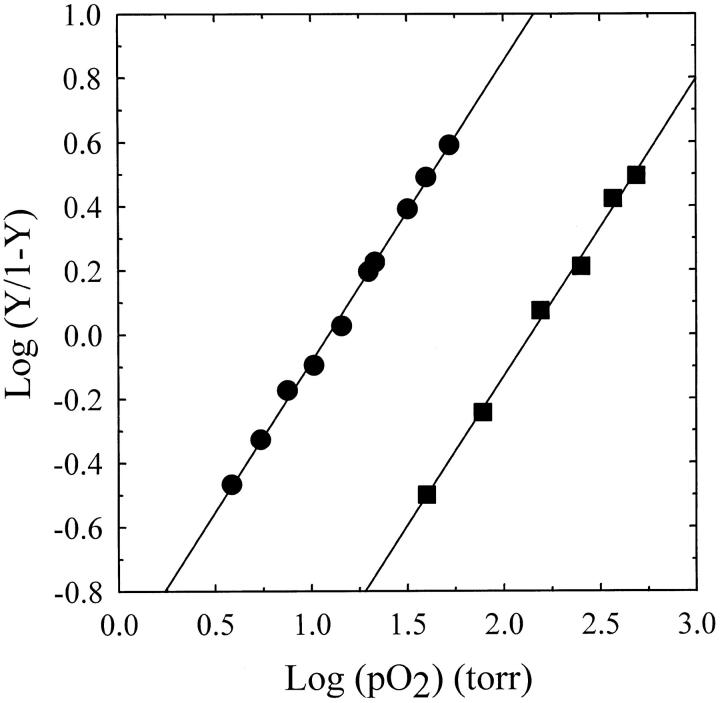
Deoxy-hemoglobin was encapsulated in silica gels under conditions that, in solution, allow the attainment of either high or low oxygen affinities for T-state hemoglobin, that is, in the absence and presence of the strong allosteric effectors bezafibrate and inositol hexaphosphate, respectively (Poyart et al. 1978; Imai 1982). Oxygen binding curves were determined by measuring absorption spectra of hemoglobin silica gels as a function of oxygen pressure. The Hill plots of the oxygen binding curves for hemoglobin gels obtained in the absence and presence of allosteric effectors show a Hill coefficient of 0.94 ± 0.02 and 0.93 ± 0.03, respectively (Table 1, Fig. 1 ▶). In the frame of the MWC model that assumes a noncooperative binding within a quaternary state (Monod et al. 1965), Hill coefficients of unity indicate that pure tertiary T-states have been isolated. The small deviation from unity can be accounted for by a residual conformational heterogeneity and, mainly, by the two- or threefold difference in oxygen affinity between α- and β-hemes (Lindstrom and Ho 1972; Shibayama et al. 1986; Bettati et al. 1996; Mozzarelli et al. 1997; Bruno et al. 2000). In fact, a two- and fourfold higher affinity of α-hemes with respect to β-hemes would result in a Hill coefficient of 0.97 and 0.91, respectively. The calculated p50s, that is, the value of oxygen pressure at half saturation, are 12.4 ± 0.2 and 139 ± 4 torr, for hemoglobin gels obtained in the absence and presence of allosteric effectors, respectively. These values are compared in Table 1 with those observed in solution for the binding of the first oxygen to T-state hemoglobin, K1, either in the absence or presence of allosteric effectors, and with the p50 determined for T-state hemoglobin crystals. Furthermore, the high-affinity hemoglobin gels—resuspended in a solution containing inositol hexaphosphate (IHP), bezafibrate, and chloride—showed a Hill coefficient of 0.91 and a fourfold increase of the p50 (Table 1), whereas the low-affinity hemoglobin gels, resuspended in an allosteric effectors-free solution, showed a Hill coefficient of 0.90 and a 1.3-fold decrease of the p50 (Table 1).
Table 1.
Oxygen binding to Hb gels, crystals and solution
| Gela | Gelb | Crystal | Solution | ||||
| Allosteric effectors | p50 (torr) | Hill n | p50 (torr) | Hill n | p50 (torr) | Hill n | K1 |
| − | 12.4c | 0.94c | 104c | 0.90c | 10.8e | ||
| + | 40.0d | 0.91d | 139d | 0.93d | 94f, 106g | ||
| −/+ | 135h | 0.99h | |||||
Solution and gels experiments were carried out at pH 7.0.
a Hemoglobin gels were prepared in the absence of allosteric effectors (protocol 1, see Materials and Methods).
b Hemoglobin gels were prepared in the presence of allosteric effectors (protocol 2, see Materials and Methods).
c 100 mM Hepes, 1 mM EDTA.
d 50 mM Bis-Tris, 50 mM Tris, 1 mM EDTA, 0.2 M Cl−, 2 mM IHP, 2 mM bezafibrate.
e 50 mM Bis-Tris, 5 mM Cl−, pH 7.0 (Poyart et al. 1978).
f 50 mM Bis-Tris, 0.15 M Cl−, 2,3 diphosphoglycerate (5 mol/mol Hb), pH 7.0 (Poyart et al. 1978).
g 50 mM Bis-Tris, pH 7.2, 1 mM IHP, 5 mM bezafibrate (the reported value is KT) (Marden et al. 1990). Since binding measurements in solution were carried out at 25°C, the values shown in the table are corrected for the temperature difference (scaling factor, 2 (Imai, 1982)).
h 10 mM phosphate, 54% (w/v) PEG 8000, 1 mM EDTA, in the absence or presence of 2 mM IHP, pH 7.2, 15°C (Rivetti et al. 1993a; Mozzarelli et al. 1997).
