Abstract
In one of the first studies of isolated intermediates in protein aggregation, we have used circular dichroism and fluorescence spectroscopy to characterize metastable oligomers that are formed in the early steps of β-lactoglobulin heat aggregation. The intermediates show typical molten globule characteristics (secondary structure content similar to the native and less tight packing of the side chains), in agreement with the belief that partly folded states play a key role in protein aggregation. The far-UV CD signal bears strong resemblance to that of a known folding intermediate. Cryo-transmission electron microscopy of the aggregates reveals spherical particles with a diameter of about 50 nm and an internal threadlike structure. Isolated oligomers as well as larger aggregates bind the dye thioflavin T, usually a signature of the amyloid superstructures found in many protein aggregates. This result suggests that the structural motif recognized by thioflavin T can be formed in small oligomers.
Keywords: β-Lactoglobulin heat aggregation, metastable intermediates, molten globule state, thioflavin T affinity
Protein aggregation has important technical implications in biotechnology as well as in food science. Deposition of protein aggregates is also observed in a number of neurodegenerative disorders like Alzheimer's, Parkinson's, and prion diseases (Johnson 2000). Several studies have shown that aggregation can be induced in vitro by conditions that favor partially folded, molten globulelike states (Fink 1998; Dobson 1999; Rochet and Lansbury 2000). The conclusion that some transitory, nonnative conformation is required to prime the process is intuitive, but why this state has to have molten globule features is not clear yet. In the case of unspecific aggregation (e.g., inclusion body formation) it is straightforward that the exposure of hydrophobic patches typical of molten globule states should increase the tendency of the molecules to stick together. For the highly regular amyloid structures, on the other hand, assembly must involve formation of specific intermolecular contacts, and it is presently not well understood why this is linked to partial unfolding.
A few studies have reported detection of oligomers together with larger aggregates after induction of aggregation (Schweers et al. 1995; Hashimoto et al. 1998; Manderson et al. 1998; Bauer et al. 2000). β-Lactoglobulin (Blg) is one of these proteins presenting oligomers (mostly dimers) in the early stages of heat aggregation (Manderson et al. 1998; Bauer et al. 2000). Blg is a globular protein with a mass of 18.3 kD, and is the most abundant protein in bovine whey. Its secondary structure consists of mainly β-sheets organized in a β-sandwich plus one α-helix (Brownlow et al. 1997).
In this report we present the characterization of isolated oligomers of the genetic A variant, that we have previously shown to be obligatory intermediates in heat aggregation (Bauer et al. 2000). The results show that the oligomers have typical features of molten globule states: secondary structure content similar to the native structure but less tight packing of the side chains. This confirms that molten globule states play a key role in the first steps of aggregation. The aggregates, as imaged by cryo-transmission electron microscopy (cryo-TEM), are spherical particles with a diameter of about 50 nm. Contrary to the native protein, the isolated oligomers as well as the aggregates bind thioflavin T, a dye used to detect amyloid (Le Vine 1995).
Results
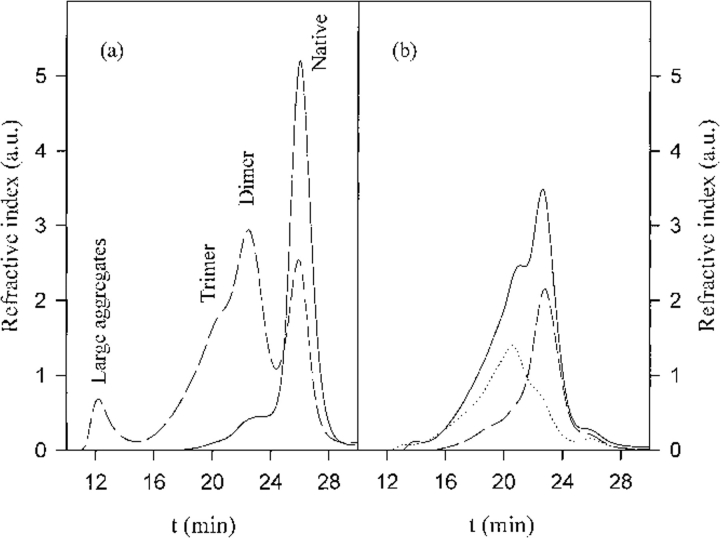
Oligomers and large aggregates were obtained by fractioning, after quenching, a partially aggregated sample by size exclusion chromatography (see Fig. 1 ▶), as reported in Materials and Methods. The most abundant oligomer is a dimer. The aggregate fraction elutes close to the void volume of the column.
