Abstract
The structure of calbindin D9k with two substitutions was determined by X-ray crystallography at 1.8-Å resolution. Unlike wild-type calbindin D9k, which is a monomeric protein with two EF-hands, the structure of the mutated calbindin D9k reveals an intertwined dimer. In the dimer, two EF-hands of the monomers have exchanged places, and thus a 3D domain-swapped dimer has been formed. EF-hand I of molecule A is packed toward EF-hand II of molecule B and vice versa. The formation of a hydrophobic cluster, in a region linking the EF-hands, promotes the conversion of monomers to 3D domain-swapped dimers. We propose a mechanism by which domain swapping takes place via the apo form of calbindin D9k. Once formed, the calbindin D9k dimers are remarkably stable, as with even larger misfolded aggregates like amyloids. Thus calbindin D9k dimers cannot be converted to monomers by dilution. However, heating can be used for conversion, indicating high energy barriers separating monomers from dimers.
Keywords: 3D domain swapping, EF-hand, calbindin D9k, folding, Ca2+ binding
Proteins are frequently metastable. Mutations or changes in the environment may induce altered conformations and aggregation as, for example, in amyloids. 3D domain swapping is a variant of this phenomenon. It has been defined (Schlunegger et al. 1997) as a process in which one domain of a multidomain protein breaks its noncovalent bonds with other domains. The disrupted interactions are restored by contacts between separate protein chains. In this way dimers or higher oligomers are created. Although many EF-hand proteins, like the S100 proteins and calpain, naturally occur as dimers (Donato 1999; Blanchard et al. 1997), no example of swapping EF-hands has yet been reported.
Calbindin D9k is an 8.5-kD protein with two EF-hands. It is thought to take part in transcellular calcium transport (Christakos et al. 1989) and magnesium uptake in the intestine (Hemmingsen et al. 1994). At physiological conditions with variations in calcium ion concentration, either magnesium is bound to one of the EF-hands (Andersson et al. 1997) or calcium binds to both EF-hands with positive cooperativity. The dimer with swapped EF-hands was obtained in an attempt to study a mutated form of calbindin D9k with reduced calcium affinity. The swapping mutant is denoted (Q22N + P43M) and contains the substitutions Gln 22 → Asn and Pro 43 → Met. The monomeric structure of calbindin D9k in its apo (Skelton et al. 1992), calcium (Szebenyi and Moffat 1986; Svensson et al. 1992), and magnesium (Andersson et al. 1997) forms is well known from X-ray crystallography and/or NMR. Calbindin D9k has one globular domain, and the separate EF-hands may be referred to as subdomains. The swapping of EF-hands is thus an example of subdomain swapping rather than swapping of entire domains.
In this paper we describe the structure of domain-swapped calbindin D9k and show which substitution is crucial for 3D domain swapping to take place. We also propose a mechanism for the formation of the 3D domain-swapped dimer.
Results and Discussion
Description of the structure
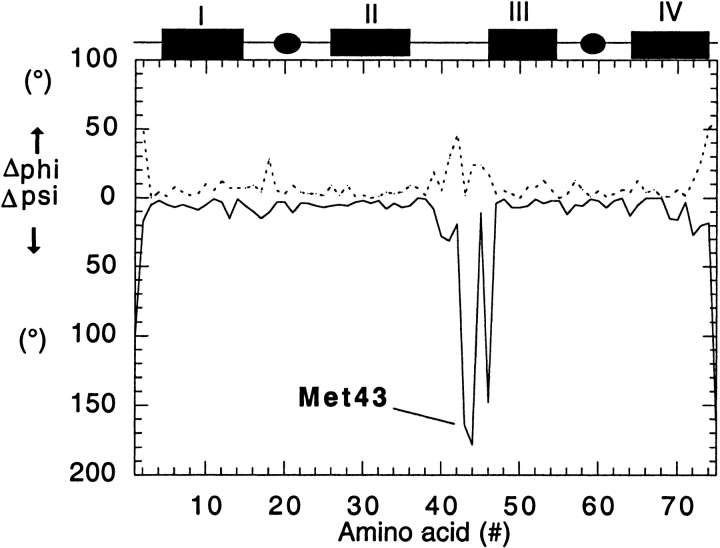
