Abstract
The crystal structure of human deoxy hemoglobin (Hb) complexed with a potent allosteric effector (2-[4-[[(3,5-dimethylanilino)carbonyl]methyl]phenoxy]-2-methylpropionic acid) = RSR-13) is reported at 1.85 Å resolution. Analysis of the hemoglobin:effector complex indicates that two of these molecules bind to the central water cavity of deoxy Hb in a symmetrical fashion, and that each constrains the protein by engaging in hydrogen bonding and hydrophobic interactions with three of its four subunits. Interestingly, we also find that water-mediated interactions between the bound effectors and the protein make significant contributions to the overall binding. Physiologically, the interaction of RSR-13 with Hb results in increased oxygen delivery to peripheral tissues. Thus, this compound has potential therapeutic application in the treatment of hypoxia, ischemia, and trauma-related blood loss. Currently, RSR-13 is in phase III clinical trials as a radiosensitizing agent in the treatment of brain tumors. A detailed structural analysis of this compound complexed with deoxy Hb has important implications for the rational design of future analogs.
Keywords: Allosteric effector, hemoglobin, oxygen delivery
Hemoglobin (Hb) is an allosteric tetrameric protein that exists in equilibrium between the deoxy (T or Tense) and oxy (R or Relaxed) states. It is composed of two αβ dimers that are arranged around a twofold axis of symmetry. This arrangement yields a large central water cavity in the deoxy state, and a narrower cavity in the oxy state. An indigenous allosteric effector of Hb, 2,3 diphospoglycerate (2,3-DPG), preferentially stabilizes deoxy Hb by forming intermolecular salt bridges between the two β subunits (Arnone 1992). This added stabilization decreases the affinity of Hb for oxygen, and subsequently allows for the release of more oxygen in the cardiovascular periphery.
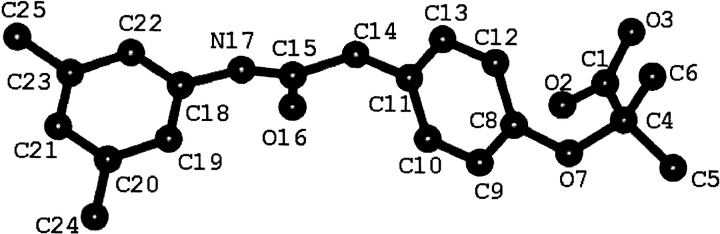
Due to the numerous potential therapeutic applications of allosteric Hb modulators, a variety of synthetic organic molecules have been examined for their effects on the Hb allosteric equilibrium. The search for antisickling agents led to the discovery that the antilipidemic fibrate agents—clofibrate (Abraham et al. 1983, 1984) and bezafibrate (Perutz and Poyart 1983)—lower the oxygen affinity of Hb in a physiologically similar fashion as 2,3 DPG. However, testing of these compounds in whole blood indicated that they had poor erythrocyte cell wall penetration, and that they were also extensively bound to serum albumin. As a result, numerous synthetic analogs of bezafibrate have been generated and tested for allosteric potency (Lalezari et al. 1988, 1990). One of these analogs, RSR-13, was synthesized in our laboratories, and possesses superior activity when tested for allosteric potency in whole blood (Randad et al. 1992) (Fig. 1 ▶).
Fig. 1.
The structure of RSR-13 with atom numbering used in the text.
A difference electron density map of an unrefined low-resolution structure of RSR-13 co-crystallized with deoxy Hb indicated that this effector binds to three of the four protein subunits (two α subunits and one β subunit) along the central water cavity, and thus lowers the oxygen affinity of Hb by stabilizing the deoxy state (Abraham et al. 1992a, 1992b; Wireko et al. 1992). However, the unrefined low-resolution structure did not allow for a detailed analysis of residue:effector and structural water:effector interactions. Therefore, to better characterize the atomic level interactions responsible for the allosteric activity of RSR-13, the high-resolution (1.85 Å) X-ray structure of this effector co-crystallized with deoxy Hb has been determined.
In contrast to the low-resolution structure, our high-resolution complex identifies several key interactions that allow for a detailed description of how RSR-13 stabilizes deoxy Hb. In particular, the high-resolution structure indicates that several structural waters mediate effector:deoxy Hb hydrogen bonds; these waters were not observed in the low-resolution structure. Importantly, we have identified a water that mediates a hydrogen bond between the methylpropionic acid moiety of the effector and the guanidinium side-chain moiety of residue α Arg141. Conversely, the low-resolution structure indicated a direct salt bridge between the effector methylpropionic acid moiety and the αArg141 side-chain guanidinium moiety (Abraham et al. 1992a, 1992b; Wireko et al. 1992). Based on this type of discrepancy between our structure and the low-resolution structure, analysis of the presented high-resolution structure is an important step towards fully understanding all of the fundamental atomic level interactions between RSR-13 and deoxy Hb, and will play a key role in the future design of more potent RSR-13 analogs.
