The preliminary X-ray crystallographic study of rabbit l-gulonate 3-dehydrogenase is described.
Keywords: λ-crystallins, l-gulonate 3-dehydrogenase, 3-hydroxyacyl CoA dehydrogenase, uronate cycle
Abstract
Rabbit l-gulonate 3-dehydrogenase was crystallized using the oil-microbatch method at 295 K. X-ray diffraction data were collected to 1.70 Å resolution from a crystal at 100 K using synchrotron radiation. The crystal belongs to the C-centred monoclinic space group C2, with unit-cell parameters a = 71.81, b = 69.08, c = 65.64 Å, β = 102.7°. Assuming the presence of a monomeric protomer in the asymmetric unit gives a V M value of 2.21 Å3 Da−1 and a solvent content of 44.4%. A cocrystal with NADH, which was isomorphous to the apo form, was also prepared and diffraction data were collected to 1.85 Å resolution using Cu Kα radiation at 100 K.
1. Introduction
l-Gulonate 3-dehydrogenase (GDH; EC 1.1.1.45) is an NAD+-dependent enzyme in the uronate cycle, an alternative glucose-metabolic pathway that plays essential roles in glucuronide formation and in the synthesis of glycosaminoglycan and ascorbic acid. The enzyme oxidizes l-gulonate to 3-dehydro-l-gulonate and exhibits low dehydrogenase activity towards some structurally related organic acids with a 3-hydroxyl group, such as l-3-hydroxybutyrate and l-threonate (Smiley & Ashwell, 1961 ▶). Recently, Ishikura and coworkers isolated a cDNA for rabbit GDH from the liver and showed that the enzyme was exactly identical to a toxon-specific λ-crystallin (Ishikura et al., 2005 ▶) known as an enzyme-related crystallin that is highly expressed in rabbit lens (Mulders et al., 1988 ▶). Thus, GDH also acts as a structural and refractive protein in the lens.
Rabbit GDH is a dimer of 36 kDa monomeric protomers (Ishikura et al., 2005 ▶) and shares marginal sequence identity (22%) with human NAD+-dependent 3-hydroxyacyl-CoA dehydrogenase (HAD), which is a critical enzyme for the oxidative metabolism of fatty acids (Schulz, 1991 ▶). The crystal structure of human HAD has been determined (Barycki et al., 1999 ▶). Based on structural information, it has been reported that human HAD is a homodimeric enzyme and that its monomeric protomer has two distinct domains. While the N-terminal α/β domain is a coenzyme-binding domain, the C-terminal domain is primarily α-helical and mediates subunit dimerization through hydrophobic interactions with the cognate C-terminal domain of the other subunit. Furthermore, the structure of human HAD allows proposal of its catalytic mechanism, in which His158 is implicated as a general base that abstracts a proton from the 3-hydroxyl group of the substrate, Glu170 neutralizes the positive charge on His158 after proton abstraction, Ser137 interacts with the substrate and coenzyme to orient them for catalysis and Asn208 is thought to stabilize the reaction product together with Ser137 (Barycki et al., 2000 ▶). These catalytically important residues Ser137, His158, Glu170 and Asn208 in human HAD are conserved in rabbit GDH as the corresponding residues Ser124, His145, Glu157 and Asn196, respectively. A site-directed mutagenesis study of rabbit GDH suggested that Ser124, His145 and Asn196 are critical for catalytic function of the enzyme. Although the catalytic importance of Glu170 of human HAD has been confirmed by a mutation experiment (Barycki et al., 2001 ▶), the role of the corresponding Glu157 in rabbit GDH may differ because no apparent kinetic alteration was observed on the replacement of Glu157 by Gln (Ishikura et al., 2005 ▶). In addition, the substrate-specificity of GDH differs remarkably compared with that of HAD, which shows broad substrate specificity, accepting 4–16 C atoms in the acyl chain (Kobayashi et al., 1996 ▶). A recent homology study proposed a novel protein family composed of 27 proteins such as GDH, HAD and λ-crystallins (Chen et al., 2003 ▶), of which HAD is the only one for which a tertiary structure has been determined. To elucidate the structure–function relationship of GDH, we have initiated a three-dimensional structure determination of the recombinant rabbit GDH. In this study, we present the first crystallization and preliminary X-ray analysis of rabbit GDH.
