Abstract
In the presence of a suitable oxidizing agent, the Ni(II) complex of glycyl–glycyl–histidine (GGH) mediates efficient and specific oxidative protein cross-linking. The fusion of GGH to the N terminus of a protein allows for the cross-linking reagent to be delivered in a site-specific fashion, making this system extremely useful for analyzing protein–protein contacts in complicated mixtures of biomolecules. Tyrosine residues have been postulated to be the primary amino acid target of this reaction, and using the dimeric serine protease inhibitor ecotin, we previously demonstrated that engineering a tyrosine at the protein interface of a dimer dramatically increased cross-linking efficiency. Cross-linking increased four-fold for GGH-ecotin D137Y in comparison to wild-type GGH-ecotin, presumably through bityrosine formation at the dimer interface. Here we report the first complete structural analysis of the cross-linked GGH-ecotin D137Y dimer. Using a combination of mass spectrometric and chemical derivatization methods, a sole novel cross-link between the N-terminal glycine residues and the engineered tyrosine at position 137 has been characterized. The dimer cross-link is localized to a single site without other protein modifications, but different reaction pathways produce structurally related products. We propose a mechanism that involves covalent bond formation between the protein backbone and a dopaquinone moiety derived from a specific tyrosine residue. This finding establishes that it is not necessary to have two tyrosine residues within close proximity in the protein interface to obtain high protein cross-linking yields, and suggests that the cross-linking reagent may be of more general utility than previously thought.
Keywords: Mass spectrometry (MS), post-source decay (PSD), ecotin, chemical cross-link, nickel oxo complex, protein oxidation, structure elucidation
The specific recognition of one protein by another plays a central role in mediating biological events (Alberts 1998). A primary step toward understanding how protein machines function is to map out the protein–protein contacts within a complex. Several methods exist to identify protein–protein interactions including immunoprecipitation, the yeast two-hybrid system, phage display, affinity chromatography, and chemical cross-linking (Phizicky and Fields 1995). There are advantages and disadvantages to each of these methods, but one clear benefit of cross-linking is that a covalent bond is formed between the proteins of interest. The resulting protein dimer is thus extremely stable to the harsh conditions that may be required for isolation and identification of proteins involved.
A drawback of conventional cross-linking agents exogenously added to the protein complex of interest is that they often lead to multiple cross-linked species each of low stoichiometry, generating a mixture of products that is difficult or even impossible to identify. Use of affinity cross-linking strategies that involve attachment of an activatable cross-linking agent to a protein avoids this problem, because local concentration of the reactive moiety leads to greater cross-linking efficiency and specificity. Such strategies usually involve post-translational modification of the protein or incorporation of a modified amino acid (Kanamori et al. 1997). To circumvent this type of chemical modification, a strategy has been developed where the protein of interest is fused to a naturally-occurring amino acid tag (GGH or his6) that upon complexation with nickel (II) and in the presence of a strong oxidant mediates protein cross-linking (Brown et al. 1998; Fancy et al. 1996).
The utility of the GGH-Ni(II) system has previously been demonstrated by cross-linking a variety of proteins in the presence of the free tripeptide, nickel (II), and an oxidant. The reaction is specific for proteins that interact in solution. The reactive species is thought to be a high-valent nickel–oxo species that oxidizes electron rich amino acid side chains, leading to the formation of protein cross-links within hydrophobic interfaces (Brown et al. 1998). Subsequent to studies using the free tripeptide, a GGH-fusion protein was prepared using the dimeric macromolecular serine protease inhibitor, ecotin, as a model system. Cross-linking of GGH-ecotin in the presence of nickel (II) and an oxidant results in efficient and specific cross-linking within the ecotin dimer and to target proteases. Mass spectrometric studies of model peptides led to the identification of bityrosyl linked dimer and postulation of a tyrosyl radical cross-linking mechanism. Based on this postulated mechanism, a tyrosine residue was introduced at the ecotin dimer interface through mutagenesis (Brown et al. 1998). The GGH-ecotin D137Y mutant increased the cross-linking yield to 60% as compared to the wild-type GGH-ecotin yield of 15%.
In recent years, several oxidative protein cross-linking strategies have been described (Campbell et al. 1998; Fancy and Kodadek 1999; Kim et al. 1999). A common theme to all these systems is generation of a high valent metal species upon addition of oxidant that in turn oxidizes protein residues, generating radicals that react to form covalent cross-linked adducts. As noted above, the proposed mechanism is cross-linking via bityrosyl or tyrosine–nucleophile adduct formation. Bityrosyl adducts resulting from oxidative cross-linking have previously been identified in model peptides by mass spectrometry or their characteristic fluorescence (Brown et al. 1998; Fancy and Kodadek 1998). There are a few examples of the identification of bityrosyl adducts in cross-linked proteins as well, detected by bityrosyl fluorescence of protease digests (Tew and Ortiz de Montellano 1988) or HPLC analysis of the acid hydrolysis products of the proteins (Fancy and Kodadek 1998; Gill et al. 1997). In many of the cross-linking systems mentioned above, the product(s) for the cross-linking reaction is only postulated and not actually identified. The use of bityrosyl fluorescence or retention time allows identification of some important reaction products, but products other than bityrosyls are overlooked. Thus, while other possible cross-linked products have been proposed, they have not yet been detected or characterized. Elucidation of the nature of the reaction mechanism will provide knowledge essential for the consistent attainment of high cross-linking yields using oxidative cross-linking strategies.
Herein, we report structural characterization of the protein–protein cross-link mediated by a GGH-fusion protein–nickel (II)–oxidant system utilizing GGH-ecotin D137Y. The nature of the cross-linking reaction on the fusion protein dimer was established using a combination of protease digestion, chemical derivatization, and mass spectrometry. For the first time, the major cross-linked species in a GGH-fusion protein covalent dimer has been identified. Identification of this novel cross-linked structure reveals that GGH-mediated cross-linking does not necessarily proceed via bityrosyl formation in the context of a protein complex's quaternary structure.
Results
GGH-Ni(II)–MMPP cross-linking reaction produces only cross-linked protein
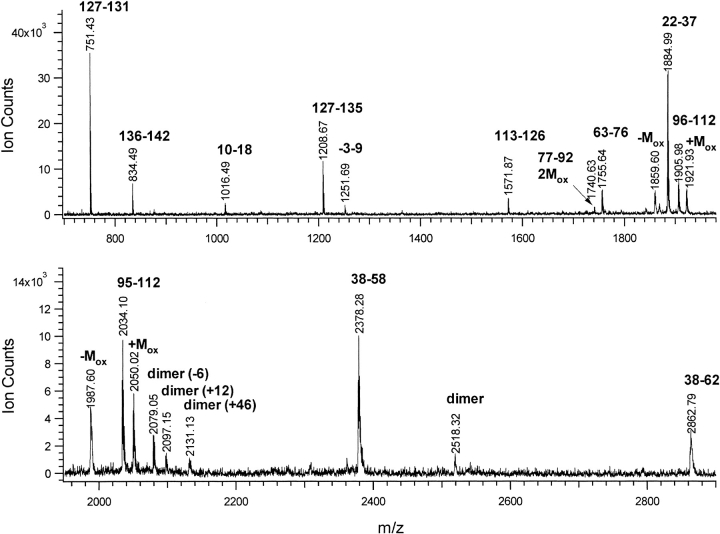
The products of the GGH-ecotin D137Y cross-linking reaction were separated by SDS-PAGE. Monomer and dimer protein bands were formed. Each band was individually extracted from the gel and then digested separately using the endoproteinase Lys-C. The MALDI-TOF spectrum of the Lys-C digest for the pure dimer band is shown in Figure 1 ▶. The peptide fragments were assigned according to their expected mass values from Lys-C digestion, yielding 97% coverage of the entire protein. Combining this data with an analogous spectrum obtained from the Asp-N digestion of the protein provided 100% coverage (data not shown). In addition, these spectra revealed a set of related modified peptides as well as the usual partial oxidation of the methionine residues. The presence of methionine sulfoxide is commonly observed following gel electrophoresis. Signals for peptides containing a single partially oxidized methionine residue will appear in the mass spectrum at mass values corresponding to both the protonated non-oxidized molecular weight (MH+) and that shifted by +16 u mass (+Mox). In addition, there is usually a broader, poorly resolved peak (metastable ion) at a mass value of about –60 u below the [M+16]H+ (Mox) resulting from dissociation of the methyl sulfoxide moiety during acceleration in the ion source. Another common chemical modification is alkylation of cysteine residues by acrylamide during gel electrophoresis (Hall et al. 1993). From comprehensive mass spectrometric analyses of the dimeric band, this study has established that this cross-linking reaction did not modify any of the amino acids in the entire protein that are not directly involved in formation of the single cross-link itself.