Results
Structural description
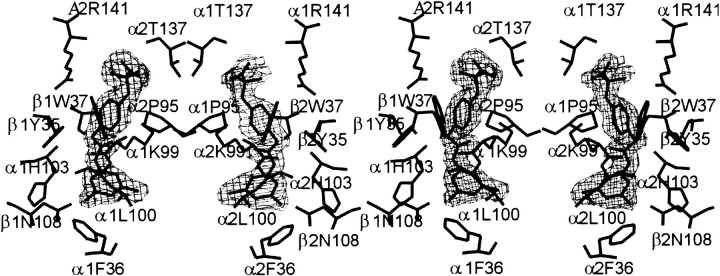
The final model for the RSR-13:deoxy Hb complex consists of 574 Hb amino acid residues, four heme groups, and 260 water molecules. Two sulfates were identified in association with the β-subunits, as described previously (Fronticelli et al. 1994), and two RSR-13 molecules were allosterically bound within the deoxy Hb central water cavity. The structure has a crystallographic Rfactor of 17.7% and an Rfree of 20.8% for all data at 1.85 Å resolution. The main chain-dihedral angles for most residues in the final model (93.8%) lie in the most favored regions of the Ramachandran plot (Laskowski et al. 1993), with the remainder in the additional allowed region. Detailed crystallographic data are summarized in Table 1. Overall, the electron density map of the structure is very good, with only weak main-chain electron densities observed for N-terminal residues β1Val1, β1His2, and β2Val1. Both effectors are well ordered, as shown by their refined 2Fo−Fc electron density maps (Fig. 2 ▶). Structure factors and the atomic coordinate set have been deposited in the Protein Data Bank, entry code 1G9V.
Table 1.
Crystallographic data and refinement statistics for RSR-13:Deoxy Hb complex a
| Data collection statistics | |
| Unit cell dimensions | 63.20 83.56 53.86 99.16 |
| Resolution (Å) | 55.0–1.85 (1.91–1.85) |
| Observed reflection | 165826 (14162) |
| Unique reflection | 44139 (3912) |
| Completeness | 93.7 (85.1) |
| Rmergeb | 5.9 (27.1) |
| Refinement statistics | |
| a Numbers in parantheses refer to the outermost resolution bin. | |
| b Rmerge = Σ(<I> − I)/ΣI. | |
| c The Rfactor and Rfree are based on 95% and 5% of the reflections used in the refinement, respectively. | |
| Resolution (Å) | 55.0–1.85 (1.91–1.85) |
| Number of reflections | 44139 (9721) |
| Rfactorc | 17.7 (32.8) |
| Rfreec | 20.8 (34.8) |
| rmsd from ideal values | |
| Bond length (Å) | 0.011 |
| Bond angle (°) | 1.67 |
| average B-values (Å2) | |
| All non-hydrogen atoms | 22.2 |
| Protein atoms | 21.3 |
| Heme atoms | 19.4 |
| Water atoms | 36.2 |
| Effector atoms | 28.4 |
| estimated positional error (Luzzatti) | |
| Rfactor | 0.21 |
| Rfree | 0.25 |
Fig. 2.
Stereo figure of the final 2Fo–Fc electron density map of bound RSR-13 effector molecules at the allosteric sites contoured at 1 σ. The figures were generated with TOM (Cambillau and Horjales 1987).
Effector:deoxy Hb residue interactions
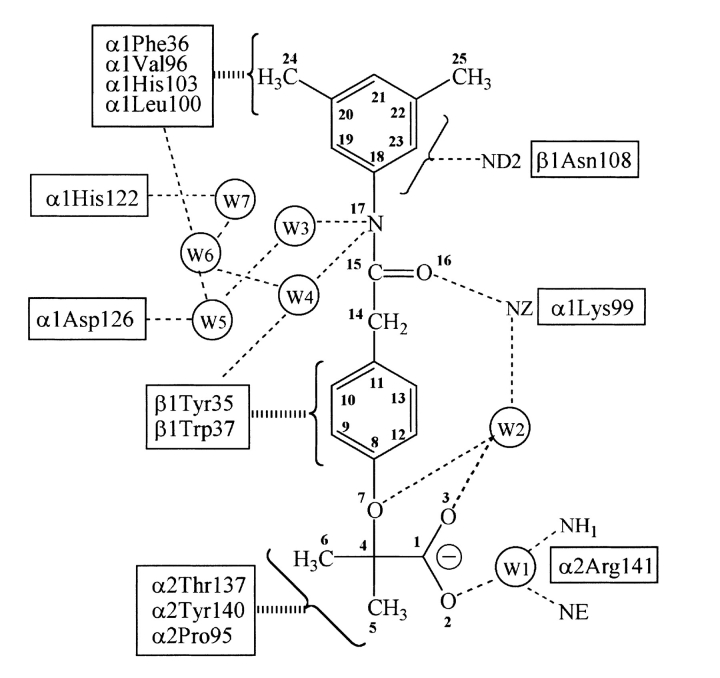
The co-crystallized structure indicates that two RSR-13 molecules, which will be referred to as 801 and 802, bind to deoxy Hb along the central water cavity in twofold symmetry-related, but not crystallographically identical, sites. Each effector interacts with three different subunits (two α-subunits and one β-subunit of the deoxy Hb tetramer). Molecule 801 engages in contacts with residues from the α1, α2, and β1 subunits; molecule 802 makes symmetrically related contacts with the α2, α1, and β2 subunits. Table 2 summarizes hydrogen-bonding interactions between the two effectors and the protein; Figure 3 ▶ sets out a two-dimensional schematic of interactions by and surrounding RSR-13 (molecule 801).
