Abstract
The HIV-1 Tat protein is a potent chemoattractant for monocytes. We observed that Tat shows conserved amino acids corresponding to critical sequences of the chemokines, a family of molecules known for their potent ability to attract monocytes. Synthetic Tat and a peptide (CysL24–51) encompassing the “chemokine-like” region of Tat induced a rapid and transient Ca2+ influx in monocytes and macrophages, analogous to β-chemokines. Both monocyte migration and Ca2+ mobilization were pertussis toxin sensitive and cholera toxin insensitive. Cross-desensitization studies indicated that Tat shares receptors with MCP-1, MCP-3, and eotaxin. Tat was able to displace binding of β-chemokines from the β-chemokine receptors CCR2 and CCR3, but not CCR1, CCR4, and CCR5. Direct receptor binding experiments with the CysL24–51 peptide confirmed binding to cells transfected with CCR2 and CCR3. HIV-1 Tat appears to mimic β-chemokine features, which may serve to locally recruit chemokine receptor-expressing monocytes/macrophages toward HIV producing cells and facilitate activation and infection.
Extracellularly, the HIV-1 Tat protein has been found to mimic both matrix molecules through its RGD (Arg-Gly-Asp) sequence (1) and angiogenic growth factors through its basic domain (2). Tat is chemotactic for human monocytes (3–5) and monocyte-derived dendritic cells (5). Tat activity has been postulated to be mediated by integrins (3) or interaction with the flt-1 tyrosine kinase receptor (4). We have recently shown that a synthetic peptide containing the cysteine-rich and core domains of Tat, CysL24–51, retains most of the chemotactic potential of Tat (6). This peptide of Tat contains a CCF (Cys-Cys-Phe) sequence, a hallmark of numerous β-chemokines.
The chemokines are a large subclass of the cytokine family showing potent chemoattractant activity (for reviews see refs. 7 and 8). CC or β-chemokines are produced by a number of different cell types and are often potent chemoattractants for monocytes (9). The chemokines are ligands for 7-transmembrane spanning G-protein coupled receptors. The β-chemokine receptor CCR5 and the α-chemokine receptor CXCR4 have been shown to act as coreceptors for HIV. Mutations of CCR5 are associated with resistance to HIV infection or progression to AIDS (for reviews see refs. 7 and 8). CCR5 usage is linked to the slow/low virus genotype, whereas CXCR4 usage is linked to the rapid/high phenotype (for review see ref. 7). Other chemokine receptors can also mediate HIV entry; for example, CCR2 and CCR3 can act as coreceptors for some dual tropic HIV-1 strains (10, 11). A CCR2 polymorphism has been found to correlate with delayed progression to AIDS (12, 13).
We report here that the HIV-1 Tat protein and the peptide encompassing the cysteine-rich and core regions induce pertussis toxin sensitive Ca2+ fluxes in monocytes. Receptor desensitization and receptor binding experiments demonstrate interaction with the β-chemokine receptors CCR2 and CCR3. Tat activation of these receptors, in particular CCR2 whose key role in monocyte chemotaxis has been established (14, 15), would act to recruit chemokine expressing cells toward a productively infected cell, favoring the spread and establishment of HIV infection.
MATERIALS AND METHODS
Cells and Reagents.
Buffy coats were obtained through the courtesy of the Blood Transfusion Center of Istituto G. Gaslini (Genoa, Italy). Monocyte-enriched populations (consisting of >85% of the monocytes as assessed by flow cytometry) were obtained after Ficoll and Percoll separation with standard methods. Lymphocyte-enriched fractions were activated in culture with phytohemagglutinin for 48 hr and then subcultured in medium (RPMI 1640 with 10% fetal calf serum) containing 100 units⋅ml−1 of hrIL-2. Cell populations consisted of >95% CD3+ T lymphocytes after 10 days of culture.
Tat1–86 protein, and peptides corresponding to amino acids 20–80 of Tat, Cys20–39, CysL24–51, Basic46–60, Pep56–70, and RGD65–80, were synthesized in solid-phase peptide synthesis by using the Fmoc/DCC/HOBt strategy as described (16) (Tecnogen, Cesna, Italy). Purity was assessed by reverse-phase–HPLC and mass spectroscopy. This Tat preparation has been shown to be biologically active in a number of assays, including HIV–long terminal repeat transactivation (16).