Fig. 1.
MALDI-TOF spectrum of Lys-C digest of GGH-ecotin D137Y dimer. Peaks are labeled with amino acid residue numbers. Methionine containing peptides are labeled additionally for sulfoxide (+Mox) or loss of methylsulfoxide (-Mox). Peptides not seen in monomer digest are labeled as dimer.
Identification of cross-linked products at a single GGH-ecotin D137Y dimer cross-linking site using multiple protease digestions coupled with mass spectrometry
As noted above, several related peaks were observed in the MALDI spectrum of the GGH-ecotin D137Y dimer which could not be assigned to unmodified Lys-C digest fragments. Specifically, four peaks having m/z values of 2079, 2097, 2131, and 2518 were observed in the digest of the cross-linked dimer band that were not present in the analogous monomer band, and thus resulted from intermolecular cross-linking. None of these mass values corresponds to the addition of any two ecotin digest fragment masses minus two mass units, as would be expected for a simple bityrosine cross-linking reaction.
The initial Lys-C digest mixture was separated by microbore reversed phase HPLC using MALDI-MS for further analysis of the reaction products and identification of fractions containing cross-linked peptides. Multiple protease digestions of these putative cross-linked peptide components were used together with tandem mass spectrometry to identify the peptides involved in the cross-link; the results of these analyses are shown in Table 1. These four "dimer-associated" peaks at m/z 2079, 2097, 2131, and 2518 eluted within a gradient window of 2%, suggesting they contain related peptide sequences.
Table 1.
Covalently linked peptides from dimeric protein cleaved by multiple enzymatic digests
| (M1 + M2)H+exp | |||||
| Enzyme | M1 | M2 | δ = −6 | δ = +12 | δ = +46 |
| Lys-C | 1250.6 GGHAESVQPLEK (−3 −9) | 833.5 IYNAVVR (136–142) | 2079.1 | 2097.1 | 2131.1 |
| Lys-C + Glu-C | 469.2 GGHAE (−3 −2) | 833.5 IYNAVVR (136–142) | 1297.6 | 1315.6 | 1349.6 |
| Lys-C + Glu-C + Aminopeptidase M | 469.2 GGHAE (−3 −2) | 620.4 YNAVVR (137–142) | 1184.6 | 1202.6 | |
| Lys-C + Glu-C + Carboxypeptidase A | 469.2 GGHAE (−3 −2) | 294.2 IY (136–137) | 758.3 | 776.3 | |
Table shows the observed covalently linked peptide masses following digestion by the enzymes specified. The Lys-C digest was performed on the protein dimer, and subsequent digests were made on isolated dimer peptides. M1 masses are calculated from the N-terminal sequence, and M2 masses are derived from the C-terminal sequence. δ is the mass change upon dimerization for three different species observed. Ecotin protein starts at A and is numbered residue 1; the GGH sequence thus begins at position −3.
Digestion of the components with m/z 2079, 2097, and 2131 with Glu-C resulted in the loss of 781.5 mass units for each peptide. This corresponds to hydrolysis of a fragment with the sequence SVQPLEK (amino acid residues 3–9). Subsequent digestion with aminopeptidase M reduced the mass by 113 u corresponding to the loss of an isoluecine/leucine residue. The loss of only one amino acid from the aminopeptidase M treatment suggested the possibility that one of the N termini of the peptide cross-link was blocked. Subsequently, a fraction of the Lys-C and Glu-C peptide digest was subjected to digestion with carboxypeptidase A, resulting in a maximal loss of 539.3 mass units, which corresponds to the hydrolytic removal of NAVVR.
Comparison of all possible pairs of digest peptides with the mass values of the dimer peptides from the multiple digestions established the location of the cross-link within the ecotin dimer. The three peaks at m/z 2079, 2097, and 2131 are derived from dimer-derived peptides with a cross-link between GGHAESVQPLEK(–3–9) at the N terminus of one monomer and IYNAVVR(136–142) at the C terminus of the other monomer. The resultant cross-linked digest products correspond to the calculated mass value of the two peptides, GGHAESVQPLEK(–3–9) and IYNAVVR(136–142), with consistent mass differences of –6, +12, and +46 mass units, respectively. Glu-C digestion removed the SVQPLEK portion of the sequence, carboxypeptidase A removed the NAVVR portion, and aminopeptidase M removed the N-terminal I from the dimer peptides. No N-terminal digestion of the GGHAE peptide was detected, suggesting that the N terminus of this peptide may be blocked. Combining the results from these digests limited the site of the cross-link to the sequence GGHAE(–3–2) and residue Y(137). This data indicated that the cross-linked moiety does indeed involve the D137Y mutation, but is not in the form of a Y–Y cross-link. No evidence for a Y–Y cross-linked peptide was observed in the analyses.
The most intense dimer-derived peak represents a loss of 6 u from the mass combination of the two separate peptides involved (Table 1). The 6 u mass loss can be explained by either a loss of six protons or by an addition/elimination reaction at one protein's N terminus coupled to tyrosine cross-linking to the dimer partner. Calibrated MALDI mass measurements of the m/z 758 and 776 peptides, accurate to 15 ppm, support the former interpretation. The measured mass of 758.3036 is 9.7 ppm from a theoretical mass of 758.3109 for loss of six hydrogen atoms from the accurate mass value of peptides GGHAE and IY. The experimental mass of 776.3154 is 7.9 ppm from the calculated mass of 776.3215 for the loss of four hydrogen atoms and addition of one oxygen atom. The difference in mass between the two peaks is 18.0118, attributable only to the composition of H2O within the accuracy of the measurement. Furthermore, in a mixture of these two species, the amount of +12 u form was seen to increase when the peptides were incubated overnight at room temperature in aqueous solutions, pH 10 (data not shown). This interconversion between the two species suggested the reversible addition of water.
The third cross-link component (+46 u) peak showed an addition of 34 u to the hydrated species. The distinctive isotope pattern seen in the MALDI spectrum of m/z 1349 was characteristic of a mono–chloro containing peptide, with enhancement of the third isotope peak due to the abundant 37Cl isotope. Interestingly, no chloro- addition was seen for the primary dimer (–6 u) form. Chloro addition was seen to a much weaker extent on some of the Y containing monomer peptides. The source of the chloro-substituent is presumed to be the sodium chloride in the reaction buffer.
The HPLC fraction containing the 2518 peptide was subject only to preliminary MALDI–PSD analysis. The PSD spectrum contained y sequence ions showing the presence of NAVVR and higher mass b sequence ions corresponding to the peptide GGHAESVQPLEK, confirming participation of the same cross-link residues as those observed for 2079, 2097, and 2131 described in detail above.