Fig. 1.
Hill plots of oxygen binding by hemoglobin silica gels prepared either in the absence (circles) or presence (squares) of allosteric effectors. Measurements were performed in 100 mM HEPES, 1 mM EDTA (circles), or 50 mM Bis-Tris, 50 mM Tris, 0.2 M Cl−, 2 mM IHP, 2 mM bezafibrate, and 1mM EDTA (squares), pH 7.0, at 15°C. The straight line through data points is the fitting to the Hill equation with p50 and Hill coefficient of 12.4 ± 0.2 torr and 0.94 ± 0.02, and 139 ± 4 torr and 0.93 ± 0.03, in the absence and presence, respectively, of allosteric effectors.
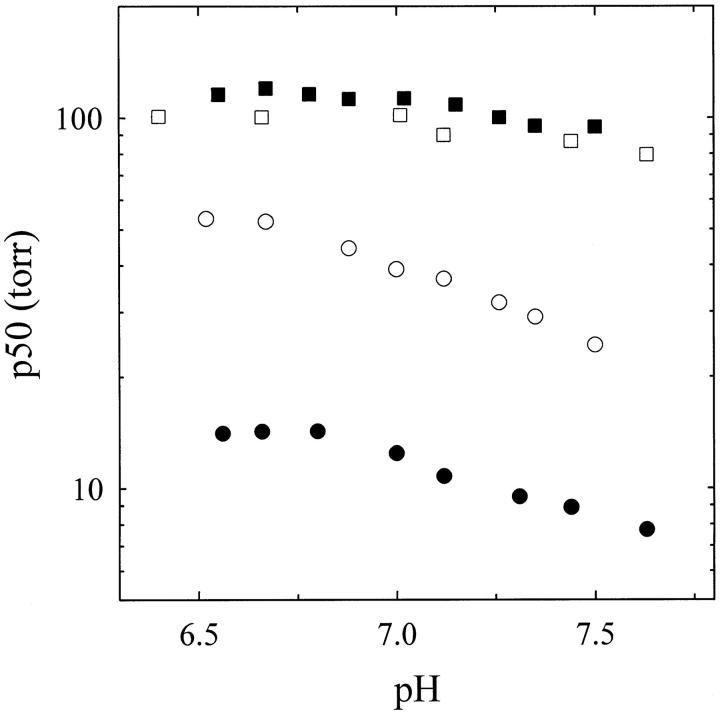
The tertiary Bohr effect of hemoglobin gels was previously investigated for a heterogeneous population of conformations (Bettati and Mozzarelli 1997). Here, we have determined the tertiary Bohr effect for the high and low oxygen-affinity hemoglobin gels, over the pH range 6.4 to 7.7. Solutions at higher pHs cannot be used because the polymeric silica network hydrolyzes and, consequently, releases hemoglobin (Bettati and Mozzarelli 1997). The effect of pH on the oxygen affinity was found to be more pronounced for the high-affinity gels than for the low-affinity gels (Fig. 2 ▶). Furthermore, the addition of allosteric effectors to the high-affinity hemoglobin gels or the removal of allosteric effectors from the low-affinity hemoglobin gels did not significantly affect the pH dependence (Fig. 2 ▶).
Fig. 2.
Bohr effect of hemoglobin gels. The values of p50 as a function of pH are calculated from the values of the fractional saturation obtained on hemoglobin gels prepared in the absence of allosteric effectors and exposed to a single oxygen pressure in a solution containing 100 mM HEPES (closed circles), 50 mM Bis-Tris, 50 mM Tris, 1 mM EDTA, 0.2 M Cl−, 2 mM IHP, and 2 mM bezafibrate (open circles), and hemoglobin gels prepared in the presence of allosteric effectors and exposed to a single oxygen pressure in a solution containing 100 HEPES (open squares) and 50 mM Bis-Tris, 50 mM Tris, 1 mM EDTA, 0.2 M Cl−, 2 mM IHP, and 2 mM bezafibrate (closed squares), 15°C.