Table 2.
Hydrogen bonds between the RSR-13 effector and Deoxy Hb at the allosteric sitea
| Interaction | Distance | |||
| Effector | Waterb | Protein | Effector 801 | Effector 802 |
| O2 | Wat 2 | α1Lys99 (NZ) | 3.3–3.0 | — |
| O3 | Wat 1 | α2Arg141 (NE) | 3.0–2.7 | 2.8–2.7 |
| O7 | Wat 2 | α1Lys99 (NZ) | 3.3–3.0 | — |
| O16 | — | α1Lys99 (NZ) | 3.3 | 3.0 |
| N17 | Wat 4 | β1Tyr35 (OH) | 2.9–3.1 | 3.3–2.9 |
| N17 | Wat 3 | α1Lys99 (O) | 3.5–2.9 | 3.5–2.8 |
| C18–C25c | — | β1Asn108 (ND2) | 3.2–3.8 | 3.3–3.8 |
aUpper limit of hydrogen bond contact set to 3.8Å.
bOnly waters that bind directly to RSR-13 are shown.
cπ-electrons of the dimethyl substituted aromatic ring engage in hydrogen bond interactions with the ND2 of βAsn108.
Fig. 3.
Two-dimensional schematic of contacts between RSR-13, surrounding Hb residues, and structurally conserved water molecules. Narrow dashed lines indicate hydrogen bond and electrostatic interactions; broad dashed lines indicate hydrophobic contacts.
The methylpropionic acid moiety of effector 801 fits into a cavity surrounded by residues α1Thr134, α2Pro95, α2Thr137, α2Tyr140, α2Arg141, and β1Trp37 (Figs. 3, 4A ▶ ▶). These contacts are mainly hydrophobic in nature, with the exception of two water-mediated hydrogen bonds between the methylpropionic acid and the protein. The phenoxy ring of effector 801 is sandwiched between, and engages in hydrophobic contacts with, residues α1Lys99, α2Arg141, β1Tyr35, and β1Trp37. Symmetry-related effector:protein interactions are found at the 802 allosteric binding site.
Fig. 4.
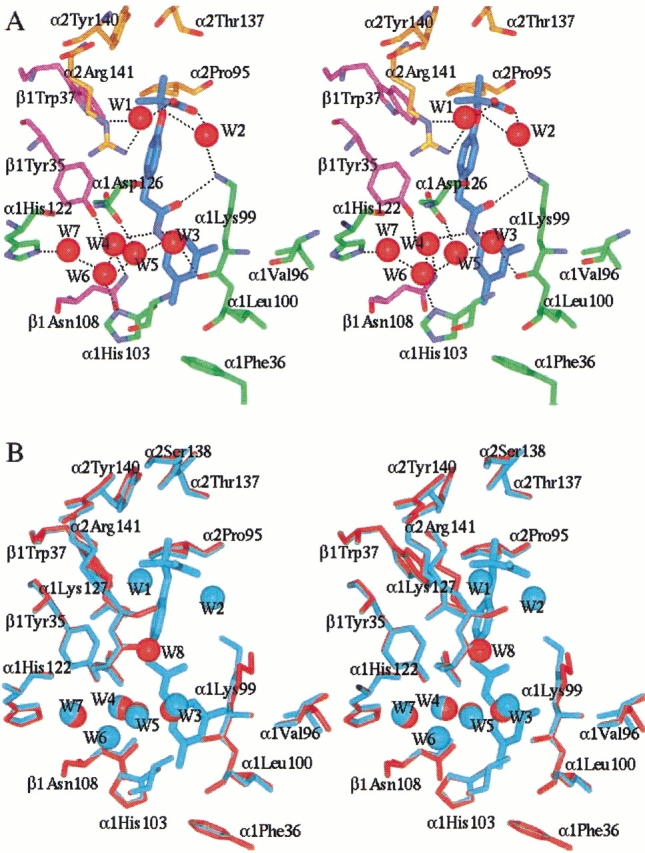
Stereoview of the allosteric site of effector 801. Similar effector environment is observed at the effector 802 site. Protein and effector atoms are shown in stick, and waters are spheres. For clarity, not all residues lining the allosteric binding site are shown. (A) Interactions between effector 801 (cyan) and Hb residues from the α1 subunit (green), α2 subunit (gold), and β1 subunit (magenta) are shown. Oxygen and nitrogen atoms are colored red and blue, respectively. Hydrogen bonds are indicated by black dashes. (B) Superimposition of the allosteric site of the native deoxy Hb structure (red) and the RSR-13:deoxy Hb complex structure (cyan). Note that the water molecules Wat 3, Wat 4, Wat 5, and Wat 7 are conserved, while Wat 1, Wat 2, and Wat 6 are unique to the complexed structure, and Wat 8 is unique to the native structure. The figures were generated using InsightII (Molecular Simulations Inc.) and labeled with Showcase.