Fig. 1.
(a) Elution profiles of the native protein (continuous line) and of the protein heated for 105 min at 67.5°C (20 mg/mL in 10 mM buffer at pH 8.7) (dashed line). (b) Elution profiles of the separated oligomer fraction (continuous line) and of two fractions after further separation: dimer fraction (dashed line) and remaining fraction (dotted line). The species corresponding to the last peak in a has also been collected and characterized, and has showed no difference with respect to the native protein.
Intermolecular bonding
It is known that the cysteines play a role in Blg heat aggregation (Shimada and Cheftel 1989). The protein contains five cysteines, one free and four involved in two disulfide bridges (Brownlow et al. 1997). By using the Elman assay we investigated the mean number of free cysteines per protein for the native protein and for the oligomers. For the native protein, as expected, one cysteine per protein was free, whereas for the full oligomer fraction we obtained 0.2 free cysteines per protein. These data show that the dimer has no free cysteines and is linked by an intermolecular disulfide bridge, a conclusion supported by the results of reducing and nonreducing SDS-PAGE (data not shown). The nature of the interactions that bind the additional monomer to the trimer is not clear.
Molten globule characteristics
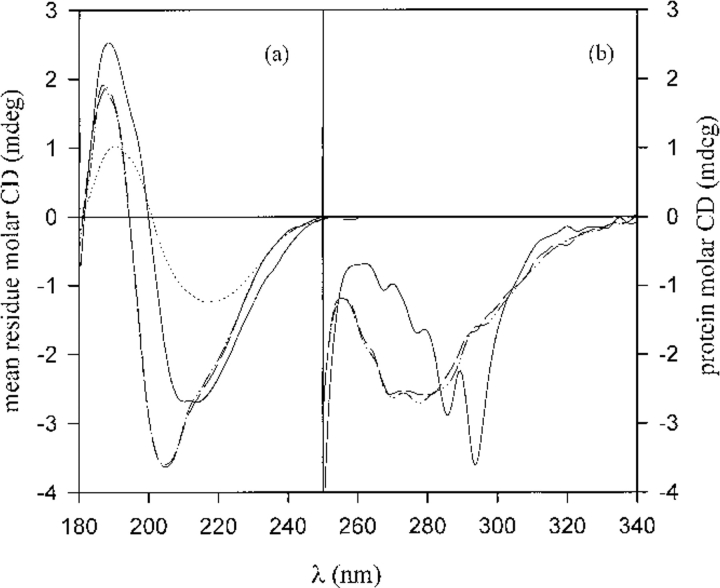
The far-UV CD spectrum for the native protein, the oligomers, and the large aggregates are reported in Figure 2a ▶. In Table 1 are reported the results of deconvolution of the spectra, according to which the oligomers partially lose helical and β-strand structure. However, the difference is not drastic. The aggregates are found to have even more β-strand structure than the native, more random coil and less α-helix. The near-UV CD spectra for the native protein and the oligomers are reported in Figure 2b ▶. In this region the circular dichroism of proteins is caused by the aromatic residues and the disulfide bridges. A change in the whole near-UV region is notable for the oligomers. The CD signal around 280 nm, usually attributed to tryptophan, is strongly reduced, and new bands show up in the range 260–290 nm. Such signals arise from disulfide bridge distortion (Fasman 1996), probably related to the intermolecular disulfide bond. The decrease of the tryptophan dichroism indicates an enhanced mobility of the chromophore. The CD data for the intermediates present typical characteristics of a molten globule state, that is, small changes in the secondary structure compared with the native and larger differences at the level of tertiary structure, reflected in the increased mobility of the side chains.
Fig. 2.
Far-UV (a) and near-UV (b) molar ellipticity. The continuous line corresponds to the native protein, the long hatched line to the oligomer fraction, the dot-hatch line to the dimer fraction purified further, and the dotted line to the aggregates, collected after 320 min.
Table 1.