To compare these results with previous studies (Shibayama and Saigo 1995; Bettati and Mozzarelli 1997), oxygen binding curves and Bohr effect were also determined for deoxy-hemoglobin gels prepared in the presence of phosphate. The oxygen binding curves were measured either in the absence or presence of allosteric effectors (Fig. 3a ▶) and as a function of pH (Fig. 3b ▶). As previously found (Bettati and Mozzarelli 1997), the values of Hill coefficients are significantly lower than unity, 0.71 and 0.78 in the absence and presence of allosteric effectors, respectively. These values cannot be simply explained by the α- to β-heme inequivalence. The observed binding curves were fitted assuming the presence of a mixture of two noninteracting and noninterconverting sites. The analysis indicates that the binding curves result from an almost equal contribution from two sites characterized by ∼10-fold difference in oxygen affinity (Fig. 3a ▶). The calculated values of p50 are remarkably close to those obtained here for the fully populated high- and low-affinity hemoglobin gels, given the limited number of data points for each curve. When the fitting was performed imposing the p50 of the high- and low-affinity states, the same relative contribution of the two states was found. The presence of a heterogeneous population of high and low oxygen-affinity conformations explains also the observed Bohr effect of these hemoglobin gels (Fig. 3b ▶).
Fig. 3.
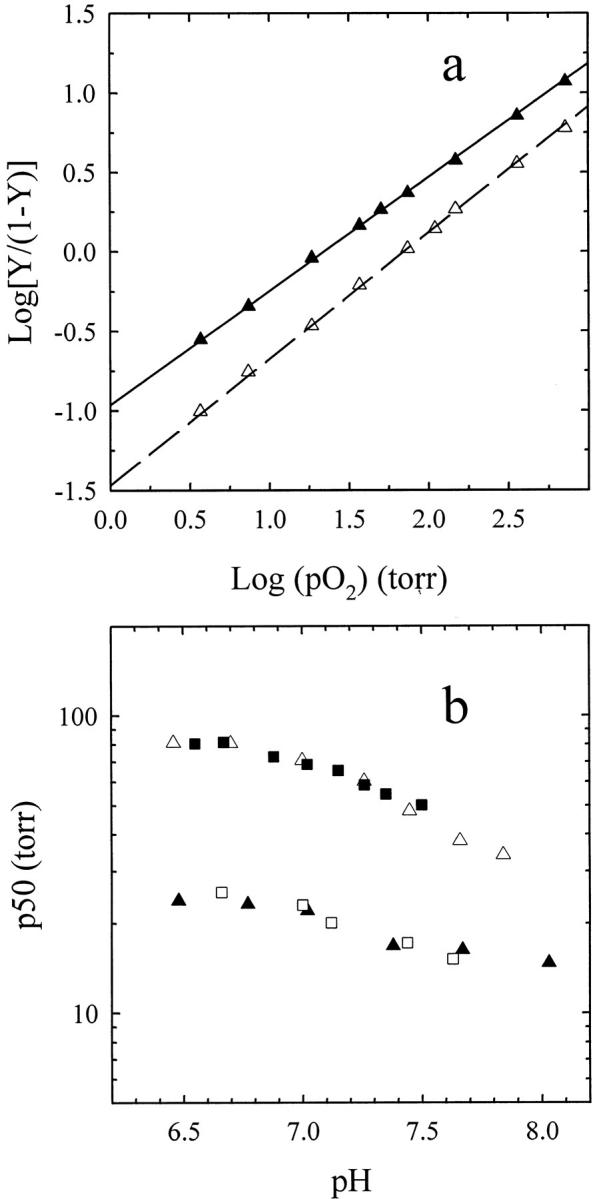
Oxygen binding and Bohr effect of hemoglobin gels prepared in phosphate buffer. Hill plot (a) and Bohr effect (b) for hemoglobin gels suspended in a solution containing 100 HEPES, 1 mM EDTA (closed triangles) and 50 mM Bis-Tris, 50 mM Tris, 1 mM EDTA, 0.2 M Cl−, 2 mM IHP, and 2 mM bezafibrate (open triangles) at 15°C. The p50 and the Hill coefficient are 22.1 ± 0.2 torr and 0.71 ± 0.01, and 70.7 ± 0.7 torr and 0.78 ± 0.01, in the absence and presence of the allosteric effectors, respectively, as calculated by a linear fit of data points. The straight lines through data points in panel a are the fitting assuming two noninterconverting and independent sites (Hill coefficient = 0.93) with distinct oxygen affinities. The calculated p50s in the presence of allosteric effectors are 27.9 ± 6 and 201 ± 48 torr with a fraction of the high affinity site of 0.54 ± 0.09. For the gel in the absence of allosteric effectors the p50s of the two sites are 8.7 ± 0.7 and 132 ± 18 torr, with a fraction of the high-affinity site of 0.64 ± 0.03. In panel b, closed triangles and open triangles represent the Bohr effect of hemoglobin gels in the absence and presence of allosteric effectors, respectively. Closed squares and open squares represent simulated values calculated assuming different contributions at each pH of the high- and low-affinity populations. The fraction of the two forms and the Hill n are obtained from the fitting of the oxygen binding curves at pH 7.0, as reported above.