The carbonyl oxygen (O16) of each effector forms a hydrogen bond with the side-chain amino group of αLys99 at both allosteric binding sites. Interestingly, residues α1Lys99 and α2Lys99, unlike other residues engaging in interactions with the effectors, experience extensive side-chain movement during effector binding. The native position of α2Lys99 partially overlaps with the allosteric binding site of effector 802. The 3,5-dimethyl benzene ring of effector 801 binds in a narrow hydrophobic pocket composed of residues α1Val96, α1Lys99, α1Leu100, α1His103, α1Phe36, and β1Asn108. As observed in Figure 4A ▶, one of the methyl groups points toward the central water cavity, while the other is oriented toward the hydrophobic binding pocket. Comparable symmetry-related interactions are observed at the effector 802 binding site. Furthermore, the π-electrons of the effectors 3,5-dimethyl benzene rings engage in hydrogen bonds with the side-chain amide nitrogens (ND2) of residue βAsn108 at both allosteric binding sites. Perutz and Poyart (1983) have observed a similar interaction between the π-electrons of a bezafibrate aromatic ring and βAsn108.
Water structure at effector binding sites
Several water-mediated hydrogen bonds exist between RSR-13 and Hb residues, and contribute significantly to effector binding. At the effector 801 binding site, seven water molecules form a hydrogen-bonding network between RSR-13 and several Hb residues (Figs. 3, 4A ▶ ▶). These water molecules are labeled Wat1–Wat7. Water molecules Wat1, Wat2, and Wat6 are not found in the native deoxy Hb structure (Fermi et al. 1984), while water molecules Wat3, Wat4, Wat5, and Wat7 are conserved, and are located in roughly the same positions as observed in the native structure. All water molecules at the 801 binding site are replicated at the 802 binding site, with the exception of Wat2.
The water labeled Wat1 mediates a hydrogen bond between the methylpropionic acid (O3) of effector 801 and the guanidinium side-chain moiety of α2Arg141, while water Wat2 bridges between the 801 methylpropionic acid (O2) and the side-chain amino group of α1Lys99. For effector 802 a symmetry-related water-mediated interaction is observed with α1Arg141. However, water-mediated interactions for the two effectors differ in that no structural water is observed to bridge between the methylpropionic acid moiety of 802 and the side-chain amino group of α2Lys99. Significantly, the positioning and orientation of the methylpropionic acid moieties of the effectors in the high-resolution structure differ substantially when compared to corresponding methylpropionic acid moiety positioning in the low-resolution structure, which were shown to engage in direct contacts with αArg141 (Abraham et al. 1992a, 1992b; Wireko et al. 1992). Water molecule Wat2 in the high-resolution structure also links the ether oxygen (O7) of effector 801 to the amino group of α1Lys99 (Fig. 4A ▶). There is no such interaction at the 802 binding site, as this water molecule is not present (vide supra).
The four conserved water molecules (Wat3, Wat4, Wat5, and Wat7) and one non-conserved water molecule, Wat6 (in the 801 binding site), form a network of hydrogen bonds that link the effector amide nitrogen atom (N17) to six Hb residues (Figs. 3, 4A ▶ ▶). Four of the water molecules (Wat3, Wat4, Wat5, and Wat6) and the effector amide nitrogen (N17) form a pentagonal network. All water molecules described above are well ordered, as indicated by their B-factors. Comparable waters and water-mediated interactions are observed at the 802 binding site.