The crystal structure of the mutant was determined at 1.8-Å resolution. The major fold of wild-type calbindin D9k (Fig. 1A ▶) is conserved and occurs twice in the dimer (Fig. 1B ▶). EF-hand I of molecule A is packed together with EF-hand II of molecule B and vice versa. Each I–II domain is virtually identical to intact monomeric wild-type calbindin D9k. An approximate twofold axis relates the domains to one another. The major changes in the dimer structure compared to wild-type calbindin D9k are found in the linker loop (residues 38–44) and the first residue (Thr 45) of helix III. Amino acids 39–45 of (Q22N + P43M) exhibit about 20° or more difference (Fig. 2 ▶) in either or both of the φ and ψ angles as compared to the wild-type structure (Svensson et al. 1992). The region around the substitution Pro 43 → Met is hence adjustable. The Gln 22 → Asn substitution is situated in EF-hand loop I. The Gln 22 side chain is not a Ca2+ ligand, but the Gln 22 → Asn substitution leads to reduced Ca2+ affinity (J. Fast, M. Håkansson, A. Muranyi, G.P. Gippert, E. Thulin, J. Evenäs, L.A. Svensson, and S. Linse, unpubl.) because of a loss of a hydrogen bond between the EF-hands. In spite of the low pH, ∼5.0, in the crystallization experiment, the calcium ions are bound in the EF-hand loops at full occupancy and with ordinary Ca2+–ligand distances (mean distance of 2.42 Å).
Fig. 1.

(A) Wild-type calbindin D9k monomer in red (Svensson et al. 1992). (B) Crystal structure of 3D domain-swapped calbindin D9k with chain A in red, chain B in blue, and calcium ions in yellow. The mutated residues are shown as green squares. (C) The side chains packing against Met 43 (chain A) are shown in ball-and-stick representation with residues in chain A red and in chain B blue. A few of the hydrophobic contacts (<4.3 Å) are indicated as dotted lines.
Fig. 2.
The difference in φ (dotted line) and ψ (solid line) angles between the wild-type monomer (Svensson et al. 1992) and the substituted dimer (molecule A). The secondary structure elements are indicated above the diagram as helices I, II, III, and IV; calcium ions are indicated by solid circles.
Interactions caused by 3D domain swapping
In a 3D domain-swapped protein there are two kinds of interfaces. The closed (C) interface is normally found between the two EF-hands in the monomer (Fig. 1A ▶) as well as twice in the 3D domain-swapped dimer (Fig. 1B ▶). This interface is disrupted but reformed upon 3D domain swapping. The open (O) interface is an additional interaction surface specific for the 3D domain-swapped protein. It is found at the contact between the monomerlike domains (Schlunegger et al. 1997). Two extensive surfaces, each of about 990 Å2 (GRASP calculation [Nicholls et al. 1991]) are buried in the C interfaces between the two EF-hands. A smaller surface of 370 Å2 (GRASP calculation [Nicholls et al. 1991]) is buried at the O interface. Although the additionally buried surface is small, it contains seven hydrogen bonds and hydrophobic contacts of both Met 43 side chains. The side chain of each Met 43 is buried in the hydrophobic core against Leu 39, Leu 40, and Glu 48 on the same chain and Thr 45 and Leu 49 on the other chain (Fig. 1C ▶). The small additional hydrophobic interaction surface and 7 polar interactions in the O interface could be compared to the 10 polar interactions and the larger hydrophobic contact areas that stabilize the C interfaces.
One or two 3D domain-swapping substitutions?