As expected, MALDI spectra of the HPLC separated digest peptides revealed peptides not detected in the unseparated digest spectrum. The most prominent new peptides were identified as missed or nonspecific enzyme cleavages, and minor amounts of chloro-substitution of non-cross-linked peptides. In the fractions containing the 2079, 2097, 2131, and 2518 peaks were a number of peptides of weak intensity with masses close to the dominant peaks. Comparison of the MALDI spectra from HPLC fractionation of digests of pure oxidized monomer versus pure dimer did not reveal any significant differences besides the peptides described above.
MALDI-PSD determination of the amino acids involved in the cross-linking reaction
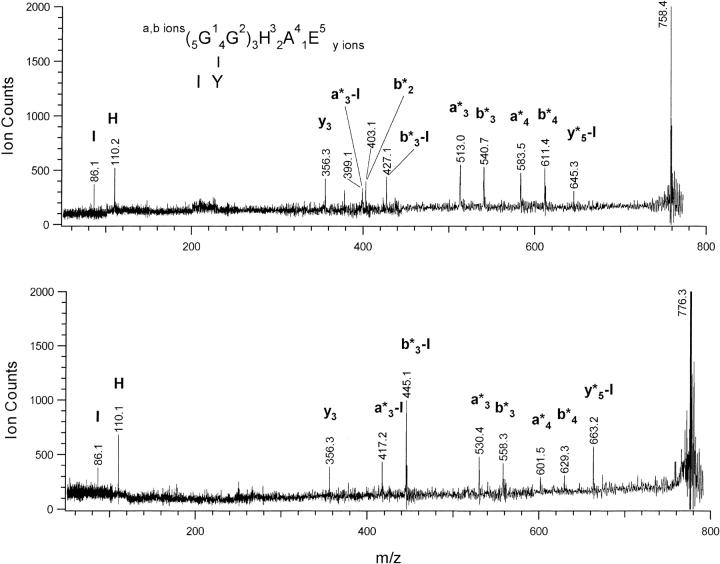
MALDI–PSD spectra of the smallest dimer-derived peptides produced via multiple protease digestion were used to establish the location of the cross-link (Fig. 2 ▶, Table 2). The peptides at m/z 758 and 776 form the cross-link moiety between the sequences GGHAE(–3–2) of one protein and IY(136–137) of the other, with 758 representing the –6 u species and 776 the +12 u species. To determine which specific amino acids are cross-linked, sequence analysis of the cross-linked peptides was carried out using MALDI-PSD. The ions most commonly observed in this technique are a, b, y, and immonium ions, with consecutive members of an ion series giving sequence information on the peptide. The a and b ions retain the charge at the N terminus while y ions retain charge at the C terminus and immonium ions are derived from individual amino acids in the peptide (Medzihradszky and Burlingame 1994; Stimson et al. 1997). The mass values of the sequence ions in a dimer-derived peptide will depend of the location and nature of the cross-link, as the cross-linked sequence ions will be shifted by the mass of IY (294 u) and either –6 u or +12 u. In these spectra the fragment ions containing the cross-link to IY are designated as a*, b*, or y*, and numbered according to position along the GGHAE sequence. The doubly fragmented peaks still containing the cross-link to Y after loss of isoleucine are designated as ion type *-I. Individual amino acids directly participating in the cross-link will no longer be found at their characteristic immonium ion mass values. Assignments are aided by comparison of the m/z 776 and 758 spectra because fragment ions containing the cross-link are shifted by +18 mass units from one spectrum to the other. Based on the multiple protease digestions, it is clear that only the D137Y residue is involved in the cross-link on one side of the cross-linked peptide. The PSD spectra allow identification of the residue(s) in the GGHAE peptide that participate in the cross-link on the other side and confirm the role of the tyrosine.
Fig. 2.
The figure shows the MALDI-PSD spectra of the dimer derived from a cross-link between the GGHAE peptide and the IY peptide. The upper panel is the –6u 758.4 peptide and the lower panel is the +12u 776.3 peptide. a, b, y, and immonium ions are labeled as for the GGHAE peptide, and those containing the cross-link to the IY peptide are denoted with an asterisk. From the fragment masses it is proven that the cross-link occurs between the Y and the GG residues (See text for details.).
Table 2.
MALDI-PSD fragment ions detected for the GGHAE-IY dimer peptides at 758 and 776 u
| m/z | 758 fragments | 776 fragments |
| 86.1 | I immonium | I immonium |
| 110.1 | H immonium | H immonium |
| 356.3 | y3(HAE) | y3(HAE) |
| 378.2 | y3Na? | y3Na? |
| 399.1 | a*3(GGH-Y) | |
| 403.1 | b*2(GG-IY) | |
| 417.2 | a*3(GGH-Y) | |
| 427.1 | b*3(GGH-Y) | |
| 445.1 | b*3(GGH-Y) | |
| 513.0 | a*3 (GGH-IY) | |
| 530.4 | a*3 (GGH-IY) | |
| 540.7 | b*3 (GGH-IY) | |
| 558.3 | b*3 (GGH-IY) | |
| 583.5 | a*4 (GGHA-IY) | |
| 601.5 | a*4 (GGHA-IY) | |
| 611.4 | b*4 (GGHA-IY) | |
| 629.3 | b*4 (GGHA-IY) | |
| 645.3 | y*5(GGHAE-Y) | |
| 663.2 | y*5(GGHAE-Y) |
Ions are numbered according to conventional notation for GGHAE sequence, with * indicating presence of cross-link to either Y or IY. Ions in bold prove only GG residues participate in N terminal portion of crosslink, ions in italics prove only Y residue participates in C-terminal portion of crosslink. See text for details.
In both spectra shown in Figure 2 ▶, the a*3, a*3-I, b*3, b*3-I, a*4, and b*4 fragment ions retain the cross-link, demonstrating that the link occurs somewhere in the GGH partial sequence. The b*2 ion in the m/z 758 spectrum further localizes the cross-link on the GG sequence. The presence of the unmodified y3 (HAE) ion at m/z 356 in both spectra and the strong unmodified H immonium ion at m/z 110 establishes that these residues are not involved. The many cross-linked fragments showing loss of I and the presence of an unmodified I immonium ion provide additional evidence that the Y residue alone is involved in the C-terminal peptide. The only other immonium ion that would normally be present is the Y immonium ion, which is notably absent in these spectra. The spectra demonstrate localization of the cross-link on the Y residue of one peptide and the N-terminal glycine residues on the other peptide. It is unlikely that only a single glycine residue is involved, as the 6 u loss upon reaction suggests multiple bond formation. All PSD ions contain either both glycine residues or none, suggesting they are both modified in the cross-linking reaction, though the low mass region is devoid of the ions needed to conclusively establish that both glycine residues participate in the cross-link. MALDI–PSD data show clearly that the cross-link occurs between the N-terminal glycine residues of one protein and the D137Y mutation of the other protein, with no other amino acids involved.
Deletion of GGH from GGH-ecotin D137Y confirms participation of the tag in the cross-link
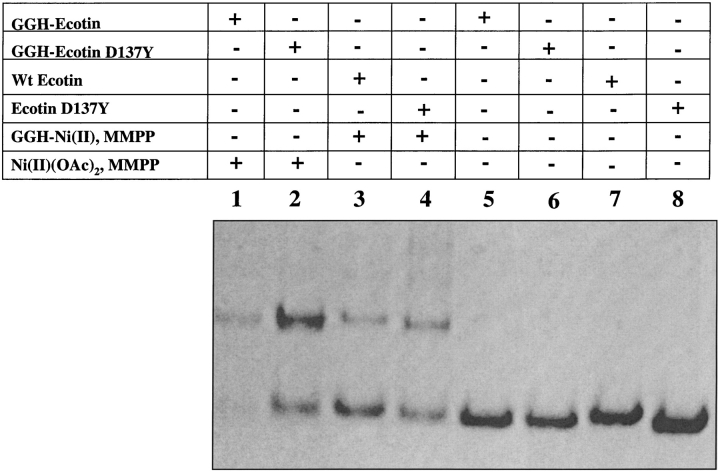
Further confirmation that the dominant cross-linking pathway for GGH-ecotin D137Y dimer involves the first two N-terminal glycine residues is obtained by deletion of the GGH tag. The ecotin mutant ecotin D137Y was expressed and purified. A comparison of the cross-linking efficiencies of ecotin and ecotin D137Y in the presence of the GGH-Ni(II) complex was carried out (Fig. 3 ▶). As seen by Coomassie brilliant blue staining, there is an enhanced cross-linking efficiency for the ecotin D137Y mutant relative to wild-type ecotin by approximately 1.5 times. However, this is significantly less than the four-fold increase observed for the fusion protein GGH-ecotin D137Y over GGH-ecotin (see Fig. 6 in Brown et al. 1998).