Discussion
The allosteric model of MWC predicts the existence of two interconverting quaternary states of hemoglobin, characterized by oxygen affinities that differ by more than two orders of magnitude (Monod et al. 1965). These structures were determined by X-ray crystallography and investigated by many other physico-chemical techniques (Perutz 1970Perutz 1989; Antonini and Brunori 1971; Edelstein 1975; Shulman et al. 1975; Imai 1982; Eaton et al. 1999). The MWC model represents a simplified view of hemoglobin function because it does not account for changes of T-state hemoglobin oxygen affinity caused by the binding of allosteric effectors (Imai 1982). To explain such behavior, either the presence of a continuous population of tertiary conformations or the modulation of the distribution between at least two tertiary conformations within each single quaternary state were proposed (Imai 1982; Rivetti et al. 1993a). However, structural and functional characterization of these tertiary conformations has been elusive because ligand binding leads to a fast redistribution of conformational states. A direct structure-function correlation was obtained by determining oxygen binding curves to T-state hemoglobin crystals (Rivetti et al. 1993a; Kavanaugh et al. 1995; Bettati et al. 1996, 1998; Mozzarelli et al. 1997; Bruno et al. 2000), their structures being uniquely defined by X-ray crystallography (Brzozowski et al. 1984; Liddington et al. 1988, 1992; Paoli et al. 1996). Those studies indicate that T-state hemoglobin in the crystal is characterized by the presence of a defined tertiary conformation endowed with the lowest oxygen affinity observed in solution (Lalezari et al. 1990; Marden et al. 1990; Miyazaki et al. 1999).
Encapsulation of hemoglobin in wet, porous silica gels has been used as an alternative method for trapping quaternary states (Shibayama and Saigo 1995Shibayama and Saigo 2001; Bettati and Mozzarelli 1997; Das et al. 1999; Juszczak and Friedman 1999; Khan et al. 2000). Oxygen binding curves to both the R and T states of hemoglobin were measured (Shibayama and Saigo 1995Shibayama and Saigo 2001; Bettati and Mozzarelli 1997). In contrast to hemoglobin in the crystal, the oxygen affinity of T-state hemoglobin in silica gels is modulated by IHP, bezafibrate, chloride, and pH (Bettati and Mozzarelli 1997), as in solution (Poyart et al. 1978; Imai 1982; Lee et al. 1988; Marden et al. 1990), indicating that the protein molecule in the gel retains a significant degree of flexibility. However, oxygen binding curves were characterized by a Hill coefficient significantly lower than unity (Shibayama and Saigo 1995Shibayama and Saigo 2001; Bettati and Mozzarelli 1997), consistent with the presence of a distribution of noninterconverting tertiary conformations (Bettati and Mozzarelli 1997). A recent study has supported this hypothesis, showing that high and low oxygen-affinity tertiary states are simultaneously pres-ent when T-state hemoglobin is encapsulated in silica gels (Shibayama and Saigo 2001). The oxygen binding curves showed a Hill coefficient of 0.5 to 0.7 and asymptotically extrapolated to two discrete components characterized by a Hill coefficient of one and a p50 of 1.1 to 2.2 and 64 to 101 torr at 20°C. The values of oxygen affinity for both the high- and low-affinity states are lower than the values of K1 reported for hemoglobin in solution under similar experimental conditions (Poyart et al. 1978; Imai 1982; Marden et al. 1990) and might result from the formation of a mixture of T and R states during the titration, as indicated by the nonperfect reversibility of the reported binding curves (Shibayama and Saigo 2001). To achieve a detailed functional characterization of the high and low oxygen-affinity T-state conformations, we have designed experimental conditions of hemoglobin encapsulation to isolate the distinct tertiary conformations of hemoglobin. The results indicate that hemoglobin silica gels fully populated with either high or low oxygen-affinity tertiary conformations have been obtained. These tertiary T states are characterized by a 10-fold difference in oxygen affinity. The observed affinities are very close to those determined in solution when the first oxygen binds to hemoglobin in the absence and presence of allosteric effectors (Poyart et al. 1978). Interestingly, the low oxygen-affinity state binds oxygen with the same affinity as hemoglobin crystals (Rivetti et al. 1993a; Mozzarelli et al. 1997). These conclusions are based on the assumption that a homogeneous population of conformations shows a Hill coefficient of one as postulated by the Monod, Wyman and Changeux model for noninteracting binding sites (Monod et al. 1965). Other models predict Hill coefficients up to 1.3 for T-state oxygen binding (Ackers et al. 2000). However, under any assumption, the hemoglobin gels that we have prepared are much more homogeneous that those previously investigated (Bettati and Mozzarelli 1997; Shibayama and Saigo 2001), opening the way to further functional and structural investigations. Furthermore, our findings indicate that the small degree of cooperativity observed within the T state in solution (Ackers et al. 2000) and in the crystalline state (Rivetti et al. 1993a; Mozzarelli et al. 1997; Eaton et al. 1999) might arise from a ligand-induced alteration of the distribution between the high and low oxygen-affinity T states.