Comparison of the deoxy Hb:RSR-13 complex and native deoxy Hb
A least-square fit of all Cα in the RSR-13:deoxy Hb complex and the native deoxy Hb structure produces an rmsd of only 0.14 Å. For all atoms, the rmsd is 0.52 Å. The two structures also display similar overall B-factors of 22.1 Å2 and 24.8 Å2, respectively. Significant differences in the two structures occur at the N-terminal residues: β1Val1, β1His2, and β2Val1, and at the allosteric binding sites. Furthermore, residue αGlu43, which is located at the α1β2 (α2β1) interface, also reorients to engage in a stronger hydrogen bond with βArg92 in the complexed structure.
Superimposition of the allosteric binding sites in the native deoxy Hb structure and the RSR-13:deoxy Hb structure clearly shows that the side chains of both αLys99 residues have reoriented to effect hydrogen bonds with the three atom bridge carbonyl oxygens of both effectors (Fig. 4B ▶). Analysis of all other residues participating in effector binding indicates very little structural change.
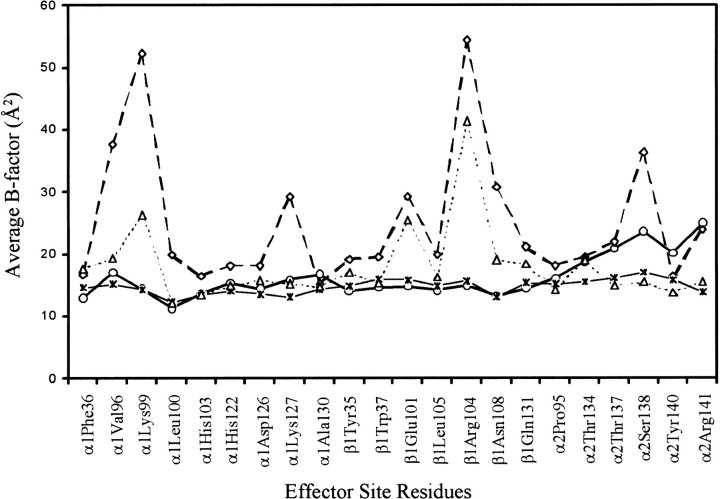
Figure 5 ▶ displays B-factor plots for residues close to, and lining, the effector 801 allosteric binding site in both the native and complexed structures. A similar plot is obtained for the effector 802 site. On average, B-factors for residues are lower in the complexed structure. Of particular interest are the residues α1Asp126, α1Lys127, α2Tyr140, and α2Arg141. In native deoxy Hb (Bolton and Perutz 1970; Ward et al. 1975; Fermi et al. 1984; Perutz and Fermi 1993) strong hydrogen bonds between α2Arg141 and α1Asp126, and α2Arg141 and α1Lys127 are important for stabilizing the deoxy state. During the transition from the T to R-state, the hydrogen bond between α2Arg141 and α1Asp126 breaks, and as a result, α2Arg141 becomes disordered (Baldwin and Chothia 1979; Shaanan 1993). The interaction between α2Arg141 and α1Lys127 is not lost during the T to R-state transition; however, this bond is significantly weakened in the T-state. Also, the penultimate residue α2Tyr140, which hydrogen bonds with α1Val93 in the T-state, becomes less ordered in the R-State (Baldwin and Chothia 1979). The observed decrease in B-factor for these residues in the effector:deoxy Hb complex is indicative of more constrained interactions between residue α2Arg141 and residues α1Asp126 and α1Lys127, and also between residues α2Try140 and α1Val93, and consequently, may lead to a more stabilized deoxy Hb tetramer. Residues α1Lys99 and β1Asn108 also display lower B-factors in the effector:deoxy Hb complex. In fact, examination of the native structure indicates that the side-chain of α1Lys99 is highly disordered, and that its position is tenuous.
Fig. 5.
B-factor plots for the native deoxy Hb structure (thick lines) and the RSR-13:deoxy Hb complex structure (thin lines). Shown are the average B-factors (Å2) for the main-chain (continuous lines) and side-chain (dashed lines) atoms. The B-factors are plotted against the Hb residues that line the effector 801 binding site. Open squares are the side-chain plot for native Hb; open triangles are the side-chain plot for the complex; open circles are the main-chain plot for the native; asterisks are the main-chain plot for the complex.
Interestingly, residues βArg104 and βGlu101 in the RSR-13:deoxy Hb structure also display significant drops in B-factors, but do not engage in direct contact with the effector. The reason for the drop in temperature for these two residues is not clear, but appears to reflect a general decrease in B-factor for all residues adjacent to, and lining the allosteric site in the complexed structure. In general, the two allosteric binding sites are less ordered in the native deoxy Hb structure, but become well ordered after effector binding.