Calcium Signaling.
For the determination of free Ca2+ cytoplasmic concentration 2.5 × 106 cells per sample were stained with 1 mM of the acetoxymethylester of Fura-2 (Sigma) (17). The fluorescence of the cellular suspension (1.5 ml in 125 mM NaCl/5 mM KCl/1 mM Na2HPO4/1 g/l glucose/25 mM Hepes/1 mM CaCl2/0.5 mM MgCl2) was monitored at 37°C with an LS-5 spectrofluorimeter (Perkin–Elmer) equipped with a thermostatically controlled cuvette holder and a stirring apparatus. Stimuli were added to the cell suspension. Fluorescence was measured at 496 nm with 345-nm excitation. The concentration of free Ca2+ was calculated by the method of Grynkiewicz et al. (17).
Blocking of the monocyte response to Tat was obtained by addition of an equimolar concentration of a specific anti-Tat monoclonal antibody (Arnika, Milan, Italy); treatment with 5 μg of trypsin at 37°C for 60 min or heat denaturation at 100°C for 30 min also abrogated the Tat response.
The effect of pertussis or cholera toxin on Ca2+ mobilization was evaluated by preincubation of monocytes with 1 μg⋅ml−1 of pertussis or cholera toxin (Sigma) or media alone for 90 min at 37°C.
Flow Cytometric Analysis of Calcium Fluxes.
Peripheral blood derived lymphoblasts or monocyte-derived macrophages were loaded with indo-1 (Molecular Probes) and stained with fluorochrome-conjugated antibodies for 15 min at room temperature. The labeled cells were collected on a dual laser FACSVantage (Becton Dickinson Immunocytometry Systems), modified with a Time Zero injection module (Cytek, Fremont, CA) and a linear ratio offset. Data were analyzed by using flowjo software program for the Macintosh (Treestar, San Carlos, CA).
Monocyte Migration Assays.
Monocytes were preincubated with 1 μg⋅ml−1 toxin or media alone for 1 hr and migration assessed in the presence of the toxins using the chemotaxis microchamber technique as described (6). Chemoattractants diluted in sfm (RMPI 1640/0.1% BSA) were used at concentrations assessed for having optimal chemotactic activity (400 ng⋅ml−1 Tat1–86/1 μg⋅ml−1 CysL24–51/100 ng⋅ml−1 MCP-3) and placed in the lower chamber.
Receptor Binding Assays.
Full-length cDNA for the CC chemokine receptors 1, 2, 3, and 5 were cloned by reverse transcription–PCR using primers based on the published sequences (18–21) and were transfected into CHO-K1 cells (22). CCR4 was cloned and transfected into HEK 293 (23). Binding assays were carried out on membrane preparations by using the scintillation proximity assay (22). The radiolabeled ligands used were 125I-MIP-1α for CCR1, CCR4, and CCR5; 125I-MCP-1 for CCR2; and 125I-MCP-3 for CCR3. Increasing concentrations of Tat (10−13 to 10−6 M) were added to allow competition with the radiolabeled chemokine.
The CysL24–51 peptide was labeled with 125I (6) to a specific activity of 950 Ci/mmol and used for binding assays on the CCR2 or CCR3 transfected Chinese hamster ovary cells described above and with HEK 293 cells (the kind gift of P. Murphy, National Institutes of Health, Bethesda) transfected with CCR2 or CCR3 in the pBABE vector (from Nathaniel Landau through the AIDS Research and Reference Reagent Program Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health), as well as on untransfected controls. Binding assays were performed as described (6). Scatchard analysis was performed by using the ligand program (Biosoft, Milltown, NJ).