Secondary structure content
| Native (X-ray)a | Native (CD)b | Oligomers (CD)b | Aggregates (CD)b | |
| α-Helix | 12% | 11% | 7% | 3% |
| β-Strand | 25% | 24% | 19% | 27% |
| Turns | 7% | 14% | 15% | 10% |
| Other | 56% | 51% | 59% | 60% |
a Calculated from the structure at pH 8.2 (Qin et al. 1999).
b Deconvolution of CD spectra.
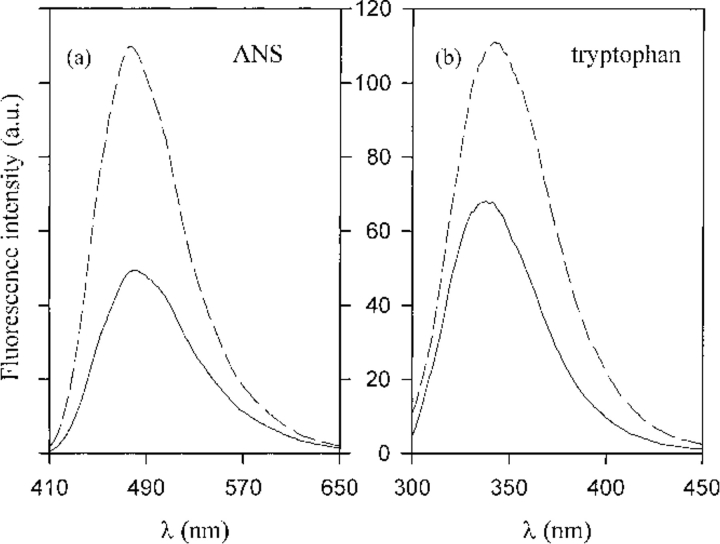
Another characteristic of molten globule states is an increased binding of the hydrophobic dye ANS. Figure 3a ▶ reports the emission spectra of ANS in the presence of the native protein and the oligomers. The increase in ANS emission intensity for the oligomers compared with the native protein reflects more exposed hydrophobic sites.
Fig. 3.
(a) ANS fluorescence for the native protein (continuous line) and the oligomers (dashed line): 100 μM ANS and 5.5 μM of protein, λex = 400 nm, 100 nm/min scan rate, excitation and emission slit width 5 nm. (b) Intrinsic tryptophan fluorescence for the native protein (continuous line) and the oligomers (dashed line). Protein concentration 5.5 μM, λex = 290 nm, 100 nm/min scan rate, excitation and emission slit width 3 nm.
We also measured tryptophan fluorescence spectra to probe the changes in the tryptophan environment after self-association. Figure 3b ▶ shows the emission spectra of the native protein and of the oligomers. Figure 4 ▶ reports both unfolding and refolding data for native BlgA (Fig. 4a ▶) and the denaturation data for the purified dimer (Fig. 4b ▶; see Fig. 1b ▶); in the inset (Fig. 4b ▶) both unfolding and refolding data for the unseparated oligomers are reported. At the conditions and protein concentrations used, there is no further association of either species. The denaturation process seems to be reversible for all the samples, and the results obtained for the dimers show that the unfolding process is still cooperative. We can describe the data by a two-state model (Fersht 1999), with free energy difference relative to the denatured state  , although we do not have complementary evidence that this is the correct model. ΔGU-FH2O is the free energy difference in water, and m is a measure of the difference in exposure of groups interacting with GuHCl. The values obtained from the fits are reported in the caption to Figure 4 ▶, but should only be seen as an empirical description of the data, because the unfolding process may in fact be more complex.
, although we do not have complementary evidence that this is the correct model. ΔGU-FH2O is the free energy difference in water, and m is a measure of the difference in exposure of groups interacting with GuHCl. The values obtained from the fits are reported in the caption to Figure 4 ▶, but should only be seen as an empirical description of the data, because the unfolding process may in fact be more complex.
Fig. 4.
Unfolding (open circles)/refolding (filled circles) in GuHCl for the native protein (a) and unfolding for the purified dimer fraction (b). In the inset unfolding (open triangles)/refolding (filled triangles) of the oligomers. Protein concentration 5.5 μM, λex = 290 nm, 100 nm/min scan rate, excitation and emission slit width 3 nm. The line represents the nonlinear best fit of the denaturation data, assuming a two-state model (Fersht 1999). The parameters of the fit are: native,  ,
,  ,
, 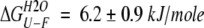 ,
, 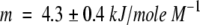 .