An interesting result of the present study is the measurement of the effect of the binding of IHP and bezafibrate on the oxygen affinity of isolated tertiary T states of hemoglobin. Binding of allosteric effectors to the high-affinity state decreases the oxygen affinity by fourfold, whereas removal of the allosteric effectors from the low-affinity state increases the oxygen affinity by 1.3-fold. Remarkably, the oxygen binding curves still showed a Hill coefficient close to unity, indicating that tertiary states do not interconvert within the gel matrix. Thus, the influence on the oxygen affinity is solely due to the interaction of the allosteric effectors with a defined tertiary state of the protein. In solution, this effect is masked by the redistribution between high and low oxygen-affinity tertiary T states induced by ligand binding.
A model proposed by Rivetti et al. (1993a) suggests that T-state tertiary conformations endowed with different oxygen affinities might be associated to the presence or absence of salt bridges. The results here reported show that the extent of Bohr effect is higher for the high-affinity tertiary T- state and almost absent for the low-affinity tertiary T-state. This indicates that oxygenation causes more Bohr effect–linked salt bridges to be broken in the high-affinity than in the low-affinity T-state. Moreover, the salt bridges that constrain the low-affinity T-state do not appear to be involved in the Bohr effect. Absence of Bohr effect was also observed for the T-state conformation of hemoglobin in the crystal (Rivetti et al. 1993a), in keeping with the crystallographic evidence of intact salt bridges even in a fully liganded state (Arnone et al. 1986; Luisi and Shibayama 1989; Luisi et al. 1990; Abraham et al. 1992; Liddington et al. 1992; Paoli et al. 1996). In solution, the K1 in the absence and presence of allosteric effectors at pH 7.6 and 7.0, respectively (Poyart et al. 1978), differs by a factor of 13. The same value is found comparing the p50 for the high-affinity state of hemoglobin gels at pH 7.6 with the p50 for the low-affinity state at pH 7.0. We propose that the tertiary Bohr effect in solution is caused by the sum of two contributions: the pH-dependent interconversion of the low- and high-affinity states, accounting for a 10-fold difference of p50, and the intrinsic tertiary Bohr effect of the high and low oxygen-affinity states, accounting for a 1.3-fold difference (Poyart et al. 1978).
When encapsulation is performed under conditions that do not lead to the predominant accumulation of either one or the other tertiary conformation, as in the presence of the weak allosteric effector phosphate, a mixture of high- and low-affinity T-states is fixed, leading to binding curves that show Hill coefficients much lower than unity and a Bohr effect that is the result of the Bohr effect of the individual components scaled by their relative distribution.
Our study shows the existence of two discrete conformations within the T quaternary state of hemoglobin and provides the experimental procedure to isolate them. Spectroscopic studies, such as ultraviolet resonance raman of hemoglobin gels (Das et al. 1999; Juczczak and Friedman 1999), will be performed to characterize the low- and high-affinity states of hemoglobin and to determine the structural changes responsible for the 10-fold difference of oxygen affinity.
Materials and methods
Chemicals and buffers
Tetramethyl orthosilicate, potassium phosphate, sodium dithionite, HEPES, Bis-Tris, Tris, sodium chloride, IHP, and bezafibrate were of the best commercially available quality and were used without further purification. All gases were research grade.
Hemoglobin purification and encapsulation
Human hemoglobin was purified as previously described (Rivetti et al. 1993a). Encapsulation of deoxy-hemoglobin in silica gels was performed using the following protocols.
Protocol 1
A solution containing 10 mM HEPES and 1 mM EDTA (pH 6.2) was added to an equal volume of tetramethyl orthosilicate and vortexed for a few minutes. The mixture was then deoxygenated by bubbling nitrogen for 20 min. A solution containing 2.7% (w/v) deoxy-hemoglobin A, 50 mM HEPES, and 1 mM EDTA (pH 6.2) was added. Gelification occurs in ∼1 h at room temperature. When the gel was formed, a solution containing 100 mM HEPES, 30 mM sodium dithionite, and 1 mM EDTA (pH 7.0) was layered on it.
Protocol 2
The encapsulation was performed following the procedure of Bettati and Mozzarelli (1997) with some modifications. A solution containing tetramethyl orthosilicate, water, and hydrochloric acid was sonicated for 20 min. An equal volume of a deoxygenated solution containing 10 mM potassium phosphate and 1 mM EDTA and the allosteric effectors 2 mM IHP (pH 6) was then added. The mixture was deoxygenated by bubbling nitrogen for 30 min. Finally, a solution containing 2.7% (w/v) deoxy-hemoglobin A, 50 mM potassium phosphate, 30 mM sodium dithionite, 1 mM EDTA, 2 mM IHP, and 2 mM bezafibrate (pH 7.2) was anaerobically added to the mixture. The gelification occured in a few minutes at 4°C. When the gel was formed, a solution containing 100 mM phosphate, 30 mM sodium dithionite, 1 mM EDTA, 2 mM IHP, and 2 mM bezafibrate (pH 7.0) was layered on it.