RESULTS AND DISCUSSION
We have recently reported that a peptide encompassing the cysteine-rich and core region of Tat (CysL24–51) is highly active in inducing monocyte chemotaxis (6). Examination of this peptide suggested that this region contained structural motifs typical of the β-chemokine family, often potent chemoattractants for monocytes. Alignment of Tat with several chemokines indicated positioning of key residues, which have been shown to be critical for CC chemokine function (Fig. 1). These include a CCF motif, an SYXR motif that has been shown to determine CXC/CC chemokine cell type selectivity (24), as well as a strongly conserved isoleucine (Fig. 1). The greatest similarity is seen with the MCP family. Both the C30C31F/Y32 and the S46Y47XR49 residues are highly conserved over numerous HIV-1 isolates and in HIV-2, even though point mutation analysis suggests that C31, S46, Y47, and R49 are not critical for HIV transactivation function (for review see ref. 25), suggesting a separate but critical function for these residues. Therefore, we investigated the possibility that HIV-Tat could bind to and signal through β-chemokine receptors by means of this chemokine-like region.
Figure 1.
Alignment of the HIV-1 Tat protein with the mature peptide sequences of the β-chemokines MCP-3, MCP-2, MCP-1, RANTES, MIP-1α, and MIP-1β. Residues conserved between Tat and the β-chemokines shown are shaded. The location of the Tat peptides along the Tat sequence used are indicated by brackets. The residues shown in italics (amino acids 87–101), not present in some HIV isolates, were not included in the Tat1–86 synthetic peptide used here.
Because free cytoplasmic Ca2+ is a key intracellular mediator of chemotactic signals by the chemokines (26), we analyzed whether HIV-1 Tat could induce Ca2+ influx in these cells. Addition of increasing amounts of Tat1–86 to human monocytes loaded with the fluorescent Ca-chelator Fura-2 produced a dose-dependent, rapid, and transient increase in the intracellular Ca2+ concentration (Fig. 2a). As little as 6 nM was sufficient to produce a significant signal. This pattern of Ca2+ mobilization is typical of chemokines, such as the response to MCP-3 (27), shown as a positive control in Fig. 2c. This activity was specific for Tat, as anti-Tat monoclonal antibodies blocked the induction of Ca2+ flux by Tat on monocytes (Fig. 2b). In addition, heat denaturation or trypsin treatment of Tat both abrogated the induction of Ca2+ flux (Fig. 2b). Tat1–86 also induced Ca2+ mobilization in the monocyte-related THP-1 leukemic cell line (Fig. 2c), whereas no effects were detected on activated human T lymphocytes, which however responded to MCP-3 (Fig. 2c).
Figure 2.
(a) Induction of Ca2+ mobilization in monocytes by increasing doses of Tat1–86. As little as 100 ng⋅ml−1 (6.6 nM) produced a significant Ca2+ mobilization. (b) Inhibition of Tat1–86 induced Ca2+ mobilization by anti-Tat monoclonal antibodies (Anti Tat Ab) or trypsin digestion of Tat1–86. (c) Tat1–86 induced Ca2+ mobilization in different cell types. Tat (20 nM) induced rapid and transient Ca2+ mobilization in monocytes and in the monocyte-related THP-1 cell line, but not in T-lymphoblasts. Responses of these cells in the same experiment to a known chemokine (10 nM MCP-3) are shown for comparison. (d) Effect of peptides encompassing different domains of Tat on Ca2+ mobilization in monocytes. The cysteine-rich and core domain peptide (CysL24–51) produced a lower calcium mobilization as compared with the same molar concentration of Tat1–86 (100 nM). Higher concentrations of this peptide did produce a strong response. A peptide corresponding to the cysteine-rich domain (Cys20–39, 100 nM) showed a weaker, but still significant, activity. A peptide corresponding to CysL24–51, but with a mutation of the CCF sequence to SSG (CysL24–51CCFmut) showed no activity. Peptides corresponding to the RGD and Basic domains were also ineffective at the doses shown or even 20-fold higher doses (2 μM, not shown). The effects of RANTES is shown for comparison.