Fig. 3.
Comparison of the cross-linking efficiencies for wild-type ecotin and ecotin D137Y without the GGH tag. Lane 1: 20 μM GGH-ecotin, 100 μM Ni(OAc)2, and 100 μM MMPP. Lane 2: 20 μM GGH-ecotin D137Y, 100 μM Ni(OAc)2, and 100 μM MMPP. Lane 3: 20 μM wt-ecotin, 100 μM GGH-Ni(II), and 100 μM MMPP. Lane 4: 20 μM ecotin D137Y, 100 μM GGH-Ni(II), and 100 μM MMPP. Lane 5: 20 μM GGH-ecotin. Lane 6: 20 μM GGH-ecotin D137Y. Lane 7: 20 μM wt-ecotin. Lane 8: 20 μM ecotin D137Y.
Cross-link reaction pathways studied by Edman sequencing, acetylation, and MALDI
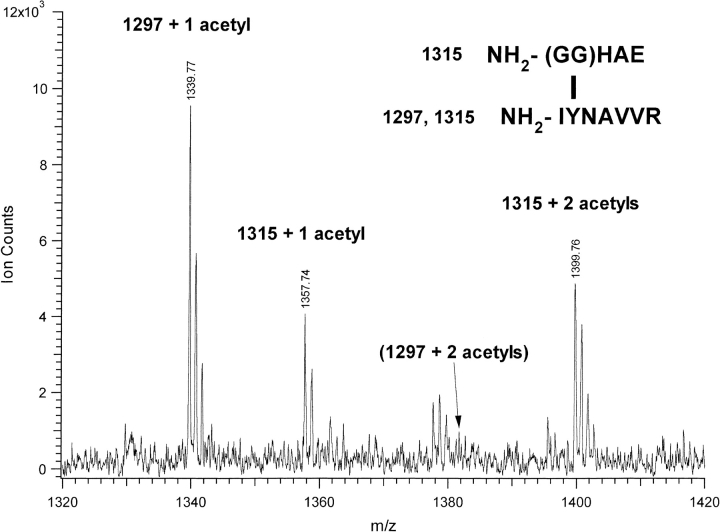
As mentioned previously, aminopeptidase M digestion removed only an isoleucine residue from IYNAVVR, while no cleavage activity occurred on the GGHAE moiety. This indicates that the N terminus of GGHAE is blocked or modified. This interpretation was further substantiated by N-terminal sequencing of the –6 u peptide species. Edman degradation yielded the sequence IXNAVV where X represents a non-natural amino acid. This confirms the participation of the Tyr residue. There is no signal due to the GGHAE N terminus or a truncated form derived from that sequence indicating that the N-terminus derived from that peptide is blocked. Further evidence of N terminus modification comes from the acetylation pattern of the dimer-derived peptides. The dimer-derived peptides at m/z 1297 and 1315, containing GGHAE cross-linked to IYNAVVR, were treated with acetic anhydride and analyzed by MALDI to determine the number of free amino functions within the peptide. Addition of only one acetyl group (+42) was observed for the 1297 peptide which shifted to mass 1339 (Fig. 4 ▶). The m/z 1315 peptide showed addition of one or two acetyl groups, indicating the presence of two free amino functions, with peaks at 1357 and 1399. One acetyl group is added to the free N-terminal amine of the isoleucine as determined by MALDI-PSD on the 1339 peak. The second acetylation site on the 1315 peptide is presumed to be the N-terminal amine of glycine, which is blocked in the 1297 peptide. This is further evidence that the N-terminal glycine has been modified in the –6 u form of the dimer, and that the addition of water to form the +12 u species liberates the N terminus.
Fig. 4.
MALDI spectrum of 1297 and 1315 u GGHAEIYNAVVR dimer peptides after treatment with acetic anhydride. 1297 peak is shifted to 1339 due to single acetylation, 1315 shifted to 1357 and 1399 due to single and double acetylation. Location for double acetylation peak of 1297 is marked. Free amines on peptides N termini, capable of acetylation shown in upper right inset.
Model peptide monomer displaying intramolecular cross-link used in deuteration studies
A model peptide was designed to include both the N-terminal GGH and a tyrosine residue, with the sequence NH2-GGHSFASYSRAF-NH2. The cross-linking reaction led to the formation of a bityrosyl dimer at 2568 u and a number of modified monomeric products. One pair of monomeric products displayed the –6 u/+12 u pairing seen in the protein dimer-derived components described above. This product pair was analyzed by MALDI–PSD and identified as an intramolecular GG-Y cross-link, analogous to the protein dimer cross-link (data not shown). Deuteration studies were conducted to measure the number of exchangeable hydrogens on the reaction products. The exchangeable hydrogens on amines, amides, alcohols, and acids are replaced with deuterons in the D2O buffer, and each contributes an additional 1 amu to the peptide mass. ESI mass measurements on the –6 u/+12 u peptides show loss of exchangeable protons for both reaction products in comparison to the synthetic peptide (Fig. 5 ▶). The composite isotope ratios for C, N, O, and non-exchangeable H were convoluted with variable levels of deuteration to match the experimental isotope pattern for the doubly charged peptide. Fig. 4A ▶ shows the experimental spectra for the deuterated synthetic peptide and the best theoretical fit. Fig. 4B ▶ shows the double charged peptide pair –6 u/+12 u after deuteration and the best theoretical fit. The pattern of deuteration used to generate the best fits is shown in Fig. 4C ▶. While the deuteration is incomplete, the maximum level of deuteration (last non-zero value) differs for the three peptides. The deuterated synthetic peptide had a maximum of 24 deuterons added, the same as the maximum number of exchangeable protons. The hydrated (+12 u) species had one less exchangeable hydrogen than the unmodified peptide, i.e., 23 deuterons maximum. For the primary dimer product, the –6u species, there is a loss of two additional exchangeable protons, with a maximum of 21 deuterons, consistent with the loss of a free amine. These results concur with the acetylation data.
Fig. 5.
Deuteration of the synthetic peptide GGHSFASYSRAFNH2 (monoisotopic mw 1284.6). (A) and (B) show experimental spectra of doubly charged peptides and theoretical fit for deuteration at levels shown in (C). (A) shows synthetic peptide and (B) shows intramolecularly cross-linked peptide pair – 6 u/+12 u. (C) shows deuteration pattern used for theoretical fits for the synthetic peptide (line), –6 u peptide (dashed line) and +12 u peptide (dotted line). Experimental error of +/−10% applies to data in (C).