2. Experimental
2.1. Protein expression and purification
The cDNA for rabbit GDH was obtained by reverse-transcription PCR from a male Japanese white rabbit, inserted into pCR T7/CT-TOPO vectors (Invitrogen) and expressed in Escherichia coli BL21 (DE3) pLysS cells as described by Ishikura et al. (2005 ▶). The purification method used was slightly modified from that described previously by Ishikura et al. (2005 ▶). Briefly, the cells were harvested by centrifugation at 20 000g for 5 min at 277 K, suspended in lysis buffer (0.1 mg ml−1 lysozyme, 0.1% Triton X-100, 0.5 M NaCl and 0.5 M EDTA in 20 mM Tris–HCl pH 8.0) and sonicated using an UD-200 ultrasonic homogenizer (Tomy). The cell extract was obtained by centrifugation at 21 600g for 15 min. The recombinant GDH was purified from the cell extract by ammonium sulfate fractionation (30–75% saturation) and consecutive column-chromatographic steps. The protein from the ammonium sulfate fraction was dialyzed against 20 mM Tris–HCl pH 8.0 (buffer A) and applied onto a Super Q Toyopearl 650M (Tosoh) column equilibrated with buffer A. The enzyme was eluted with a linear gradient of 0–0.3 M NaCl in the buffer. After the NaCl in the enzyme fraction had been removed using a HiPrep 26/10 desalting column, the sample was applied onto a Source 15Q (GE Healthcare BioScience) column equilibrated with buffer A and the enzyme was eluted with a linear gradient of 0–0.3 M NaCl in the buffer. The buffer in the enzyme fraction was replaced with 10 mM potassium phosphate buffer pH 7.0 using a HiPrep 26/10 desalting column and the enzyme sample was applied onto a Bio-Scale CHT-20-I column (Bio-Rad) equilibrated with the phosphate buffer. The enzyme was eluted with a linear gradient of 10–250 mM potassium phosphate buffer pH 7.0. The enzyme fraction was concentrated by ultrafiltration (Vivaspin, 10 kDa cutoff) and loaded onto a HiLoad 16/60 Superdex 200 prep-grade column (GE Healthcare BioScience) equilibrated with buffer A containing 0.2 M NaCl. SDS–PAGE of the combined peak fractions eluted from the column revealed a single 36 kDa protein band on Coomassie Brilliant Blue staining. N-terminal sequence analysis confirmed the identity of the purified sample as GDH and showed that its amino-acid sequence started from the second alanine residue; the first methionine residue may have been cleaved off. Finally, the purified rabbit GDH was concentrated to 22.2 mg ml−1 by ultrafiltration and stored at 203 K. The preparation of the E. coli cell extract and purification of the enzyme were carried out at 278 K.
2.2. Crystallization and X-ray data collection
The Hampton Crystal Screen kit (Jancarik & Kim, 1991 ▶) was used to determine the crystallization conditions for rabbit GDH. Crystallization was carried out by the oil-microbatch method (Chayen et al., 1990 ▶) using Nunc HLA plates (Nalge Nunc International). Each crystallization drop was prepared by mixing 0.5 µl precipitant solution and 0.5 µl protein solution (22.2 mg ml−1 protein, 200 mM NaCl and 20 mM Tris–HCl pH 8.0). The crystallization drop was overlaid with a 1:1 mixture of silicone and paraffin oils, allowing slow evaporation of water in the drop, and stored at 295 K. After one week of incubation, plate-like crystals of the apoenzyme occasionally grew using precipitant solution No. 10 from Crystal Screen Cryo comprising 0.17 M ammonium acetate, 25.5%(w/v) polyethylene glycol 4000, 15%(v/v) glycerol and 85 mM acetate–NaOH pH 4.6. Crystals of the GDH–NADH complex (holoenzyme) were obtained by cocrystallization. NADH was added to the protein solution to a final concentration of 5 mM. Crystals grew under the same conditions as used for the apoenzyme in one week. All X-ray diffraction data sets were collected from crystals that had been directly flash-cooled from the crystallization drop at 100 K. Treatment with cryoprotectant solution was not required because of the high concentration of polyethylene glycol and glycerol in the crystallization solution. The diffraction data for the apoenzyme were collected using a Rigaku R-AXIS V image-plate detector and synchrotron radiation at beamline BL26B1 of SPring-8, Japan (Ueno et al., 2006 ▶). The diffraction data for the holoenzyme were collected in-house using a Rigaku R-AXIS VII image-plate detector and a MicroMax-007 generator operating with a copper target. All the measured diffraction spots were indexed, integrated and scaled using the HKL-2000 program package (Otwinowski & Minor, 1997 ▶).
3. Results
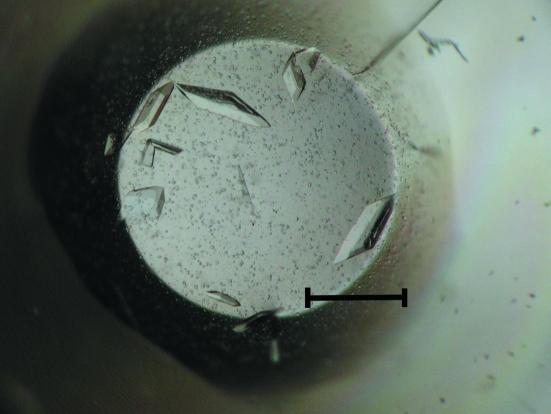
Crystals of rabbit GDH apoenzyme appeared one week after setup and grew to typical dimensions of 0.10 × 0.10 × 0.15 mm in two weeks (Fig. 1 ▶). Data-collection statistics are summarized in Table 1 ▶. The crystal belongs to the C-centred monoclinic space group C2, with unit-cell parameters a = 71.81, b = 69.08, c = 65.64 Å, β = 102.7°. Assuming the presence of a monomeric protomer of rabbit GDH in the asymmetric unit led to a crystal volume per protein weight (V M) of 2.21 Å3 Da−1, corresponding to a solvent content of 44.4% (Matthews, 1968 ▶). Structural determination of the apoenzyme crystal by the molecular-replacement method is in progress, using the coordinates of chain A of HAD from Archaeoglobus fulgidus (PDB code 1zej), which has 22% amino-acid sequence identity to rabbit GDH, as a search model. Calculations of cross-rotation and translation functions using the program MOLREP (Vagin & Isupov, 2001 ▶) from the CCP4 suite (Winn et al., 2002 ▶; Collaborative Computational Project, Number 4, 1994 ▶) showed a clear solution and a reasonable molecular arrangement without steric clashes in the asymmetric unit: the initial R factor and correlation coefficient at 4 Å resolution were 57.4% and 0.194, respectively. The cocrystal of the GDH–NADH binary complex (holoenzyme) was isomorphous with the apoenzyme crystal and a diffraction data set was collected to 1.85 Å resolution (Table 1 ▶). Structure determination of the holoenzyme by the difference Fourier method based on the apoenzyme structure is in progress using the program CNS (Brünger et al., 1998 ▶). Electron density for the bound cofactor NADH was clearly observed in the initial difference Fourier map. The refined structures will be published elsewhere.