.
It is problematic to study the isolated oligomers under the exact conditions of aggregation (67.5°C) because they tend to associate further at this temperature. This is the reason that all the measurements we report are made at 25°C. As an exception, we acquired tryptophan fluorescence spectra of the oligomers in 0 M and 4 M GuHCl immediately after heating to 67.5°C. The two spectra obtained are well distinct, the latter sample showing a red-shifted fluorescence (data not shown). Comparison of fluorescence at 25°C and 67.5°C shows that the denatured state, in 4 M GuHCl, is the same at both temperatures. A slight difference can be noted in the spectra for the oligomers in 0 M GuHCl, the sample at higher temperature showing a red shift. These results suggest that the molten globule state is populated during aggregation, at 67.5°C, although a fraction of the molecules might be in the denatured state.
Thioflavin T binding
Many proteins aggregate forming ordered and structured fibrils, with a high content of β-sheet and amyloid superstructure (Fink 1998; Dobson 1999; Rochet and Lansbury 2000; Wilkins et al. 2000). These amyloid fibrils are usually detected by using staining molecules such as thioflavin T or congo red, which change their spectroscopic properties by interaction with the aggregates (Le Vine 1995). Measurements of thioflavin T fluorescence in the presence of oligomers as well as aggregates isolated after 105 min of heating show that there is, indeed, an interaction between thioflavin T and the aggregates, as reported in Figure 5 ▶. An interaction is observed also for the oligomers, whereas no interaction is detected for the native protein.
Fig. 5.
Emission spectra of 5 μM thioflavin T in the presence of native protein (continuous line), oligomers (dashed line), and large aggregates (dotted line). Protein concentration 9.4 μM, λex = 442 nm, 100 nm/min scan rate, excitation and emission slit width 5 nm.
Aggregate structure
Figure 6 ▶ shows a cryo-TEM image of the aggregate fraction. It is seen to be composed mainly of approximately spherical particles, with diameters between 35 and 70 nm. Between and inside these particles one observes threadlike objects, with a width of 5 nm or less (limited by the resolution of the image). In comparison, the diameter of a Blg monomer is about 3.5 nm (Qin et al. 1999). The diameters of the protofibrils formed by several amyloidogenic proteins are of the order 4–6 nm as measured by AFM (Walsh et al. 1997; Ionescu-Zanetti et al. 1999; Rochet and Lansbury 2000).
Fig. 6.

Cryo-TEM images of β-lactoglobulin aggregates obtained after 210 min of heating. Length of bar 100 nm. Aggregate concentration prior to application to the grid was 3.2 mg/mL.
Discussion
The present work is one of the first characterizations of multimeric intermediates in protein aggregation, and offers direct information about the assembly process. The results of the Ellman assay confirm that the dimer is linked by a disulfide bond, as previously proposed (Manderson et al. 1998; Bauer et al. 2000). Formation of the dimer therefore requires at least unfolding of the protein's main helix (Qi et al. 1997), which protects the free cysteine from the solvent in the native structure. We have previously shown that the Blg oligomers are productive intermediates in aggregation: assembly of aggregates coincides with consumption of oligomers, and purified oligomers at high concentration aggregate very rapidly (Bauer et al. 2000). The molten globule character of the oligomers observed here is perfectly in line with previous studies on other proteins (Booth et al. 1997; Kayed et al. 1999; McParland et al. 2000; Rochet and Lansbury 2000), demonstrating that aggregation is accelerated by conditions that favor this type of state. The cooperativity of the dimer denaturation curve as well as the fact that the full oligomer fraction has exactly the same far- and near-UV CD spectrum as the dimer part indicate that the same state of the protein is preferred under a variety of conditions. For Blg, this state is stabilized by an intermolecular disulfide bond, as is also observed for the dimer of the τ protein (Schweers et al. 1995).