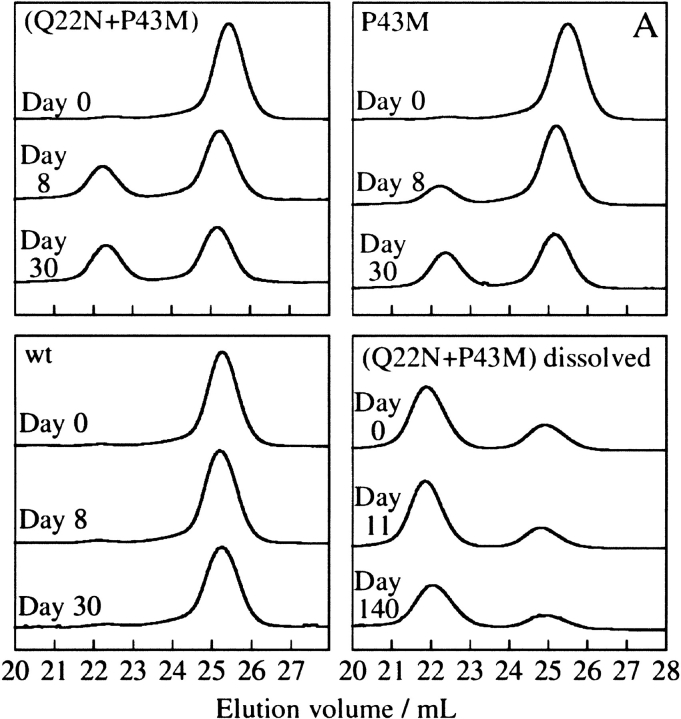
The 3D domain-swapped calbindin D9k is a protein with two substitutions, Gln 22 → Asn and Pro 43 → Met. The Pro 43 → Met substitution was initially introduced to facilitate cleavage of the EF-hands (Finn et al. 1992) and was not believed to have any effect on the structure because it is situated in a mobile loop region. To discriminate between the roles of the two substitutions for the dimerization, crystallization trials of wild-type, P43M, and (Q22N + P43M) calbindin D9k were followed by gel filtration. Wild-type calbindin D9k does not dimerize to any significant extent, whereas P43M and (Q22N + P43M) form dimers (Fig. 3A ▶), implying that the Pro 43 → Met substitution is the major reason for the 3D domain swapping.
Fig. 3.
Gel filtration on a sephadex-75 column. (A) Chromatograms obtained when crystallization drops were dissolved in 56 μL of buffer at 0, 8, or 30 d after starting the crystallization experiment for (Q22N + P43M), P43M, and wild type. After the crystals had appeared, drops were dissolved and gel filtrated after 0, 11, and 140 d, as shown in the lower right quadrant for (Q22N + P43M). (B) Dimer formation for (Q22N + P43M) (filled circles), P43M (filled squares), P43G (open squares), and wild type (open triangles) represented as the percentage of the chains found in dimers. The calculation was made from the areas of the dimer and monomer peaks in the chromatograms obtained as in panel A. (C) Percentage of the chains found in dimers after heating crystallized (Q22N + P43M) to 90°C at pH 5.5 (filled circles) and 4.85 (open squares).
Loss of proline and/or gain of methionine?
It is known that mutation of residues in linkages between domains can lead to 3D domain swapping (Schlunegger et al. 1997). In the present structure, a proline in the linker between the swapping EF-hands was replaced by a methionine. Prolines in the cis conformation restrict the flexibility of the preceding residue (Tonelli 1974). Since it is known that Pro 43 exists as a mixture of cis and trans isomers (Svensson et al. 1992), the rotation of the preceding glycine residue is restrained. However, in the dimer structure we see a stabilization of the linker region owing to a hydrophobic cluster around Met 43 (Fig. 1C ▶), rather than an increased flexibility. To estimate the importance of the methionine side chain, crystallization trials on P43G calbindin D9k were followed by gel filtration. Like wild-type calbindin D9k, only a small percentage of the P43G protein was observed to form dimers (Fig. 3B ▶). Thus, the 3D domain swapping does not seem to be caused primarily by the deletion of a proline but more importantly by the gain of the methionine side chain. This side chain seems to induce and stabilize a hydrophobic assembly of the linker region that is not found in the monomer.
It has been suggested based on other studies that prolines in hinge regions favor oligomerization; however, oligomerization is seldom found when a glycine precedes a proline as in the present case (Bergdoll et al. 1997).
Additional factors influencing domain swapping
The Gln 22 → Asn and Pro 43 → Met substitutions lead to 2.7-fold and 1.4-fold reductions in calcium affinity, respectively (Linse et al. 1993; J. Fast, M. Håkansson, A. Muranyi, G.P. Gippert, E. Thulin, J. Evenäs, L.A. Svensson, and S. Linse, unpubl.), which seem to increase the rate of 3D domain swapping (Fig. 3B ▶). In analogy, the rate of dimer formation is about 2-fold higher for (Q22N + P43M) than for P43M. NMR studies of P43M at pH 6.0 do not reveal dimers (Johansson et al. 1993). Therefore, the low pH ≈ 5.0 at the crystallization conditions seems necessary for dimer formation. Crystallization was also enhanced by decreasing the concentration of Ca2+ and by the addition of 100 mM MgCl2. Since, the low pH and the added Mg2+ also decrease the affinity of calbindin D9k for Ca2+ (Andersson et al. 1997), one can assume that the apo form or transitions between different forms of calbindin D9k are important for 3D domain swapping to take place.