Discussion
In the presence of an oxidant such as monoperoxyphthalic acid, the complex between the tripeptide gly–gly–his and Ni(II) mediates cross-linking between proteins that interact in solution (Brown et al. 1995). Previously, this tripeptide was expressed at the N terminus of the dimeric macromolecular serine protease inhibitor, ecotin, resulting in a fusion protein that functions as an efficient and specific cross-linking agent in the presence of Ni(II) and oxidant (Brown et al. 1998). The ecotin dimer complex was chosen as a model system for studying the characteristics of cross-linking mediated by a gly–gly–his fusion protein because it has been well characterized from a biochemical and structural standpoint (Yang et al. 1998). Herein we report the structural identification of the novel inter-protein cross-link mediated by this GGH-fusion protein. The results are discussed with a focus on addressing the issue of the cross-linking reaction mechanism in a protein context.
The chemical mechanism by which the gly–gly–his–Ni(II) complex mediates cross-linking between proteins is not entirely known. It has been proposed that oxidation of this complex results in formation of a high valent nickel–oxo species that in turn oxidizes protein residues, creating radicals that react to form covalent bonds (Brown et al. 1998). There are several indications that tyrosine residues play a crucial role in the process. First, free tyrosine in solution inhibits the reaction (Brown et al. 1995). Second, mass spectrometric studies of model peptide dimers indicate that cross-linking occurs through formation of intermolecular bityrosyl products (Brown et al. 1998). Third, the D137Y variant of GGH-ecotin, which contains an engineered tyrosine at the ecotin dimer interface in close proximity to a wild-type tyrosine on the other monomer, exhibits enhanced cross-linking efficiency (Brown et al. 1998).
Bityrosyl products have been detected in proteins after oxidative cross-linking in related systems (Fancy and Kodadek 1998; Tew and Ortiz de Montellano 1988; Gill et al. 1997), and this mechanism has been proposed in other instances where the cross-linked moiety was not isolated (Campbell et al. 1998; Fancy and Kodadek 1999). The most common methods employed for analysis of oxidatively cross-linked proteins have involved detection of bityrosyl products by their characteristic bityrosyl fluorescence after protease digestion or by measurement of chromatographic retention time following acid hydrolysis. While these methods permit detection of this type of cross-linked product, they are discriminatory against all other possible reaction products. Detection of the presence of the bityrosyl moiety does not prove that this is the sole or even dominant species responsible for any cross-linking observed.
Mass spectrometric studies of a cross-linked GGH-fusion protein now conclusively reveal that GGH-mediated cross-linking of proteins does not always proceed via formation of the bityrosyl moiety. HPLC and MALDI analysis of cross-linked GGH-wt-ecotin protease digests does not reveal any relatively abundant "dimer-derived" peptides, indicating that although the protein cross-links to itself with an approximate yield of 15% (Brown et al. 1998), either no single predominant cross-linked product is formed or it is not detected by the MALDI MS method employed. A likely explanation for the former is that several isomeric species of cross-linked product(s) are formed, each of which is scarce enough to make identification by mass spectrometry difficult. In contrast to wild-type GGH-ecotin, MALDI analysis of cross-linked GGH-ecotin D137Y protease digests reveals dimer-derived peptides not observed in the analysis of GGH-ecotin D137Y oxidized monomer. Detailed structural analysis of three of these dimer-derived peptides has been carried out in this study and establishes that they are all formed from a single cross-linking reaction involving the N-terminal GG residues on one ecotin monomer and the 137Y residue on the other monomer. Only dimer-derived peptides containing the GG-Y cross-link produced reasonable signal in the MALDI spectra from HPLC fractionation, indicating a single, residue specific cross-link localization. It is remarkable that this is the case, as there are two tyrosine residues in close proximity at the GGH-ecotin D137Y dimer protein interface. This demonstrates that the pathway taken to formation of cross-links in the context of a protein is dependent on restrictions imposed by nickel chelate center and the quaternary structure and is thus not restricted to a single mechanism. The GG-Y cross-link also stands in contrast to the model peptide dimers that we have characterized previously (Brown et al. 1998) which all proceed through formation of a bityrosyl cross-link. Indeed, the model employed in this study, GGHSFASYSRAF, was able to form the intramolecular GG-Y cross-link, although much less abundantly than the bitryosyl intermolecular cross-link product. The results confirm the importance of tyrosine in oxidative cross-linking reactions and explain the dramatic improvement in dimerization efficiency for the D137Y point mutation.
In the case of the GGH-ecotin D137Y dimer, the cross-linked peptide was visible by differences between the MALDI spectra of pure oxidized monomer and pure dimer. This dramatic difference is helped by the good ionization efficiency of the cross-linked peptide and the small size of the protein, making for relatively uncomplicated spectra. However, HPLC separation of the digests is required to truly compare two different species due to ion suppression effects and the inherently lower signal expected for any cross-linked species. Even in a situation with a single type of cross-link producing a single cross-linked peptide mass, the yield for that species will be only 50% relative to most of the other digest peptides. If the cross-link is between peptides A and B`, the un-cross-linked peptides A` and B will also be present in the dimer digest. Additionally, the higher mass of the cross-linked peptide may result in lower detection efficiency, especially when using MALDI for detection. In the case presented here, the single dominant cross-link site is represented by at least three different major reaction pathways, and more minor ones. These each give peaks of different mass, further reducing signal intensity relative to other peptides. Thus, the success of the digest–HPLC–MALDI analysis of a cross-linked species cannot be predicted a priori. Additionally, finding a cross-link site by this method does not guarantee that this is the sole or even dominant site of cross-linking. In this particular case, use of two enzymes for the initial digest, Asp-N and Lys-C, helped to alter the peptide composition of digest fragments to increase the chances of finding the cross-linked peptides; in both digests, the same cross-link was identified. HPLC separation was also used to ensure that possible candidates were not overlooked. Finally, the site was confirmed by expression of the ecotin D137Y mutant and demonstration of a weakened cross-linking enhancement relative to the GGH-ecotin D137Y mutant. The advantage of using this type of analysis is its comprehensive nature compared to other available methods. For example, in this case a bityrosyl species was expected initially, with a cross-link between D137Y of one protein and Y127 of its partner. This species would have mass 1595 after Lys-C digest, and such a species was searched for in all spectra and never found. Methods based solely on detection of YY by fluorescence or amino acid analysis could have missed this GG-Y cross-link. In fact, the oxidative environment of most cross-linking reactions can lead to numerous side reactions and byproducts to complicate any type of absorption or fluorescence based analysis. So while there are limitations to a digest–HPLC–MALDI/ESI–MS based approach to protein cross-link analysis, there are advantages in identifying novel species and determining mechanisms.