Molten globule states are encountered in equilibrium denaturation of many proteins, in particular at low pH, and have in a number of cases been shown to be identical to folding intermediates. The equilibrium denaturation and refolding kinetics of bovine and equine Blg have been the subjects of several studies. Titration of the bovine protein with GuHCl at pH 1.8 gave rise to an equilibrium intermediate with above-native content of α-helical structure that was further stabilized by the addition of trifluoroethanol (Hamada and Goto 1997; Kuwata et al. 1998). The equine protein likewise presents an equilibrium intermediate (the A state) when the pH is lowered to 1.5. CD measurements indicated a helix content above that of the native protein (Ikeguchi et al. 1997), although the extra helices could not be localized by hydrogen exchange measurements (Kobayashi et al. 2000). The folding intermediate at pH 3 has been investigated both by H/2H exchange pulse labeling for the bovine protein (Forge et al. 2000; Kuwata et al. 2001) and by stopped-flow CD for the bovine protein as well as the equine protein (Hamada et al. 1996; Arai et al. 1998; Fujiwara et al. 1999; Forge et al. 2000), and the folding intermediate resembles the A state quite well. Remarkably, these far-UV CD data are very similar to the spectrum that we find for the oligomers (Fig. 2a ▶), strongly suggesting that the Blg molecules linked together in the oligomers are in a conformation rather similar to the low-pH intermediate. The helix content predicted by our deconvolution (Table 1) is significantly smaller than the values found at low pH (Hamada et al. 1996; Ikeguchi et al. 1997), despite the resemblance of the data. As noted above, the main helix of the protein must unfold in order for the dimer to form, and this might account for the balance. Another difference is that our analysis takes into account the important spectral region between 180 and 205 nm, which is inaccessible in the cited works owing to high concentrations of HCl and/or GuHCl.
The H/2H pulse labeling data of Forge et al. (2000) show that three β-strands (F, G, and H) are formed in the folding intermediate. The pulse labeling results of Kuwata et al. (2001) also show protection in the large α-helix and part of an additional β-strand (A), in agreement with steady-state hydrogen-exchange measurements on the A state (Kobayashi et al. 2000). Kuwata et al. (2001) propose that the residues of the A strand might be in a marginally stable nonnative helix. In the native structure, the A, F, G, and H strands together form a β-sheet, with the free cysteine Cys121 in the middle of the sheet, and with the large α-helix shielding Cys121 from the solvent. The α-helix cannot interact with the β-sheet in the oligomers, but we propose that the AFGH-sheet in each Blg molecule instead is stabilized by interactions with the sheet of the molecule to which it is linked.
We have previously shown that the oligomers aggregate by hydrophobic interactions, and that the assembly is nucleated, with at least four oligomers in the nucleus (Bauer et al. 2000). The presence of threadlike objects in Figure 6 ▶ suggests that the oligomers initially assemble into small strings, which then aggregate into the larger particles, giving a very open structure as previously observed (Bauer et al. 2000). The character of the nucleation step cannot presently be ascertained. The deconvolution of our CD data indicates that the content of β-sheet is below-native for the oligomers but above-native for the aggregates. As discussed, the intact β-sheet in the oligomers appears to be located at the monomer–monomer interface. Thus, formation of additional β-sheet structure upon aggregation probably takes place in the part of the oligomers facing away from this interface. The joining together of oligomers by contacts between these parts is consistent with the appearance of small strings with approximately the width of one monomer.
The increased affinity for thioflavin T found for the oligomers shows that binding of this dye is not restricted to complete amyloid fibrils. It might be argued that the binding to the Blg oligomers could be purely hydrophobic and unspecific, but this is not likely, because the difference in affinity between the native state and the oligomers (Fig. 5 ▶) is much larger than for ANS (Fig. 3a ▶), the classical hydrophobic dye (ANS can also form ionic contacts to proteins [Matulis and Lovrien 1998], yet this mode of binding does not lead to increased fluorescence). Rather, our results indicate that Blg adopts a structural motif with increased thioflavin T binding very early in the aggregation process at the same time as the loss of tertiary structure. Based on the ability of many different proteins to form extremely stable amyloid fibrils, it has recently been proposed (Dobson 2000) that this structure in fact represents the global free energy minimum for a polypeptide chain (at normal concentrations). In this light, it is not at all surprising that β-lactoglobulin apparently takes on a related conformation as soon as the tertiary interactions that constrain the chain to the native structure have been disrupted.