Protocol 3
The procedure is the same as protocol 2. The only difference is that none of the solutions contained the allosteric effectors IHP and bezafibrate.
Oxygen binding measurements
Before measurements, silica gels were washed under anaerobic conditions once with the storing buffer containing 30 mM dithionite (pH 7) and six times with a deoxygenated buffer solution at a defined pH between 6.5 and 7.8. Exposure of gels to higher pHs leads to slow hemoglobin release. Gels were anaerobically loaded in a Dvorak-Stotler flow cell (Dvorak and Stotler 1971) covered with a gas-permeable silicon copolymer membrane. The flow cell was mounted on the thermostated stage of a Zeiss MPM03 microspectrophotometer (Rivetti et al. 1993a; Bettati and Mozzarelli 1997). Silica gels are optically isotropic and transparent. Unpolarized light was used, and the absorbtion was measured through the gel and the surrounding solution. Spectra were recorded in the wavelength range 450 to 700 nm at 1-nm intervals using gels that absorb usually less than one optical density unit.
Oxygen pressures between 0 and 760 torr were prepared by mixing oxygen and helium with a gas mixture generator (Environics, series 200). The gas mixture, humidified by bubbling through a solution containing 100 mM phosphate buffer, was flowed into the sample cell and then to a polarographic oxygen meter. The oxygen electrode was calibrated with three ceied oxygen gas mixtures—1%, 5%, and 21%—and pure oxygen. Experiments were performed by first exposing hemoglobin gels to helium and then to a defined oxygen pressure. Spectra were recorded for several hours to monitor both the equilibration with oxygen, which is usually complete within 30 min, and a very slow successive process that has been attributed to the T-to-R quaternary transition (Bettati and Mozzarelli 1997). To minimize the formation of oxidized hemoglobin and the T-to-R transition, measurements were performed at 15°C and on a new sample at each oxygen pressure. Under these conditions, <5% hemes are oxidized and the quaternary transition shows a lifetime of >10 h (C. Viappiani, G. Abbruzzetti, S. Bruno, and A. Mozzarelli, unpubl.).
Data analysis
Fractional saturation with oxygen and fractional concentration of oxidized hemes were determined by fitting each individual spectrum to a linear combination of deoxy, oxy, and oxidized hemoglobin spectra (reference spectra; Rivetti et al. 1993a; Bettati and Mozzarelli 1997), recorded in solution, plus a baseline and a slope to take into account the nonperfect optical quality of the gel surface. This procedure provides a more precise determination of the binding curve with respect to single wavelength measurements.
Acknowledgments
This work was supported by grants from the Italian National Research Council (98.01117.CT14), the Italian Ministry of Instruction, University and Research; and the Italian National Institute for the Physics of Matter. Support was also provided by National Institutes of Health grant 1 P01 GM58890 from the United States Public Health Service.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.20501.
References
- Abraham, D.J., Peascoe, R.A., Randad, R.S., and Panikker, J. 1992. X-ray diffraction study of di-ligated and tetra-ligated T-state hemoglobin from high salt crystals. J. Mol. Biol. 227 480–492. [DOI] [PubMed] [Google Scholar]
- Ackers, G.K., Doyle, M.L., Myers, D.M., and Daugherty, M.A. 1992. Molecular code for cooperativity in hemoglobin. Science 255 54–63. [DOI] [PubMed] [Google Scholar]
- Ackers, G.K., Holt, J.M., Huang, Y., Grinkova, Y., Klinger, A.L., and Denisov, I. 2000. Confirmation of a unique intra-dimer cooperativity in the human hemoglobin α1β2 half-oxygenated intermediate supports the symmetry rule model of allosteric regulation. Proteins 4 23–43. [DOI] [PubMed] [Google Scholar]
- Antonini, E. and Brunori, M. 1971. Hemoglobin and myoglobin in their reactions with ligands. North-Holland Publishing Co., Amsterdam.