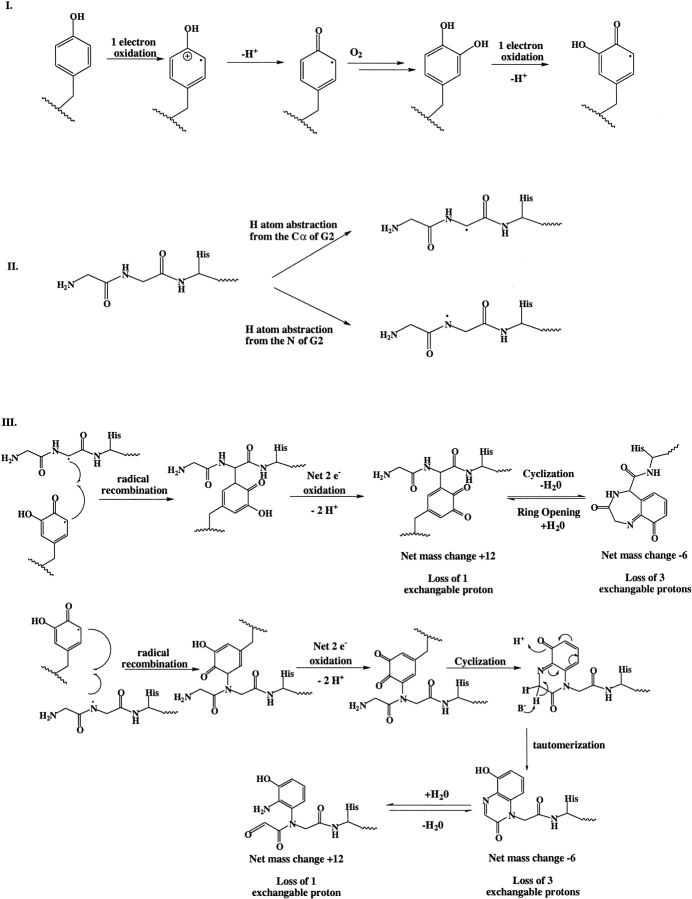
Based on experimental characterization of the dimer-derived peptides, including acetylation, aminopeptidase M digestion, and deuteration studies, a mechanism for the formation of this GG-Y cross-link is proposed (Scheme 1). The tyrosine residue is converted to a DOPA species by a one-electron oxidation followed by addition of molecular oxygen. Another one-electron oxidation results in the formation of a semiquinone radical. This reaction is analogous to those proposed for oxidative reactions of tyrosine residues mediated by metal complexes or hydroxyl radicals (Fu et al. 1998; Dean et al. 1997). Hydrogen atom abstraction from the alpha carbon of glycine 2 results in radical formation at the GGH tag. The two intermediate radical species then recombine and the resulting species undergoes a two-electron oxidation, leading to the +12 u form of the dimer. The molecular weight, free N terminus, and loss of one exchangeable tyrosine proton of this species make this proposed structure consistent with the experimental data. This species then undergoes an intramolecular condensation to form the –6 u Schiff base adduct with a loss of water. Reversibility to the +12 u species at higher pH is consistent with Schiff base formation. Loss of the free N terminus and its two exchangeable protons also makes the proposed structure for this species consistent with the deuterium exchange data. Alternatively, hydrogen atom abstraction could occur from the amide nitrogen of the first glycine. This species can recombine with the semiquinone radical as in the first case and undergo further oxidation, resulting in a net loss of two hydrogen atoms. Cyclization followed by tautomerization results in a fused bicyclic compound that is consistent with a –6 mu dimer species that has lost three exchangable protons. Addition of H20 to this species results in the +12 mu species with loss of one exchangable proton. From the current data, these two mechanisms cannot be distinguished. It should be noted that a similar mechanism can be imagined except that the hydrogen atom abstraction occurs from the C-α of the first glycine residue; however, the loss of H2O would result in an unlikely four-member ring. The third major product detected in dimer peptides from a Lys-C digest of cross-linked GGH-ecotin D137Y is a +46 u species that arises by addition of a chlorine. The position of the chloro-substitution is unknown. However, because the chloro-substituent is observed only for the hydrated form, it is possible that the presence of the chloro-substituent prevents cyclization. Oxidative addition of a chloro-substituent to tyrosine has been observed previously (Chowdhury et al. 1995).
While this GG-Y species has previously not been observed in proteins after oxidative cross-linking, there are examples of non-bityrosyl products in other systems. A similar mechanism has been proposed for the oxidation of protein bound 3,4-dihydroxyphenylalanine (DOPA) (Gieseg et al. 1993). In this case, DOPA undergoes a two-electron oxidation to form the corresponding quinone. The amide nitrogen of the peptide backbone undergoes a 1,4 addition to yield an indole quinone. This intermediate can then undergo another two-electron oxidation. Furthermore, lysyl oxidase has a cofactor that is comprised of a tyrosine residue-derived dopaquinone covalently linked to a lysyl ɛ-amine (Wang et al 1996; Klinman 1996). Treatment of β-hemoglobin with nickel and an oxidant leads to deamination of an N-terminal alanine, resulting in a carbonyl that allows cross-linking via Schiff base condensation with a lysine. The N-terminal XHX nickel binding motif of β-hemoglobin also produces non-bityrosyl cross-links, but these low level products have not been structurally characterized (Levine et al. 1998).
The first four amino acids in the crystal structure of the ecotin–ecotin complex are disordered and it is thus impossible to predict the exact location of the N-terminal GGH tag (Shin et al. 1996; Perona et al. 1997). However, there is sufficient chain length for the tag to access the dimer overlap region. It is not clear why the placement of the engineered tyrosine close to the GGH tag increases the cross-linking yield so dramatically. Three possible reasons can be envisioned. First, the tyrosine residue is in close proximity of the GGH-Ni(II) site which allows for more facile electron transfer from the aromatic ring. Second, the engineered tryosine residue is located in a proximal position to the peptide backbone (in this case, the location of the GGH tag), thus facilitating the hydrogen atom abstraction from this position. Lastly, it is possible that the rate of hydrogen atom abstraction from the C-α carbon of the peptide backbone is increased because of its location relative to the metal binding and this increases the yield of coupling to a nearby reactive group, i.e., a tyrosyl radical. However, this seems unlikely as free GGH-Ni(II) is an efficient cross-linking reagent and there is no evidence for degradation of the GGH during the cross-linking reaction or for oxidative coupling of GGH to proteins during cross-linking.
Cross-linking mediated by his6-tagged proteins in the presence of nickel and an oxidant is believed to proceed via a similar mechanism to that of the GGH-Ni(II) system. A report on cross-linking of glutathione S-transferase with an N-terminal his6 tag and a C-terminal TEV protease cleavage site tag demonstrated that a tyrosine to alanine mutation in the C-terminal tag drastically reduces the yield of dimer cross-linking (Fancy and Kodadek 1998). Based on modeling of both tags onto the glutathione S-transferase crystal structure, it has been speculated that proximity of an exposed tyrosine to the reactive metal-binding tag in the fusion protein increases the yield of dimer cross-linking, although the mechanism for this yield enhancement remains unknown.
Along these lines, mutants designed to place tyrosine residues at the ecotin–protease interface have been expressed and purified. The ecotin mutant GGH-ecotin L52Y places a tyrosine residue at the ecotin–protease interface, and shows enhanced cross-linking efficiency to trypsin by ∼20–30% (S. Mahrus and C.S. Craik, unpubl.). However, the increase in yield of cross-linking for the ecotin–protease was not as dramatic as that observed for the case of GGH-ecotin D137Y. This could be due to the location of the GGH tag in relation to the engineered tyrosine or due to non-optimized cross-linking partners on the trypsin site. The factors affecting cross-linking efficiency are most likely a combination of proximity to the tag relative to a tyrosine residue to initiate the cross-linking reaction and presence of a tyrosine group at the interface.
Mass spectrometric analysis of cross-linked GGH-ecotin D137Y has demonstrated that the only major modification of the protein besides the cross-link is the probably unrelated oxidation of methionine and the small amount (<5%) of chlorotyrosine observed. While oxidation of tryptophan and modification of histidine residues has been observed in studies of model peptides (Brown et al. 1998), no evidence of these reactions was found in the comprehensive analysis of the protein carried out in this study. The conditions used for the cross-linking reaction thus result in specific oxidation which should not disrupt protein tertiary or quaternary structure. Coupled with the specificity inherent to cross-linking mediated by GGH-fusion proteins, the mild oxidizing conditions make this system extremely appealing for use in the identification of protein–protein interactions and the isolation of novel interacting proteins in complex biochemical settings. Identification of the cross-linked species in this study demonstrates that two tyrosine residues at the protein–protein interface are not required, but affirms the critical role played by tyrosine in the overall process. In fact, the cross-linking may be even more general than previously thought and may require only a single tyrosine link to the peptide backbone. This would explain why this reagent has been an effective cross-linking reagent for many protein complexes (K.C. Brown, unpubl.). It is still unknown why the proximity of the GGH tag to a tyrosine residue increases the cross-linking yield. However, extension of the N-terminal linker region would allow for greater mobility of the GGH tag to the site of cross-linking, and incorporation of a tyrosine into this mobile region could be used as "bait" for identifying potential protein partners in multicomponent systems.