In a recent study, the dimers of τ protein have been isolated and studied spectroscopically (Schweers et al. 1995; von Bergen et al. 2000). In contrast to our case, no binding of thioflavin dyes was observed prior to formation of filaments (Schweers et al. 1995). Metastable dimers have also been observed during fibrilization of α-synuclein (Hashimoto et al. 1998), which is involved in Parkinson's disease. It would be very interesting to perform a spectroscopic investigation on this system.
Materials and methods
All chemicals used were of analytical grade. All buffers were 10 mM Na2HPO4 at pH 8.7, except for the Elman assay. Blg A was obtained from Sigma as dried powder and dialyzed against the buffer.
Aggregation was induced by incubating the protein (20 mg/mL) at 67.5°C for 105 min. The partially aggregated sample was separated on a Superdex 200 column (Pharmacia). The species passing through the column were detected and sized as previously described (Bauer et al. 2000), by using a static light-scattering instrument (Dawn F with a K2 cell; Wyatt Technology) and a refractive index detector (RID-10A, Shimadzu, Japan). Figure 1a ▶ shows the elution profiles of a Blg A solution before and after heating. We collected fractions of large aggregates, oligomers, and nativelike species. The dimer was purified further from the oligomer fraction. Figure 1b ▶ shows the quality of separation.
CD measurements were performed on a JASCO J715 at 20°C, with a 20-nm/min scan rate and 0.5-nm resolution, and far-UV spectra were deconvoluted using an SVD-based algorithm (Manavalan and Johnson 1987). CD measurements on the oligomers, isolated from samples heated for different times, showed unchanged conformations, whereas structural changes were observed in the aggregates, reflecting their progressive growth (results not shown). Secondary structure content in the X-ray structure at pH 8.2 (Qin et al. 1999) was calculated using the program MOLMOL (Koradi et al. 1996). 1-Anilinonaphthalene-8-sulfonic acid (ANS) binding, intrinsic tryptophan fluorescence, and unfolding/refolding in guanidine hydrochloride (GuHCl) probed by tryptophan emission and thioflavin fluorescence were measured on a Perkin Elmer LS50B spectrofluorimeter. The Elman assay was performed on 3 M urea solutions in 100 mM Tris buffer at pH 8, with a ratio of DTNB/protein = 2 and using 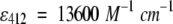 to probe the reaction. Protein concentration was estimated using
to probe the reaction. Protein concentration was estimated using 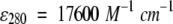 . Absorption was measured on a HP8453 spectrophotometer.
. Absorption was measured on a HP8453 spectrophotometer.
Cryo-TEM was performed on aggregates isolated after heating at 67.5°C for 105 or 210 min, with very similar results. The samples were frozen in liquid nitrogen immediately after size-exclusion chromatography and kept at −20°C until just before application to the microscope grid. Application to the grid was done in a controlled environment vitrification system (CEVS), with the chamber temperature at 30°C and the humidity close to saturation to avoid evaporation of water from the sample during preparation. A small amount of the sample (5 μL) was put on a lacey carbon film supported by a copper grid and gently blotted with filter paper to get a thin liquid film on the grid (<0.3 μm). The grid was quenched in liquid ethane at its freezing point and transferred into liquid nitrogen (Bellare et al. 1988). The vitrified specimens were stored under liquid nitrogen and transferred to the transmission electron microscope (Philips CM120 BioTWIN Cryo) equipped with a post-column energy filter (Gatan GIF 100), using an Oxford CT3500 cryoholder and its workstation. The acceleration voltage was 120 kV, and the working temperature was −180°C. The images were recorded with a CCD camera (Gatan 791) under low dose conditions and utilizing the zero loss peak (slit width of 8 eV). The defocus was approximately 1 μm.
Acknowledgments
This work was supported by the Carlsberg Foundation, the Danish Research Academy, and the Danish Natural Science Foundation. We thank Tommy Nylander from Lund University and Niels C. Kaarsholm from Novo Nordisk for helpful discussions and the latter also for the use of the CD facility. We gratefully acknowledge the competent technical support of Christian H. Andersen and Anne-Marie Kolstrup.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
Blg, β-lactoglobulin
Cryo-TEM, Cryo-transmission electron microscopy
SDS-PAGE, sodium dodecyl sulfate polyacryl-amide gel electrophoresis
ANS, 1-anilino-8-naphthalene sulfonate
Trp, tryptophan
GuHCl, guanidine hydrochloride
DTNB, 5,5`-dithiobis(2-nitrobenzoic acid)
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.42501.