Discussion
The ability of allosteric effectors to modulate Hb oxygen affinity is based on the natural allosteric equilibrium between the T- and R-states of this protein. RSR-13 shifts this equilibrium by stabilizing the T-state, leading to a decrease in the oxygen affinity of Hb. The high-resolution structure presented in this study provides a significantly more detailed description of the RSR-13:deoxy Hb complex than previously reported (Abraham et al. 1992a, 1992b; Wireko et al. 1992). Examination of our structure indicates that two allosterically bound effectors engage in several important interactions that include not only direct effector–protein contacts, but also water-mediated hydrogen bonds. Several of these contacts were not identified for each bound effector in the previously reported low-resolution structure.
Based on analysis of our structure, interactions between αLys99 residues and effector carbonyl oxygens (O16) at both allosteric binding sites are extremely important in regulating the allosteric equilibrium, as these interactions serve to significantly stabilize the deoxy Hb dimer–dimer interface. Similar analogs of RSR-13, with their carbonyl oxygen pointing away from αLys99, do not lower the Hb oxygen affinity as effectively as RSR-13 (Wireko et al. 1992). These analyses also indicate that insertion of the effector 3,5-dimethyl benzene group, and particularly one of its methyl substituents, into a hydrophobic groove of the G-helix constrains deoxy Hb by preventing subunit rotation during the allosteric transition. This is corroborated by studies that have shown decreased potency for analogs missing one or both of these methyl substituents (Randad et al. 1992). Our RSR-13:deoxy Hb structure also indicates that interactions between the amide ND2 of βAsn108 and the π-electrons of the effectors 3,5-dimethyl benzene rings are important for stabilizing the T-state. Kroeger and Kundrot have shown that mutation of βAsn108 to Lys results in a marked decrease in Hb oxygen affinity (Kroeger and Kundrot 1997). In the native deoxy Hb structure, βAsn108 does not engage in any direct residue–residue interactions, but does participate in a water-mediated hydrogen bond with residue βTyr35. Upon mutation, a direct interaction between mutant βLys108 and βTyr3β was observed to stabilize the T-state. Similarly, RSR-13 hydrogen bonds with βAsn108 should also serve to further stabilize the T-state.
The high-resolution structure reveals that water-mediated interactions, which were not identified in the low-resolution structure, are also important for RSR-13 binding. In particular, water-mediated interactions between the effector methylpropionic acid moieties and the side-chain guanidinium of αArg141, in both allosteric binding sites, appear to be the key for allosteric potency. Preliminary structural analyses of RSR-13 analogs that do not maintain these water-mediated interactions, but instead engage in direct hydrogen bonds with αArg141, are weaker allosteric effectors (Abraham, unpubl.). This observed decrease in potency may be attributed to the movement of residue αArg141 upon effector binding, which subsequently weakens an important deoxy Hb stabilizing interaction between it and residue αAsp126. During transition from the T- to the R-state, αArg141 moves by 7.5 Å, and a salt bridge between it and αAsp126 is lost. Mutant Hb αArg141–His (Poyart et al. 1980) and αAsp126–Asn (Moo-Penn et al. 1977) have both been shown to disrupt this salt bridge, and consequently result in Hb with increased oxygen affinity (i.e., the T-state of these mutants is destabilized). Our high-resolution structure reveals that the new water-mediated interaction between αArg141 and RSR-13 does not significantly change the original position of this residue compared with the native deoxy Hb structure. Thus, original residue–residue contacts made by αArg141 with αAsp126 are not significantly perturbed upon effector binding. Conversely, when the methylpropionic acid moieties of effectors engage in direct interactions with the αArg141 side-chain guanidinium moiety, this residue reorients to facilitate the new interaction with the effector, and its salt bridge with αAsp126 is weakened. As a result, the deoxy state is less constrained, and these effectors are less potent allosteric effectors than RSR-13. In the high-resolution RSR-13:deoxy Hb complex, B factors for both αArg141 and αAsp126 are lower than observed in the native deoxy Hb structure, which confirms that the binding of this effector confers stability to these important residues, and further stabilizes the T-state.
Finally, the extensive water-mediated interactions between the amide nitrogens (N17) of the RSR-13 effectors (at both binding sites) and surrounding protein residues also contributes significantly to effector binding, and additionally stabilizes the T-state.
Conclusions
The high-resolution crystal structure of the RSR-13 co-crystallized with deoxy Hb has provided new insights into the atomic level intermolecular interactions that lead to the allosteric modification of Hb by this effector. The presented analysis of interactions between RSR-13 and deoxy Hb will aid in the identification of new sites of effector modification, which will aid in the development of more potent allosteric effectors. The well-ordered water molecules around the effector may be targets for occupation by substituent polar atoms. In particular, the water molecules labeled Wat3 and Wat4 may be replaced with electronegative atoms, which may directly interact with protein residues (see Fig. 4A ▶). Furthermore, the 3,5-dimethyl aromatic ring may also be targeted as a site for the generation of additional hydrophobic interactions.