Figure 1.
Crystals of rabbit GDH apoenzyme. The scale bar represents 0.2 mm.
Table 1. Data-collection statistics for rabbit GDH.
Values in parentheses correspond to the highest resolution shell.
| Apoenzyme | Holoenzyme | |
|---|---|---|
| Space group | C2 | C2 |
| Unit-cell parameters | ||
| a (Å) | 71.81 | 72.02 |
| b (Å) | 69.08 | 69.52 |
| c (Å) | 65.64 | 65.10 |
| β (°) | 102.7 | 102.7 |
| Wavelength (Å) | 1.00000 | 1.5418 |
| Resolution range (Å) | 30.0–1.70 (1.76–1.70) | 30.0–1.85 (1.92–1.85) |
| No. of unique reflections | 34467 | 26610 |
| Redundancy | 4.0 (4.0) | 3.2 (3.2) |
| Completeness (%) | 100.0 (99.9) | 99.1 (97.8) |
| Mean I/σ(I) | 9.0 (3.3) | 11.2 (4.9) |
| Rmerge† (%) | 7.5 (53.7) | 5.2 (22.9) |
R
merge = 
 , where I
i(hkl) and 〈I(hkl)〉 are the observed intensities of reflections with index hkl for measurement i and the mean intensity, respectively.
, where I
i(hkl) and 〈I(hkl)〉 are the observed intensities of reflections with index hkl for measurement i and the mean intensity, respectively.
Acknowledgments
We thank M. Yamamoto and his staff for their assistance with data collection at the beamline BL26B1 of SPring-8. This work (HTPF50004) was supported by the ‘National Project on Protein Structural and Functional Analyses’ funded by the MEXT of Japan.
References
- Barycki, J. J., O’Brien, L. K., Bratte, J. M., Zhang, R., Sanishvili, R., Strauss, A. W. & Banaszak, L. J. (1999). Biochemistry, 38, 5786–5798. [DOI] [PubMed]
- Barycki, J. J., O’Brien, L. K., Strauss, A. W. & Banaszak, L. J. (2000). J. Biol. Chem.275, 27186–27196. [DOI] [PubMed]
- Barycki, J. J., O’Brien, L. K., Strauss, A. W. & Banaszak, L. J. (2001). J. Biol. Chem.276, 36718–36726. [DOI] [PubMed]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed]
- Chayen, N. E., Shaw Stewart, P. D., Maeder, D. L. & Blow, D. M. (1990). J. Appl. Cryst.23, 297–302.
- Chen, J., Yu, L., Li, D., Gao, Q., Wang, J., Huang, X., Bi, G., Wu, H. & Zhao, S. (2003). Gene, 302, 103–113. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Ishikura, S., Usami, N., Araki, M. & Hara, A. (2005). J. Biochem.137, 303–314. [DOI] [PubMed]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411.
- Kobayashi, A., Jiang, L. L. & Hashimoto, T. (1996). J. Biochem.119, 775–782. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Mulders, J. W., Hendriks, W., Blankesteijn, W. M., Bloemendal, H. & de-Jong, W. W. (1988). J. Biol. Chem.263, 15462–15466. [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Schulz, H. (1991). Biochim. Biophys. Acta, 1081, 109–120. [DOI] [PubMed]
- Smiley, J. D. & Ashwell, G. (1961). J. Biol. Chem.236, 357–364.
- Ueno, G., Kanda, H., Hirose, R., Ida, K., Kumasaka, T. & Yamamoto, M. (2006). J. Struct. Funct. Genomics, 7, 15–22. [DOI] [PubMed]
- Vagin, A. A. & Isupov, M. N. (2001). Acta Cryst. D57, 1451–1456. [DOI] [PubMed]
- Winn, M. D., Ashton, A. W., Briggs, P. J., Ballard, C. C. & Patel, P. (2002). Acta Cryst. D58, 1929–1936. [DOI] [PubMed]