References
- Arai, M., Ikura, T., Semitsonov, G.V., Kihara, H., Amemiya, Y., and Kuwajima, K. 1998. Kinetic refolding of β-lactoglobulin. Studies by synchrotron X-ray scattering, and circular dichroism, absorption and fluorescence spectroscopy. J. Mol. Biol. 275 149–162. [DOI] [PubMed] [Google Scholar]
- Bauer, R., Carrotta, R., Rischel, C., Øgendal, L. 2000. Characterization and isolation of intermediates in β-lactolobulin heat aggregation. Biophys. J. 79 1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellare, J.R., Davis, H.T., Scriven, L.E., and Talmon, Y. 1988. Controlled environment vitrification system: An improved sample preparation technique. J. Electron Microsc. Technol. 10 87–111 [DOI] [PubMed] [Google Scholar]
- Booth, D.R., Sunde, M., Bellotti, V., Robinson, C.V., Hutchinson, W.L., Fraser, P.E., Hawkins, P.N., Dobson, C.M., Radford, S.E., Blake, C.C.F., et al. 1997. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385 787–793. [DOI] [PubMed] [Google Scholar]
- Brownlow, S., Cabral, J.H.M., Cooper, R., Flower, D.R., Yewdall, S.J., Polikarpov, I., North, A.C.T., and Sawyer, L. 1997. Bovine β-lactolobulin at 1.8 Å resolution—Still an enigmatic lipocalin. Structure 5 481–495. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M. 1999. Protein misfolding, evolution and disease. Trends Biochem. Sci. 24 329–332. [DOI] [PubMed] [Google Scholar]
- ———. 2000. Protein folding, evolution and disease. Eur. Biophys. J. 29 233. [Google Scholar]
- Fasman, G.D. 1996. Circular dichroism and conformational analysis of biomolecules. Plenum Press, New York.
- Fersht, A. 1999. Structure and mechanism in protein science. Freeman, New York.
- Fink, A.L. 1998. Protein aggregation: Folding aggregates, inclusion bodies and amyloid. Folding & Design 3 R9–R23. [DOI] [PubMed] [Google Scholar]
- Forge, V., Hoshino, M., Kuwata, K., Arai, M., Kuwajima, K., Batt, C.A., and Goto, Y. 2000. Is folding of β-lactoglobulin non-hierachic? Intermediate with native-like β-sheet and non-native α-helix. J. Mol. Biol. 296 1039–1051. [DOI] [PubMed] [Google Scholar]
- Fujiwara, K., Arai, M., Shimizu, A., Ikeguchi, M., Kuwajima, K., and Sugai, S. 1999. Folding–unfolding equilibrium and kinetics of equine β-lactolobulin: Equivalence between the equilibrium molten globule state and a burst-phase folding intermediate. Biochemistry 38 4455–4463. [DOI] [PubMed] [Google Scholar]
- Hamada, D. and Goto, Y. 1997. The equilibrium intermediate of b-lactoglobulin with non-native α-helical structure. J. Mol. Biol. 269 479–487. [DOI] [PubMed] [Google Scholar]
- Hamada, D., Segawa, S., and Goto, Y. 1996. Non-native α-helical intermediate in the refolding of β-lactoglobulin, a predominantly β-sheet protein. Nat. Struct. Biol. 3 868–873. [DOI] [PubMed] [Google Scholar]
- Hashimoto, M., Hsu, L.J., Sisk, A., Xia, Y., Takeda, A., Sundsmo, M., and Masliah, E. 1998. Human recombinant NACP/α-synuclein is aggregated and fibrillated in vitro: Relevance for Lewy body disease. Brain Research 799 301–306. [DOI] [PubMed] [Google Scholar]
- Ikeguchi, M., Kato, S., Shimizu, A., and Sugai, S. 1997. Molten globule state of equine β-lactoglobulin. Proteins: Struct. Funct. Genet. 27 567–575. [DOI] [PubMed] [Google Scholar]