- Arnone, A., Rogers, P., Blough, N., McGourty, J., and Hoffman, B. 1986. X-ray diffraction studies of a partially liganded hemoglobin, [α(FeII-CO)β(MnII)]2. J. Mol. Biol. 188 693–706. [DOI] [PubMed] [Google Scholar]
- Baldwin, J. and Chothia, C. 1979. Hemoglobin: The structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 129 175–220 [DOI] [PubMed] [Google Scholar]
- Bettati, S. and Mozzarelli, A. 1997. T state hemoglobin binds oxygen noncooperatively with allosteric effects of protons, inositol hexaphosphate, and chloride. J. Biol. Chem. 272 32050–32055. [DOI] [PubMed] [Google Scholar]
- Bettati, S., Mozzarelli, A., Rivetti, C., Rossi, G.L., Tsuneshige, A., Yonetani, T., Eaton, W.A., and Henry, E.R. 1996. Oxygen binding by single crystals of hemoglobin: The problem of cooperativity and inequivalence of alpha and beta subunits. Proteins 25 425–437. [DOI] [PubMed] [Google Scholar]
- Bettati, S., Kwiatkowski, L.D., Kavanaugh, J.S., Mozzarelli, A., Arnone, A., Rossi, G.L., and Noble, R.W. 1997. Structure and oxygen affinity of crystalline desHis146β human hemoglobin in the T state. J. Biol. Chem. 272 33077–33084. [DOI] [PubMed] [Google Scholar]
- Bettati, S., Mozzarelli, A., and Perutz, M.F. 1998. Allosteric mechanism of haemoglobin: Rupture of salt-bridges raises the oxygen affinity of the T structure. J. Mol. Biol. 281 581–585. [DOI] [PubMed] [Google Scholar]
- Bruno, S., Bettati, S., Manfredini, M., Mozzarelli, A., Bolognesi, M., Deriu, D., Rosano, C., Tsuneshige, A., Yonetani, T., and Henry, E.R. 2000. Oxygen binding by α(Fe2+)2β(Ni2+)2 hemoglobin crystals. Protein Sci. 9 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski, A., Derewenda, Z., Dodson, E., Dodson, G., Grabowski, M., Liddington, R., Skarzynski, T., and Vallely, D. 1984. Binding of molecular oxygen to T state human haemoglobin. Nature 307 74–76. [DOI] [PubMed] [Google Scholar]
- Das, T.K., Khan, I., Rousseau, D.L., and Friedman, J.M. 1999. Temperature dependent quaternary relaxation in sol-gel encapsulated hemoglobin. Biospectroscopy 5 S64—S70. [DOI] [PubMed] [Google Scholar]
- Dvorak, J.A. and Stotler, W.F. 1971. A controlled-environment culture system for high resolution light microscopy. Exp. Cell. Res. 68 144–148. [DOI] [PubMed] [Google Scholar]
- Eaton, W.A., Henry, E.R., Hofrichter, J., and Mozzarelli, A. 1999. Is cooperative oxygen binding by hemoglobin really understood? Nat. Struct. Biol. 6 351–358. [DOI] [PubMed] [Google Scholar]
- Edelstein, S.J. 1975. Cooperative interactions of hemoglobin. Annu. Rev. Biochem. 44 209–232. [DOI] [PubMed] [Google Scholar]
- Imai, K. 1982. Allosteric effects in hemoglobin. Cambridge University Press, Cambridge.
- Juszczak, L.J. and Friedman, J.M. 1999. UV resonance Raman spectra of ligand binding intermediates of sol-gel encapsulated hemoglobin. J. Biol. Chem. 274 30357–30360. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, J.S., Chafin, D.R., Arnone, A., Mozzarelli, A., Rivetti, C., Rossi, G.L., Kwiatkowski, L.D., and Noble, R.W. 1995. Structure and oxygen affinity of crystalline desArg141α human hemoglobin A in the T state. J. Mol. Biol. 248 136–150. [DOI] [PubMed] [Google Scholar]
- Khan, I., Shannan, C.F., Dantsker, D., Friedman, A.J., Gonzales-de-Apodeca, J.P., and Friedman, J.M. 2000. Sol-gel trapping of functional intermediates of hemoglobin: Geminate and bimolecular recombination studies. Biochemistry 39 16099–16109. [DOI] [PubMed] [Google Scholar]
- Koshland, D.E., Nemethy, G., and Filmer, D. 1966. Comparison of experimental binding data and theoretical methods in proteins containing subunits. Biochemistry 5 365–385. [DOI] [PubMed] [Google Scholar]
- Lalezari, I., Lalezari, P., Poyart, C., Marden, M., Kister, J., Bohn, B., Fermi, G., and Perutz, M.F. 1990. New effectors of human hemoglobin: Structure and function. Biochemistry 29 365–385. [DOI] [PubMed] [Google Scholar]
- Lee, A., Karplus, M., Poyart, C., and Bursaux, E. 1988. Analysis of proton release in oxygen binding by hemoglobin: Implications for the cooperative mechanism. Biochemistry 27 1285–1301. [DOI] [PubMed] [Google Scholar]
- Liddington, R., Derewenda, Z., Dodson, G., and Harris, D. 1988. Structure of the liganded T state of haemoglobin ideies the origin of cooperative oxygen binding. Nature 331 725–728. [DOI] [PubMed] [Google Scholar]
- Liddington, R., Derewenda, Z., Dodson, E., Hubbard, R., and Dodson, G. 1992. High-resolution crystal-structures and comparisons of T-state deoxyhaemoglobin and two liganded T-state haemoglobins: T(α-oxy)haemoglobin and T(met)haemoglobin. J. Mol. Biol. 228 551–579. [DOI] [PubMed] [Google Scholar]