3D domain swapping step by step
A series of events leading to the misfolded 3D domain-swapped structure of calbindin D9k may be proposed (Fig. 4 ▶).
Fig. 4.
Free energy diagram illustrating the proposed mechanism for 3D domain swapping. The closed Ca2+-loaded state is in equilibrium with the closed apo calbindin D9k. The apo state may convert to the 3D domain-swapped dimer via the open apo form, here drawn from the monomer in the dimer. The open apo form may be viewed as an unstable transition state because of the exposure of many hydrophobic groups to solvent. The kinetic barrier between the apo and dimeric states may be lowered in (Q22N + P43M) by interactions of Met 43 with Leu 39 and Leu 40 side chains, which are shown as ball-and-stick models. The diagram is simplistic and omits intermediates between the open transition state and the 3D domain-swapped dimer, for example, a dimer with one domain closed. The drawing of apo calbindin D9k was made from calcium-loaded wild-type calbindin D9k (Svensson et al. 1992) because apo calbindin D9k (Skelton et al. 1995) is known to be similar to calcium calbindin D9k.
Step 1. Dissociation of Ca2+
Although the [Ca2+]2 form of calbindin D9k is the dominating species at pH 5.0, the [Ca2+]2 ↔ apo equilibrium is slightly shifted toward the apo form, because several acidic residues of importance for attraction and coordination of Ca2+ are protonated at this pH, leading to reduced Ca2+ affinity (T. Kesvatera, B. Jönsson, E. Thulin, and S. Linse, unpubl.). Likewise, the Gln 22 → Asn substitution facilitates 3D domain swapping because it reduces the Ca2+ affinity. Subsaturating concentration of Ca2+ and the addition of Mg2+ also help to increase the population of apo calbindin D9k.
Step 2. Opening
Apo calbindin D9k opens up more frequently than the [Ca2+]2 form. NMR studies of amide proton exchange rates have shown that [Ca2+]2-calbindin D9k opens up on a time scale of years, whereas the apo state opens up with a half-life of a few hours (Linse et al. 1990; Skelton et al. 1992). The lower stability of the apo state is, among other factors, caused by fewer hydrogen bonds, less optimized hydrophobic packing, and electrostatic repulsion between the EF-hands. In the apo form the two EF-hands have net charges of −1 and −6, compared to +1 and −4 in the [Ca2+]2 state. Nevertheless, the rate of opening is so low that most apo molecules have time to bind Ca2+ instead of opening up, explaining the low rate of dimer formation (Fig. 3B ▶).
The open state has many different conformations and may be viewed as a transition state, which is highly unstable. Structural analysis of wild-type [Ca2+]2-calbindin D9k (Svensson et al. 1992) suggests that the side chain of Met 43 is exposed in the monomer. The side chain of Met 43 may slightly stabilize the open state by forming a hydrophobic cluster with Leu 39 and Leu 40, thereby lowering the kinetic barrier for 3D domain swapping.
Step 3. Dimerization
One EF-hand of the open monomer binds Ca2+ and encounters an EF-hand from a different chain, which also binds or has bound Ca2+, and thus one domain of the swapped dimer has been formed. Subsequently the other domain binds Ca2+ and closes. The side chains of both Met 43s stabilize the dimer by packing in the hydrophobic core. Thereby two hydrophobic residues are protected from the solvent, the hydrophobic effect, and the van der Waals interaction surface increase (Fig. 1C ▶), and Met 43 may accordingly favor the 3D domain-swapped dimer relative to the monomer.
Thermodynamic or kinetic stabilization of the dimer?