Materials and methods
Materials
Ni(OAc)2 and monoperoxyphthalate (MMPP) were purchased from Aldrich and used without further purification. Enzymes for DNA manipulations were purchased from New England Biolabs and were used according to the manufacturer's instructions. DNA oligonucleotides were synthesized by standard phosphoramidite chemistry on a Perkin-Elmer/Applied Biosystems 391 DNA synthesizer and purified with a NENSORB column from DuPont NEN. The Escherichia coli strain DH5α was used for cloning of ecotin variants, and the protease-deficient E. coli strain 27C7 was used for expression. Ecotin concentrations were determined by absorbance measurements at 280 nm using a calculated extinction coefficient of 23,140 M–1 cm–1 (Gill and von Hippel 1989). GGH-ecotin and GGH-ecotin D137Y were prepared as previously described (Brown et al. 1998).
Expression of ecotin D137Y
The Tyr mutation at position 137 was accomplished by subcloning the eco D137Y fragment from the pBS-eco D137Y vector into the expression vector pTacTac ecotin. The vector pBS-eco D137Y (Brown et al. 1998) was cut with PflMI and HindIII restriction enzymes and the resulting gene fragment was separated on a 1% agarose gel. The fragment was purified using a Promega PCR wizard kit and then subcloned into pBS-eco vector that does not contain the GGH-tag. The gene was then subcloned into pTacTacecotin between the BamHI and HindIII sites and the sequence was confirmed by DNA sequencing. Protein expression and purification was accomplished using the same procedure described for wild-type ecotin and GGH-ecotin (Brown et al. 1998; Wang et al. 1995). 17 mgs of purified protein were isolated from 1 L of expression media.
Small-scale cross-linking reactions
The reactions were performed with the final concentrations of 10 μM of ecotin variants (ecotin, ecotin D137Y, GGH-ecotin, or GGH-ecotin D137Y), 100 μM Ni(OAc)2, and 100 μM MMPP in a final volume of 15 μL. All reactions were buffered with 50 mM sodium phosphate (pH 7.5), 150 mM NaCl. 100 μM GGH was added to ecotin samples that did not contain the GGH tag. Buffer was substituted for MMPP in control samples that were not cross-linked. The Ni(OAc)2 and MMPP solutions were prepared immediately before use. Solutions of GGH-ecotin variants and the Ni(OAc)2 solution were prepared in water to avoid precipitation of Ni(II) salts. Appropriate volumes of each were added together and incubated at room temperature for 15 min to allow formation of the GGH-Ni(II) complex. The reactions were initiated by the addition of MMPP to a final concentration of 100 μM. The cross-linking reactions were incubated for 1 min and then quenched by addition of 3.75 μL of 5X loading buffer (0.3 M Tris, 10% SDS, 3.6 M β-mercaptoethanol, 50% glycerol, and 0.5% bromophenol blue). The samples were boiled for 5 min and analyzed by SDS-PAGE using 10% tricene gels. Protein bands were visualized by staining with Coomassie brilliant blue.
Large scale cross-linking reactions
The reactions were performed at the same concentrations as described for the small-scale reactions with the volumes scaled up to accommodate 2.0 mg of GGH-ecotin D137Y. The reaction was quenched by the addition of thiourea to a final concentration of 10 mM. The samples were dialyzed into water (MWCO 10 kD) and then lyophilized to dryness. The sample was resuspended in 300 μL H2O and 100 μL 4X loading buffer. The samples were boiled for 5 min and the resultant protein adducts were separated by SDS–PAGE using an 11cm × 15 cm 10% tricene gels. The protein bands were visualized by UV shadowing. Protein monomer and dimer bands were cut out with a razor blade and the proteins electroeluted within a dialysis bag. Small molecule contaminants were removed by dialysis into water (MWCO 10 kD). The detergents were removed with a Pierce 5 mL extracti-gel detergent removal column.
Digest and acetylation reactions on GGH-D137Y ecotin dimer
Endoproteinases Lys-C (Wako) and Glu-C and exopeptidases aminopeptidase M and carboxypeptidase A (all from Boehringer Mannheim except Lys-C) were used for digestion of the ecotin dimer or the dimer peptides. Digestions were carried out in 30 mM ammonium bicarbonate buffer at pH 8, using 2% Lys-C to protein with 2–4 h digestion at room temperature. The digest was analyzed by MALDI-TOF mass spectrometry and separated by rp-HPLC. Dimer peptides were digested in the same buffer using 100% enzyme to peptide, for 4–24 h at room temperature or 37°C. The sample was periodically analyzed by MALDI-TOF to assess the extent of digestion. Acetylation of the peptides was achieved by drying down the peptide in the Speed-Vac and then resuspending in 2–3 μL of acetic anhydride (Sigma) for 5–10 min, then repeating the procedure.
Model peptide synthesis and deuteration
The model peptide GGHSFASYSRAF-NH2 was synthesized by standard FMOC chemistry using an Applied Biosystems model 432 synthesizer (PE Biosytems) and purified by rp-HPLC. Cross-linking reactions were performed as for the GGH-ecotin protein but with peptide concentration of 100 μM, and the products separated by rp-HPLC. Deuteration studies were performed on both synthetic peptide and synthetic cross-linked peptides. The samples were by dried down and resuspended in 0.1% formic acid, 80% D2O, and 20% acetonitrile twice. The deuterated peptides were analyzed using electrospray ionization mass spectrometry.
Chromatography
Microbore reversed phase high performance liquid chromatography (rp-HPLC) separations were performed on an Applied Biosystems 140B solvent delivery system with a 785A detector. A Vydac C-18 1 × 150 mm, 300 μm pore size column was used with a gradient using 0.1% formic acid in water as solvent A and 0.08% formic acid in ethanol/1-propanol (5:2) as solvent B. A 5–60% B linear gradient was applied over a 2 h time period and fractions were collected manually for mass spectrometric analysis.
Mass spectrometry
Matrix Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) spectra were taken on PE Biosystems Voyager Elite and Voyager De-Str instruments using the reflectron detector in the positive ion mode. Each instrument is equipped with a nitrogen laser operating at 337 nm. Standard method parameters were used, with 200 ns delay time. MALDI-Post Source Decay (PSD) spectra were taken on the DE-STR instrument using standard methods. Air was used as the collision gas for collecting PSD ions below m/z = 200 at a pressure of 3 × 10–6 torr. The matrix used was Hewlett-Packard α-cyano-4-hydroxycinnamic acid, mixed 1:1 with the sample and drop dried on a gold target in a total volume of 1 μL. A mixture of Bio-rad cze standards at 1 ng/μL and ACTH (Sigma) at 2.5 ng/μL was used for internal calibration of the MALDI spectra during accurate mass measurements, and was diluted to match the concentration of the sample. Five peptides from the standard were used for the multipoint calibration, and six independent measurements were averaged for the final mass. The MALDI technique generates predominantly singly protonated molecular species.
Electrospray ionization (ESI) spectra were acquired on a PE Biosystems Mariner orthogonal acceleration (oa) TOF instrument operating in the positive ion mode. The peptides were infused at 1 μL/min and the spectra were acquired over the m/z range of 350–2000, with 5 sec acquisition time. Approximately 15 min of infusion scans were averaged to produce isotope profiles for deuterated samples.
N-terminal sequencing
N-terminal sequencing was performed by the Biomolecular Resource Center at UCSF. The peptide sample was isolated by HPLC as described. Samples containing 1–10 pmole of protein were subjected to Edman degradation using an Applied Biosystems 470A gas-phase sequencer. The phenylthiohydantoin (PTH)-derivatives were identified and quantitated by reverse-phase HPLC using an on-line Applied Biosystems 120A PTH Analyzer.
Figure .