- Ionescu-Zanetti, C., Khurana, R., Gillespie, J.R., Petrick, J.S., Trabachino, L.C., Minert, L.J., Carter, S.A., and Fink, A.L. 1999. Monitoring the assembly of Ig light-chain amyloid fibrils by atomic force microscopy. Proc. Natl. Acad. Sci. USA 96 13175–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, W.G. 2000. Late-onset neurodegenerative diseases—The role of protein insolubility. J. Anat. 196 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed, R., Bernhagen, J., Greenfield, N., Sweimeh, K., Brunner, H., Voelter, W., and Kapurniotu, A. 1999. Conformational transitions of Islet amyloid polypeptide (IAPP) in amyloid formation in vitro. J. Mol. Biol. 287 781–796. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Ikeguchi, M., and Sugai, S. 2000. Molten globule structure of equine β-lactoglobulin probed by hydrogen exchange. J. Mol. Biol. 299 757–770. [DOI] [PubMed] [Google Scholar]
- Koradi, R., Billeter, M., and Wütrich, K. 1996 MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graphics 14 51–55. [DOI] [PubMed]
- Kuwata, K., Hoshino, M., Era, S., Batt, C.A., and Goto, Y. 1998. α → β transition of β-lactoglobulin as evidenced by heteronuclear NMR. J. Mol. Biol. 283 731–739. [DOI] [PubMed] [Google Scholar]
- Kuwata, K., Shastry, R., Cheng, H., Hoshino, M., Batt, C.A., Goto, Y., and Roder, H. 2001. Structural and kinetic characterization of early folding events in β-lactoglobulin. Nat. Struct. Biol. 8 151–155. [DOI] [PubMed] [Google Scholar]
- Le Vine iii, H. 1995. Thioflavine T interaction with amyloid β-sheet structures. Amyloid: Int. J. Exp. Clin. Invest. 2 1–6. [Google Scholar]
- Manderson, G.A., Hardman, M.J., and Creamer, L.K. 1998. Effect of heat treatment on the conformation and aggregation of β-lactoglobulin A, B and C. J. Agricul. Food Chem. 46 5052–5061. [Google Scholar]
- Matulis, D. and Lovrien, R. 1998 1-Anilino-8-naphthalene sulfonate anion–protein binding depends primarily on ion pair formation. Biophys. J. 74 422–429. [DOI] [PMC free article] [PubMed]
- McParland, V.J., Kad, N.M., Kalverda, A.P., Brown, A., Kirwin-Jones, P., Hunter, M.G., Sunde, M., and Radford, S.E. 2000. Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry 39 8735–8746. [DOI] [PubMed] [Google Scholar]
- Qi, X.L., Holt, C., McNulty, D., Clarke, D.T., Brownlow, S., and Jones, G.R. 1997. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7 as determined by CD and IR spectroscopy: A test of the molten globule hypothesis. Biochem. J. 324 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, B.Y., Bewley, M.C., Creamer, L.K., Baker, H.M., Baker, E.N., Geoffrey, B., and Jameson, G.B. 1999. Structural basis of the Tanford transition of bovine β-lactoglobulin. Biochemistry 37 14014–14023. [DOI] [PubMed] [Google Scholar]
- Rochet, J.C. and Lansbury, P.T. 2000. Amyloid fibrilloenesis: Themes and variations. Curr. Opin. Struct. Biol. 10 60–68. [DOI] [PubMed] [Google Scholar]
- Schweers, O., Mandelkow, E.-M., Biernat, J., and Mandelkow, E. 1995. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein τ controls the in vitro assembly of paired helical filaments. Proc. Natl. Acad. Sci. USA 92 8463–8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, K. and Cheftel, J.C. 1989. Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J. Agricul. Food Chem. 37 161–168. [Google Scholar]
- von Bergen, M., Friedhoff, J., Heberle, J., Mandelkow, E.-M., and Mandelkow, E. 2000. Assembly of τ protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. USA 97 5129–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, D.M., Lomakin, A., Benedek, G.B., Condron, M.M., and Teplow, D.B. 1997. Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem. 272 22364–22372. [DOI] [PubMed] [Google Scholar]
- Wilkins, D.K., Dobson, C.M., and Groβ, M. 2000. Biophysical studies of the development of amyloid fibrils from a peptide fragment of cold shock protein B. Eur. J. Biochem. 267 2609–2616. [DOI] [PubMed] [Google Scholar]