Dimerization is a slow process, occurring over about 30 d (Fig. 3A ▶). With 40% of the protein chains found in dimers at 4.5 mM protein, the equilibrium constant for the reaction 2 Monomers ⇌ Dimer may be calculated as K = [Dimer]/[Monomer]2 = 123 M−1, and the corresponding free energy change as ΔG° = −RT ln K = −12 kJ/mole (Fig. 4 ▶). When dimers crystallize and are removed from solution, new dimers form to keep the solution surrounding the crystals at equilibrium. Drops with crystals, which are dissolved and 20-fold diluted (in buffer at pH 5.5), contain 75%–90% dimer, and return to equilibrium at an exceedingly low rate (Fig. 3A ▶). No conversion to monomers was observed over a period of 4.5 mo. The very high kinetic barrier that seems to separate dimers from monomers probably arises because both Ca2+-binding domains have to release Ca2+ and open up at the same time to unwind the intertwined dimer. As expected for a kinetically trapped system, heating experiments show that it is possible to convert dimer to monomer by raising the temperature (Fig. 3C ▶). The suggested effect of Ca2+ release is supported by the presence of a lower kinetic energy barrier at lower pH (Fig. 3C ▶). Surprisingly, the heated samples seem to overshoot the equilibrium. Maybe the opened and dissociated monomers self-close at a higher rate than that at which they encounter another chain. The higher dimer fractions in samples taken to very high temperatures suggest that increased diffusion may help to reestablish the equilibrium. Monomeric wild-type Ca2+-calbindin D9k is thermostable with a denaturation temperature well above the boiling point (Wendt et al. 1988).
Implications for stability of amyloid deposits
The kinetic barriers between monomers and dimers of calbindin D9k are attributable to the nature of the swapped dimer, requiring simultaneous opening of two domains for dissociation to occur. Similar or even more severe kinetic barriers are likely to exist between monomers and other misfolded aggregates like amyloid fibrils. Amyloid formation is a slow process, and the diseases take years to develop. However, the kinetic barrier is even higher when viewed from the fibril side, because a very large number of noncovalent bonds need to be broken at once for the fibril to dissolve. Therefore, once formed, the amyloid deposits practically never disappear.
Conclusion
There is a very strong preference for the native pairing of EF-hand I with EF-hand II. The two EF-hands may be on the same polypeptide chain as in the regular monomeric form of calbindin D9k or come from different chains and form a dimeric structure with two I–II domains. In the wild-type protein, the sequence and the properties of the linker between the EF-hands prevent dimerization. However, at low pH, a single point mutation is enough to switch the equilibrium toward the dimeric form. We propose that the Pro 43 → Met substitution induces 3D domain swapping in two ways: by stabilization of a partially unfolded open form of calbindin D9k and by stabilization of the hydrophobic core of the dimeric calbindin D9k.
Materials and methods
Crystallization
Mutagenesis, expression, and purification was performed as described previously (Brodin et al. 1986; Johansson et al. 1990). The crystals were grown in plastic Petri dishes sealed with parafilm using the hanging drop technique. The mutant crystals were obtained by mixing 3 μL of 35–40-mg/mL protein solution containing 1.7–3 equivalents of CaCl2 with 3 μL of reservoir solution, 60%–70% (NH4)2SO4, at pH 5.5. In all but the first trial, the speed of the crystallization was enhanced from taking months to taking only two-to-three weeks by the addition of 100 mM MgCl2. The Petri dishes allow a slow evaporation, and at the same time the pH decreases owing to the evaporation of NH3. The pH value in drops with crystals is ∼4.8–4.9. A heavy atom derivative was obtained by soaking a crystal with 12 mM trimethyllead actetate (Holden and Rayment 1991).