- Lindstrom, T.R. and Ho, C. 1972. Functional nonequivalence of α and β hemes in human hemoglobin. Proc. Natl. Acad. Sci. 69 1707–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi, B. and Shibayama, N. 1989. Structure of hemoglobin in the deoxy quaternary state with ligand bound at the α haems. J. Mol. Biol. 206 723–736. [DOI] [PubMed] [Google Scholar]
- Luisi, B., Liddington, R., Fermi, G., and Shibayama, N. 1990. Structure of deoxy-quaternary hemoglobin with liganded β subunits. J. Mol. Biol. 214 7–14. [DOI] [PubMed] [Google Scholar]
- Marden, M.C., Bohn, B., Kister, J., and Poyart, C. 1990. Effectors of hemoglobin: Separation of allosteric and affinity factors. Biophys. J. 57 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, G., Morimoto, H., Yun, K.-M., Park, S.-Y., Nakagawa, A., Minagawa, H., and Shibayama, N. 1999. Magnesium(II) and Zinc(II)-protoporphyrin IX's stabilize the lowest oxygen affinity state of human hemoglobin even more strongly than deoxyheme. J. Mol. Biol. 292 1121–1136. [DOI] [PubMed] [Google Scholar]
- Monod, J., Wyman, J., and Changeux, J.-P. 1965. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12 88–118. [DOI] [PubMed] [Google Scholar]
- Mozzarelli, A., Rivetti, C., Rossi, G.L., Henry, E.R., and Eaton, W.A. 1991. Crystals of hemoglobin with the T quaternary structure bind oxygen noncooperatively with no Bohr effect. Nature 351 416–419. [DOI] [PubMed] [Google Scholar]
- Mozzarelli, A., Rivetti, C., Rossi, G.L., Eaton, W.A., and Henry, E.R. 1997. Allosteric effectors do not alter the oxygen affinity of hemoglobin crystals. Protein Sci. 6 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli, M., Liddington, R., Tame, J., Wilkinson, A., and Dodson, G. 1996. Crystal structure of T state haemoglobin with oxygen bound at all four haems. J. Mol. Biol. 256 775–792. [DOI] [PubMed] [Google Scholar]
- Perrella, M. 1999. Understanding mechanisms in a cooperative protein: The CO ligation intermediates of hemoglobin. Biophys. Chem. 81 157–178. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. 1970. Stereochemistry of cooperative effects in haemoglobin. Nature 228 726–739. [DOI] [PubMed] [Google Scholar]
- ———. 1989. Mechanisms of cooperativity and allosteric regulation in proteins. Quart. Rev. Biophys. 22 139–236. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F., Wilkinson, A., Paoli, M., and Dodson G, 1998. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 27 1–34. [DOI] [PubMed] [Google Scholar]
- Poyart, C.F., Bursaux, E., and Bohn, B. 1978. An estimation of the first binding constant of O2 to human hemoglobin A. Eur. J. Biochem. 87 75–83. [DOI] [PubMed] [Google Scholar]
- Rivetti, C., Mozzarelli, A., Rossi, G.L., Henry, E.R., and Eaton, W,A. 1993a. Oxygen binding by single crystals of hemoglobin. Biochemistry 32 2888–2906. [DOI] [PubMed] [Google Scholar]
- Rivetti, C., Mozzarelli, A., Rossi, G.L., Kwiatkowski, L.D., Wierzba, A., and Noble, R,W. 1993b. Effect of chloride on oxygen binding to crystals of hemoglobin Rothschild (β37 Trp→Arg) in the T quaternary structure. Biochemistry 32 6411–6418. [DOI] [PubMed] [Google Scholar]
- Russo, R., Benazzi, L., and Perrella, M. 2001. The Bohr effect of hemoglobin intermediates and the role of salt bridges in the tertiary/quaternary transitions. J. Biol. Chem. 276 13628–13634. [DOI] [PubMed] [Google Scholar]
- Shibayama, N. 1999. Kinetics of the allosteric transition in hemoglobin within silicate sol-gel. J. Am. Chem. Soc. 121 444–445. [Google Scholar]
- Shibayama, N. and Saigo, S. 1995. Fixation of the quaternary structures of human adult hemoglobin by encapsulation in transparent porous silica gels. J. Mol. Biol. 251 203–209. [DOI] [PubMed] [Google Scholar]
- –––. 1999. Kinetics of the allosteric transition in hemoglobin within silicate sol-gels. J. Am. Chem. Soc. 121 444–445. [Google Scholar]
- –––. 2001. Direct observation of two distinct affinity conformations in the T state human deoxyhemoglobin. FEBS Lett. 492 50–53. [DOI] [PubMed] [Google Scholar]
- Shibayama, N., Morimoto, H. and Kitagawa, T. 1986. Oxygen equilibrium study and light absorption spectra of Ni(II)-Fe(II) hybrid hemoglobins. J. Mol. Biol. 192 322–329. [DOI] [PubMed] [Google Scholar]
- Shulman, R.G., Hopfield, J.J., and Ogawa, S. 1975. Allosteric interpretation of hemoglobin properties. Quart. Rev. Biophys. 8 325–342. [DOI] [PubMed] [Google Scholar]