Scheme I
Acknowledgments
Thanks are due to Dr. Haydn Ball for peptide synthesis. We are grateful to Dr. Kati Medzihradszky for assistance in interpretation of MALDI-PSD spectra. The N-terminal peptide sequencing was performed at the UCSF Biomolecular Research Center. Support was provided by the Biomedical Research Technology Program of the National Center for Research Resources, NIH NCRR BRTP RR01614. KCB was supported by the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation (fellowship DRG-1297). Financial support was also provided by NSF grant MCB9604379 (CSC) and NIH grant CA72006 (CSC).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.46601
References
- Alberts, B. 1998. The cell as a collection of protein machines – Preparing the next generation of molecular biologists. Cell 92 291–294. [DOI] [PubMed] [Google Scholar]
- Brown, K.C., Yang, S.H., and Kodadek, T. 1995. Highly specific oxidative cross-linking of proteins mediated by a nickel-peptide complex. Biochemistry 34 4733–4739. [DOI] [PubMed] [Google Scholar]
- Brown, K.C., Yu, Z., Burlingame, A.L., and Craik, C.S. 1998. Determining protein–protein interactions by oxidative cross-linking of a glycine– glycine–histidine fusion protein. Biochemistry 37 4397–4406. [DOI] [PubMed] [Google Scholar]
- Campbell, L.A., Kodadek, T., and Brown, K.C. 1998. Protein cross-linking mediated by metalloporphyrins. Bioorg. Med. Chem. 6 1301–1307. [DOI] [PubMed] [Google Scholar]
- Chowdhury, S.K., Eshraghi, J., Wolfe, H., Forde, H., Hlavac, A.G., and Johnston, D. 1995. Mass Spectrometric Identification of Amino Acid Transformations during Oxidation of Peptides and Proteins: Modifications of Methionine and Tyrosine. Anal. Chem. 67 390–398. [DOI] [PubMed] [Google Scholar]
- Dean, R.T., Fu, S.L., Stocker, R., and Davies, M.J. 1997. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 324 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy, D.A., Melcher, K., Johnston, S.A., and Kodadek, T. 1996. New chemistry for the study of multiprotein complexes: The six-histidine tag as a receptor for a protein crosslinking reagent. Chem. Biol. 3 551–559. [DOI] [PubMed] [Google Scholar]
- Fancy, D.A. and Kodadek, T. 1998. A critical role for tyrosine residues in His6Ni-mediated protein cross-linking. Biochem. Biophys. Res. Commun. 247 420–426. [DOI] [PubMed] [Google Scholar]
- Fancy, D.A. and Kodadek, T. 1999. Chemistry for the analysis of protein–protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. 96 6020–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, S.L., Fu, M.X., Baynes, J.W., Thorpe, S.R., and Dean, R.T. 1998. Presence of dopa and amino acid hydroperoxides in proteins modified with advanced glycation end products (ages) –Amino acid oxidation products as a possible source of oxidative stress induced by age proteins. Biochem. J. 330 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseg, S.P., Simpson, J.A., Charlton, T.S., Duncan, M.W., and Dean, R.T. 1993. Protein-Bound 3,4-Dihydroxyphenylalanine is a Major Reductant Formed During Hydroxyl radical Damage to Proteins. Biochemistry 32 4780–4786. [DOI] [PubMed] [Google Scholar]
- Gill, G., Richter-Rusli, A.A., Ghosh, M., Burrows, C.J., and Rokita, S.E. 1997. Nickel-dependent oxidative cross-linking of a protein. Chem. Res. Toxicol. 10 302–309. [DOI] [PubMed] [Google Scholar]
- Gill, S.C. and von Hippel, P.H. 1989. Calculation of protein extinction coefficients from amino acid sequence data [published erratum appears in Anal. Biochem. 1990 Sep; 189(2): 283]. Anal. Biochem. 182 319–326. [DOI] [PubMed] [Google Scholar]
- Hall, S.C., Smith, D.M., Masiarz, F.R., Soo, V.W., Tran, H.M., Epstein, L.B., and Burlingame, A.L. 1993. Mass spectrometric and Edman sequencing of lipocortin I isolated by two-dimensional SDS/PAGE of human melanoma lysates. Proc. Natl. Acad. Sci. 90 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori, T., Nishikawa, S.-I., Shin, I., Schultz, P.G., and Endo, T. 1997. Probing the Environment Along the Protein Import Pathways in Yeast Mitochondria by Site-Specific Photocrosslinking. Proc. Natl. Acad. Sci. 94 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K., Fancy, D.A., Carney, D., and Kodadek, T. 1999. Photoinduced protein cross-linking mediated by palladium porphyrins. J. Am. Chem. Soc. 121 11896–11897. [Google Scholar]
- Klinman, J.P. 1996. New quinocofactors in eukaryotes. J. Biol. Chem. 271 27189–27192. [DOI] [PubMed] [Google Scholar]
- Levine, J., Weickert, M., Pagratis, M., Etter, J., Mathews, A., Fattor, T., Lippincott, J., and Apostol, I. 1998. Identification of a nickel(II) binding site on hemoglobin which confers susceptibility to oxidative deamination and intramolecular cross-linking. J. Biol. Chem. 273 13037–13046. [DOI] [PubMed] [Google Scholar]
- Medzihradszky, K.F. and Burlingame, A.L. 1994. The advantages and versatility of a high-energy collision-induced dissociation-based strategy for the sequence and structural determination of proteins. Methods: A Companion to Methods in Enzymology 6 284–303. [Google Scholar]
- Perona, J.J., Tsu, C.A., Craik, C.S., and Fletterick, R.J. 1997. Crystal structure of an ecotin-collagenase complex suggests a model for recognition and cleavage of the collagen triple helix. Biochemistry 36 5381–5392. [DOI] [PubMed] [Google Scholar]
- Phizicky, E.M. and Fields, S. 1995. Protein–protein interactions: Methods for detection and analysis. Microbiol. Revs. 59 94–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D.H., Song, H.K., Seong, I.S., Lee, C.S., Chung, C.H., and Suh, S.W. 1996. Crystal Structure Analyses of Uncomplexed Ecotin in Two Crystal Forms: Implications for its Function and Stability. Protein Sci. 5 2236–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson, E., Truong, O., Richter, W.J., Waterfield, M.D., and Burlingame, A.L. 1997. Enhancement of charge remote fragmentation in protonated peptides by high-energy CID MALDI-TOF-MS using "cold" matrices. Int. J. Mass. Spectrom Ion. Proc. 169/170 231–240. [Google Scholar]
- Tew, D. and Ortiz de Montellano, P.R. 1988. The myoglobin protein radical. Coupling of Tyr-103 to Tyr-151 in the H2O2-mediated cross-linking of sperm whale myoglobin. J. Biol. Chem. 263 17880–17886. [PubMed] [Google Scholar]
- Wang, C.-I., Yang, Q., and Craik, C.S. 1995. Isolation of a High Affinity Inhibitor of Urokinase-type Plasminogen Activator by Phage Display of Ecotin. J. Biol. Chem. 270 12250–12256. [DOI] [PubMed] [Google Scholar]
- Wang, S.X., Mure, M., Medzihradszky, K.F., Burlingame, A.L., Brown, D.E., Dooley, D.M., Smith, A.J., Kagan, H.M., and Klinman, J.P. 1996. A crosslinked cofactor in lysyl oxidase: Redox function for amino acid side chains. Science 273 1078–1084. [DOI] [PubMed] [Google Scholar]
- Yang, S.Q., Wang, C.I., Gillmor, S.A., Fletterick, R.J., and Craik, C.S. 1998. Ecotin: A serine protease inhibitor with two distinct and interacting binding sites. J. Mol. Biol. 279 945–957. [DOI] [PubMed] [Google Scholar]