Structure solution and refinement
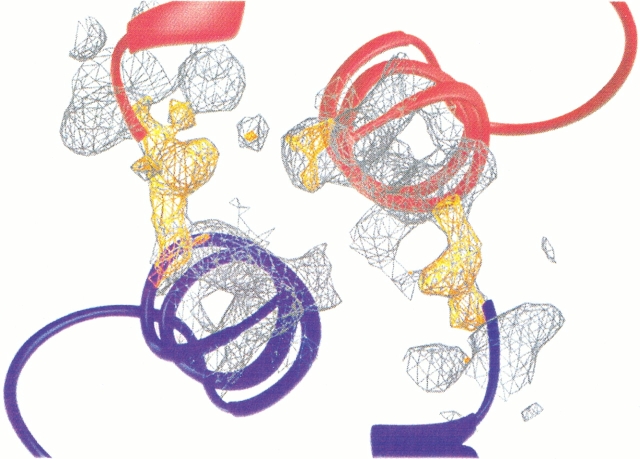
X-Ray diffraction data were collected to 1.75-Å resolution making use of a Rigaku RU-200B rotating anode and an image plate detector (MAR Research System). The XDS program system was used for data reduction (Kabsch 1993), and data processing was done with the CCP4 program suite (Collaborative Computational Project Number 4, 1994). The space group was P21 with cell parameters a = 32.60 Å, b = 48.24 Å, c = 41.53 Å, and β = 96.98°. The Matthew coefficient, VM (Matthew 1977), indicated two molecules per asymmetric unit (VM = 1.9 Å3/D), but at this stage we were not able to solve the structure by molecular replacement. Instead, the heavy atom derivative trimethyllead acetate was used for the SIRAS technique. Solvent flattening was applied using the DM program (Kleywegt and Jones 1997). A map calculated between 15 and 3 Å resolution using the new phases was investigated with the real-space search program ESSENS (Cowtan 1994). Using a template of 54 Cα atoms of wild-type calbindin D9k (Svensson et al. 1992) and a rotational step size of 2°, the first molecule was easily found, whereas the second molecule could not be correctly positioned. Instead, the first molecule refined with X-PLOR version 3.851 (Brünger 1992) was used as a search model in AMoRe (Navaza 1994), and in that way the position of the first as well as the second molecule could be found. The structures were built with the O program (Jones et al. 1991). The B factors were initially set to 18 Å2 and rigid-body refinement; molecular dynamics refinement with a slow cool run and bulk-solvent correction was performed in X-PLOR (Brünger et al. 1992). The resulting partial structure factors were transferred to the PROTIN/REFMAC/ARPP procedure (Lamzin and Wilson 1997; Murshudov et al. 1997) for subsequent refinement with individual B factors and automatic water editing. No NCS restraints were applied. After 8 rounds of refinement, Ser 44 was omitted, and an OMIT map was calculated. This showed positive electron density, indicating main chain connections between two independent molecules (Fig. 5 ▶). The model was then redefined and refined as the 3D domain-swapped dimer (for statistics, see Table 1). The structure has been deposited in the protein data bank with accession code 1HT9.
Fig. 5.
The initial electron density maps, gray and yellow, used to convert monomers, blue and red, to dimers. The 2FoFc map (gray) is shown at 1 σ level and the positive FoFc map (yellow) at 3 σ level. The maps were generated with Ser 44 omitted. By moving Ser 44 into the positive density it was possible to redefine the monomers to a single 3D domain-swapped dimer.
Table 1.
Statistics of structure determination
| Q22N + P43M | Pb soakeda | |
| Resolution range (Å) | 44–1.75 (1.80–1.75) | 20–2.45 (2.45–3.5) |
| Observed reflections | 50352 | 9162 |
| Unique reflections | 11923 | 4337 |
| Completeness | 0.915 (0.601) | 0.917 (0.932) |
| I/σ(I) | 14.5 (3.7) | 12.6 (9.1) |
| bRiso, cRano (%) | 21.7, 8.7 | |
| Sites (#) | 1 | |
| dPhasing power | 2.78 | |
| Refined resolution range (Å) | 20–1.76 | |
| eR-factor, R-free | 0.162, 0.192 | |
| fR.m.s.d. for bond lengths (Å) | 0.011 | |
| fR.m.s.d. for bond angles (°) | 2.3 | |
| fR.m.s.d. for dihedrals (°) | 22.7 | |
| Number of water molecules | 86 |
a Trimethyllead acetate derivative.
b Riso = Σ(|FPH − FP|)/ΣFP.
c Rano = [Σ(|±ΔFobs| − |±ΔFcalc|)2/Σ(±ΔFobs)2]1/2.
d Phasing power = (Σ|FH|2/Σ(|FP| − |FH|)2)1/2.
e R-factor/R-free = Σ|Fobs − Fcalc|/ΣFobs, where 95% of Fobs are used in refinement and for calculation of the R-factor and the other 5% used for calculation of R-free.
f Root mean square deviations for bond lengths, angles, and dihedrals from ideal stereochemical values.