Materials and methods
Crystallization, X-ray data collection, and processing
The synthesis of RSR-13 is described elsewhere (Randad et al. 1992). For RSR-13: deoxy Hb complex formation and crystallization, a solution of 3.72 × 10−2 mM of RSR-13 Na salt (13.5 mg) was added to 7.44 × 10−3 mM of oxygenated Hb solution in 50 mM Bis Tris, pH 6.8 (60 mg/mL). The complex was deoxygenated under vacuum for 1 h. The reduced solution was reconstituted to 60 mg/mL with 10 mM phosphate buffer at pH 7.4 in a nitrogen-filled glove box. The solution had a final ratio of five effector molecules to one Hb molecule. Crystals were then grown in 3.6 M sulfate/phosphate precipitant (pH = 6.5) by the batch method as described by Perutz (1968). X-ray quality crystals grew within 5 days. Crystals for diffraction were mounted in a capillary between layers of mother liquor under nitrogen atmosphere.
X-ray diffraction data were collected using an R-axis II image plate detector equipped with a Rigaku RU-200 generator operating at 50 kV and 180 mA. The space group is P21 with one molecule in the asymmetric unit. The crystal is isomorphous to that of the native deoxy Hb structure (Fermi et al. 1984). Diffraction data were collected at room temperature to 1.85 Å, and processed using the program Biotex from Molecular Structure Corporation and the CCP4 suite of programs (CCP4 1994). The final structure factor file contains anomalous differences, and was used to refine the structure. Data collection and processing statistics are summarized in Table 1.
Structure refinement
The isomorphous 1.7 Å native deoxy Hb structure (PDB code 1HHB) (Fermi et al. 1984), excluding the water and phosphate molecules, was used as the starting model for the refinement of the complex. The refinement was performed with the X-PLOR program (Brunger 1992a), with a bulk solvent correction applied. A statistically random selection of 5% of the total reflection data was excluded from the refinement and used to calculate the free Rfactor (Rfree) as a monitor of model bias (Brunger 1992b). The model was subjected to positional refinement with data from 55.0 to 2.0 Å and extended to 1.85 Å. The 2Fo−Fc and Fo−Fc electron density maps indicated clear densities for two sulfates at the β-subunits, and also two molecules of RSR-13 at the allosteric binding sites, as previously observed (Abraham et al. 1992a, 1992b; Wireko et al. 1992). The sulfate and RSR-13 molecules were added to the model. The model was then subjected to three alternate rounds of positional and simulated annealing, with the addition of water molecules and manual adjustments of some side chains. Only water molecules with B-factors less than 55 Å2 were retained in the model. All model building and model corrections were carried out using TOM (Cambillau and Horjales 1987). Refinement statistics are summarized in Table 1.
Acknowledgments
This work was supported by grants from the National Institutes of Health HL 32793 (D.J.A.).
The publication costs of this article were defrayed in part by payment of page charges.This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.50601.
References
- Abraham, D.J., Perutz, M.F., and Philips, S.E. 1983. Physiological and x-ray studies of potential antisickling agents. Proc. Natl. Acad. Sci. USA 80 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham, D.J., Kennedy, P.E., Mehanna, A.S., Patwa, D.C., and Williams, F.L. 1984. Design, synthesis, and testing of potential antisickling agents. 4. Structure–activity relationships of benzyloxy and phenoxy acids. J. Med. Chem. 27 967–978. [DOI] [PubMed] [Google Scholar]
- Abraham, D.J., Peascoe, R.A., Randad, R.S., and Panikker, J. 1992a. X-ray diffraction study of di- and tetra-ligated T-state hemoglobin from high salt crystals. J. Mol. Biol. 227 480–492. [DOI] [PubMed] [Google Scholar]
- Abraham, D.J., Wireko, F.C., Randad, R.S., Poyart, C., Kister, J., Bohn, B., Liard, J.F., and Kunert, M.P. 1992b. Allosteric modifiers of hemoglobin: 2-[4-[[3,5-dimethylanilino[(carbonyl)methyl]-phenoxy]-2-methylpropionic acid derivatives that lower the oxygen affinity of hemoglobin in red cell suspensions, in whole blood, and in vivo in rats. Biochemistry 31 9141–9149. [DOI] [PubMed] [Google Scholar]
- Arnone, A. 1992. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237 146–149. [DOI] [PubMed] [Google Scholar]
- Baldwin, J. and Chothia, C. 1979. Hemoglobin: The structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 129 175–220. [DOI] [PubMed] [Google Scholar]
- Bolton, W. and Perutz, M.F. 1970. Three dimensional Fourier synthesis of horse deoxy Hb at 2.8 Å. Nature 228 551–552. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T. 1992a. X-PLOR, version 3.840: A system for X-ray crystallography and NMR. Yale Univeristy Press, New Haven, CT.