Gel filtration
Crystallization experiments were set up as described above but with wild-type, (Q22N + P43M), P43M, and P43G calbindin D9k. Prior to chromatography, each drop was dissolved in 60 μL of buffer (15 mM CaCl2, 150 mM NaCl, and 100 mM NaOAc at pH 5.5). Samples of 50 μL were injected to a Superdex 75 HR 10/30 column. The running buffer (1 mM calcium chloride, 100 mM NaOAc at pH 5.5, and 150 mM NaCl) and a flow rate of 0.55 mL/min were used.
Dissolution experiment
Single crystallization drops of (Q22N + P43M) calbindin D9k were dissolved in 60 μL of buffer (1 mM calcium chloride, 7 mM MgCl2, 100 mM NaOAc at pH 5.5, and 150 mM NaCl) and placed in carefully sealed Eppendorf tubes at room temperature. At different times ranging up to 4.5 mo, gel filtration was performed as above with 50-μL samples.
Thermal conversion
Thermal dimer-to-monomer conversion of (Q22N + P43M) was followed by gel filtration. Three to five crystallization drops, prepared as above, were dissolved by adding an equal volume of buffer (100 mM NaOAc, 150 mM NaCl, 1 mM CaCl2 at pH 5.5 or pH 4.85), and stored at room temperature (25°C). Aliquots of 4 μL were heated from 5°C to 35°–95°C on an Eppendorf mastercycler. The heating and cooling speed was 0.3°C/sec, and the sample was kept at every degree, between 5°C and the highest temperature, for 30 sec. Directly after the heating procedure the sample was diluted 10 times with buffer (100 mM NaOAc at pH 5.5 or pH 4.85, 150 mM NaCl, and 1 mM CaCl2), and gel filtration was performed, as above. All data points were collected during the same day, or in a few cases, the day after the stock solution was prepared. The percentage of dimer was calculated from the peak heights of the dimer and monomer signals.
Acknowledgments
We thank Eva Thulin for production and purification of the protein and Anders Liljas for helpful discussions. The Swedish Natural Science Research Council (NFR) has funded this work.
The publication costs of this article were defrayed in part by payment of page charges.This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.47501.
References
- Andersson, M., Malmendal, A., Linse, S., Ivarsson, I., Forsén, S., and Svensson, A. 1997. Structural basis for the negative allostery between Ca2+- and Mg2+-binding in the intracellular Ca2+-receptor calbindin D9k. Protein Sci. 61139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdoll, M., Remy, M.-H., Cagnon, C., Masson, J.-M., and Dumas, P. 1997. Proline-dependent oligomerization with arm exchange. Structure 5391–401. [DOI] [PubMed] [Google Scholar]
- Blanchard, H., Grochulski, P., Li, Y., Arthur, S.C., Davies, P.L., Elce, J.S., and Cygler, M. 1997. Structure of a calpain Ca2+-binding domain reveals a novel EF-hand and Ca2+-induced conformational changes. Nature Struct. Biol. 4 532–538. [DOI] [PubMed] [Google Scholar]
- Brodin, P., Grundström, T., Hofmann, T., Drakenberg, T., Thulin, E., and Forsén, S. 1986. Expression of bovine intestinal calcium binding protein from a synthetic gene in Escherichia coli and characterization of the product. Biochemistry 25 5371–5377. [DOI] [PubMed] [Google Scholar]
- Brünger, A.T. 1992. X-PLOR (Version 3.1), A System for X-ray Crystallography and NMR. Yale University Press, New Haven, CT.
- Christakos, S., Gabrielides, C., and Rhoten, W.B. 1989. Vitamin D-dependent calcium binding proteins: Chemistry, distribution, functional considerations, and molecular biology. Endocr. Rev. 10 3–26. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4. 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallog. D 50 760–763. [DOI] [PubMed] [Google Scholar]
- Cowtan, K. 1994. `dm': An automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EASBM Newsletter on Protein Crystallography 31 34–38. [Google Scholar]
- Donato, C. 1999. Review: Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450 191–231. [DOI] [PubMed] [Google Scholar]
- Finn, B.E., Kördel, J., Thulin, E., Sellers, P., and Forsén, S. 1992. Dissection of calbindin D9k into two Ca2+-binding subdomains by a combination of mutagenesis and chemical cleavage. FEBS Lett. 298 211–214. [DOI] [PubMed] [Google Scholar]
- Hemmingsen, C., Staun, M., and Olgaard, K. 1994. Effects of magnesium on renal and intestinal calbindin-D. Miner Electrolyte Metabolites 20 265–273. [PubMed] [Google Scholar]
- Holden, H.M. and Rayment, I. 1991. Trimethyllead Acetate: A first-choice heavy atom derivative for protein. Arch. Biochem. Biophys. 291 187–194. [DOI] [PubMed] [Google Scholar]
- Johansson, C., Brodin, P., Grundström, T., Thulin, E., Forsén, S., and Drakenberg, T. 1990. Biophysical studies of engineered mutant proteins based on calbindin D9k modified in the pseudo EF-hand. Eur. J. Biochem. 187 455–460. [DOI] [PubMed] [Google Scholar]
- Johansson, C., Ullner, M., and Drakenberg, T. 1993. The solution structures of mutant calbindin D9k's, as determined by NMR, show that the calcium-binding site can adopt different folds. Biochemistry 32 8429–8438. [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.-Y., Cowtan, S.W., and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta. Crystallog. A 47 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch, W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26 795–800. [Google Scholar]
- Kleywegt, G.J. and Jones, T.A. 1997. Template convolution to enhance or detect structural features in macromolecular electron-density maps. Acta Crystallog. D 53 179–185. [DOI] [PubMed] [Google Scholar]
- Lamzin, V.S. and Wilson, K.S. 1997. Automated refinement for protein crystallography. Methods Enzymol. 277 269–305. [DOI] [PubMed] [Google Scholar]
- Linse, S., Teleman, O., and Drakenberg, T. 1990. Ca2+ binding to calbindin D9k strongly affects backbone dynamics: Measurements of exchange rates of individual amide protons using 1H NMR. Biochemistry 29 5925–5934. [DOI] [PubMed] [Google Scholar]
- Linse, S., Thulin, E., and Sellers, P. 1993. Disulfide bonds in homo- and heterodimers of EF-hand subdomains of calbindin D9k: Stability, calcium binding, and NMR studies. Protein Sci. 2 985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew, B.W. 1977. X-Ray structures of proteins. In The proteins (eds. H. Neurath and R.L. Hill), Vol. III, p. 476. Academic Press, New York.
- Murshudov, G.N., Vagin, A.A., and Dodson, E.J. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallog. D 53 240–255. [DOI] [PubMed] [Google Scholar]
- Nicholls, A., Sharp, K.A., and Honig, B. 1991. Protein folding and association: Insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11 281–296. [DOI] [PubMed] [Google Scholar]
- Schlunegger, M.P., Bennett, M.J., and Eisenberg, D. 1997. Oligomer formation by 3d domain swapping: A model for protein assembly and misassembly. Adv. Protein Chem. 50 61–122. [DOI] [PubMed] [Google Scholar]
- Skelton, N.J., Kördel, J., Akke, M., and Chazin, W.J. 1992. Nuclear magnetic resonance studies of the internal dynamics in apo, (Cd2+)1 and (Ca2+)2 calbindin D9k. J. Mol. Biol. 227 1100–1117. [DOI] [PubMed] [Google Scholar]
- Skelton, N.J., Kördel, J., and Chazin, W.J. 1995. Determination of the solution structure of apo calbindin D9k by NMR spectroscopy. J. Mol. Biol. 249 441–462. [DOI] [PubMed] [Google Scholar]
- Svensson, L.A., Thulin, E., and Forsén, S. 1992. Proline cis–trans isomers in calbindin D9k observed by X-ray crystallography. J. Mol. Biol. 223 601–606. [DOI] [PubMed] [Google Scholar]
- Szebenyi, D.M.E. and Moffat, K. 1986. The refined structure of vitamin D-dependent calcium-binding protein from bovine intestine. J. Biol. Chem. 261 8761–8777. [PubMed] [Google Scholar]
- Tonelli, A.E. 1974. Conformational characteristics of polypeptides containing isolated l-proline residues with cis peptide bonds. J. Mol. Biol. 86 627–635. [DOI] [PubMed] [Google Scholar]
- Wendt, B., Hofman, T., Martin, S.R., Bayley, P., Grundström, T., Thulin, E., Linse, S., and Forsén, S. 1988. Effect of amino acid substitutions and deletions on the thermal stability, the pH stability and unfolding by urea of bovine calbindin D9k. Eur. J. Biochem. 175 439–445. [DOI] [PubMed] [Google Scholar]