- Brunger, A.T. 1992b. Free R-value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 355 472–475. [DOI] [PubMed] [Google Scholar]
- Cambillau, C. and Horjales, E. 1987. TOM: A Frodo subpackage for protein–ligand fitting with interactive energy minimization. J. Mol. Graph. 5 174–177. [Google Scholar]
- CCP4. Collaborative Computing Project Number 4. 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D50 760–763. [DOI] [PubMed] [Google Scholar]
- Fermi, G., Perutz, M.F., Shaanan, B., and Fourme, R. 1984. The crystal structure of human deoxyhemoglobin at 1.7Å resolution. J. Mol. Biol. 175 159–174. [DOI] [PubMed] [Google Scholar]
- Fronticelli, C., Pechick, I., Brinigar, W., Kowalczyk, J., and Gilliland, G. 1994. Chloride ion independence of the Bohr effect in a mutant human hemoglobin (βV1M +H2 deleted). J. Biol. Chem. 269 23965–23969. [PubMed] [Google Scholar]
- Kroeger, K.S. andKundrot, C.E. 1997. Structures of hemoglobin-based blood substitute: Insights into the function of allosteric proteins. Structure 5 153–311. [DOI] [PubMed] [Google Scholar]
- Lalezari, I., Rahbar, S., Lalezari, P., Fermi, G., and Perutz, M.F. 1988. LR16, a compound with potent effects on the oxygen affinity of hemoglobin, on blood cholesterol, and on low density lipoprotein. Proc. Natl. Acad. Sci. USA 85 6117–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari, I., Lalezari, P., Poyart, C., Marden, M., Kister, J., Bohn, B., Fermi, G., and Perutz, M.F. 1990. New effectors of human hemoglobin: Structure and function. Biochemistry 29 1515–1523. [DOI] [PubMed] [Google Scholar]
- Laskowski, R.A., Macarthur, M.W., Moss, D.S., and Thornton, J.M. 1993. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26 283–291. [Google Scholar]
- Moo-Penn, W.F., Jue, D.L., Johnson, M.H., Wilson, S.M., Therrell, B., and Schmidt, R.M. 1977. Hemoglobin Tarrant: 126(H9) Asp leads to Asn. A new hemoglobin variant in the alpha1beta1 contact region showing high oxygen affinity and reduced cooperativity. Biochim. Biophys. Acta 490 443–451. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. 1968. Preparation of hemoglobin crystals. J. Crystal. Growth 2 54–56. [Google Scholar]
- Perutz, M.F. and Fermi, G. 1993. A novel allosteric mechanism in hemoglobin. J. Mol. Biol. 233 536–545. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. and Poyart, C. 1983. Bezafibrate lowers oxygen affinity of haemoglobin. Lancet ii 881–882. [DOI] [PubMed] [Google Scholar]
- Poyart, C., Bursaux, E., Arnone, A., Bonaventura, J., and Bonaventura, C. 1980. Structural and functional studies of hemoglobin Suresnes (arg141 alpha 2 replaced by His beta). Consequences of disrupting an oxygen-linked anion-binding site. J. Biol. Chem. 255 9465–9473. [PubMed] [Google Scholar]
- Randad, R.S., Mahran, M.A., Mehanna, A.S., and Abraham, D.J. 1992. Allosteric modifiers of hemoglobin. Design, synthesis, testing and structure–allosteric activity relationship of novel hemoglobin oxygen affinity decreasing agents. J. Med. Chem. 34 752–757. [DOI] [PubMed] [Google Scholar]
- Shaanan, B. 1983. Structure of oxyhemoglobin at 2.1 Å resolution. J. Mol. Biol. 171 31–59. [DOI] [PubMed] [Google Scholar]
- Ward, K.B., Wishner, B.C., Lattman, E.E., and Love, W.E. 1975. Structure of deoxyhemoglobin a crystals grown from polyethylene solutions. J. Mol. Biol. 98 161–177. [DOI] [PubMed] [Google Scholar]
- Wireko, F.C., Kellogg, G.E., and Abraham, D.J. 1992. Allosteric modifiers of hemoglobin. 2. Crystallographic determined binding sites and hydrophobic binding/interaction analysis of novel hemoglobin oxygen effectors. J. Med. Chem. 34 758–767. [DOI] [PubMed] [Google Scholar]