The CysL24–51 peptide encompassing the chemokine-like region of Tat was also effective in mediating Ca2+ influx in monocytes (Fig. 2d). Another peptide (Cys20–39) encompassing only the cysteine-rich domain (the CCF sequence without the SYXR motif) showed weaker Ca2+ mobilization (Fig. 2d). Mutation of the CCF sequence in the CysL24–51 peptide to SSG abrogated its ability to induce Ca2+ fluxes (Fig. 2d), indicating a critical role for these residues, whereas the SYXR motif may be more critical for chemokine receptor selectivity, as previously reported (24). Similar concentrations of a peptide based on the MIP-1α sequence covering the same region as the CysL24–51 peptide had little activity (not shown), suggesting that the secondary structure conferred by the additional cysteines in Tat play an important role in receptor interaction. The three-dimensional structure of Tat has been solved by NMR (28) and shows no resemblance to the chemokine fold. We therefore hypothesize that the bulk of the Tat protein acts as a scaffold, which presents the chemokine receptor binding region (in the loop comprised between residues 24–51) to the receptor, thereby enabling its activation by Tat. The chemokine-like activity of molecules with little similarity to chemokines is not without precedent; results obtained with the HIV envelope demonstrate that a high sequence homology is not necessary for receptor binding and signaling (29, 30).
Although peptides corresponding to the basic domain (aa 46–60) and the RGD domain (aa 65–80) interact with other cell types (2, 31), these peptides did not induce Ca2+ influx (Fig. 2d), even at high concentrations (2 μM). This is consistent with the expected mechanisms of action for these two peptides, i.e., activation of tyrosine kinase receptors by the basic peptide (31) or integrin binding by the RGD peptide (1).
Ca2+ mobilization induced by HIV–Tat was inhibited by an average of 80% when extracellular Ca2+ was blocked by the addition of the Ca2+ chelator EGTA. Similar inhibition by EGTA was obtained when control chemokines were used in the same experiments. This indicated that the increase in Ca2+ concentration induced by Tat involves both extracellular and intracellular components, in accordance with the mechanisms through which chemokines induce Ca2+ mobilization (32).
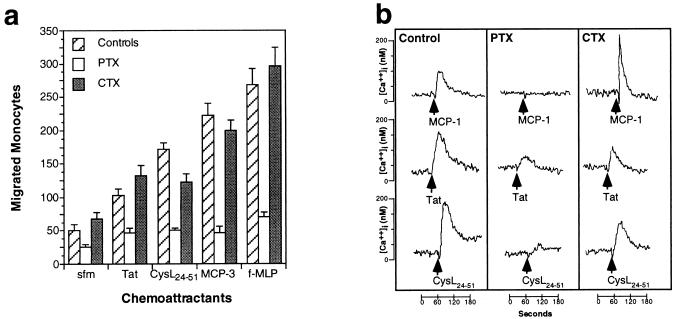
Chemokine activity is mediated through seven transmembrane domain receptors coupled to pertussis-toxin sensitive, cholera-toxin insensitive, Gi-proteins (26). We therefore analyzed the effects of pertussis and cholera toxins on Tat-induced migration and Ca2+ mobilization. Pertussis toxin completely blocked the chemotactic response to Tat, to the CysL24–51 peptide, and to the controls, MCP-3 and f-MLP (Fig. 3a). Cholera toxin only showed a slight inhibition of the chemotactic response to the CysL24–51 peptide. Accordingly, pertussis toxin strongly inhibited the Ca2+ influx induced by Tat1–86 or by CysL24–51 peptide and completely blocked the response to control chemokines, such as MCP-1 (Fig. 3b). Cholera toxin had no effect on control chemokine-induced Ca2+ influx, and only marginally affected the response to Tat1–86 or the CysL24–51 peptide. These data indicate that both the chemotactic response and the Ca2+ mobilization induced by Tat are largely mediated by Gi proteins. The small pertussis resistant Ca2+ response to Tat suggests that Tat interacts with other cell surface receptors as well, consistent with previous observations (1, 31).
Figure 3.
(a) Effects of pertussis toxin (PTX) and cholera toxin (CTX) on monocyte migration in response to 400 ng ml−1 Tat1–86, 1 μg⋅ml−1 CysL24–51, and 100 ng⋅ml−1 MCP-3 and f-MLP. PTX caused the marked inhibition of chemotaxis to all the chemoattractants tested, CTX did not substantially affect migration. (b) Effects of PTX and CTX on calcium mobilization induced by 10 nM MCP-1, 20 nM Tat1–86, or 100 nM CysL24–51 peptide in monocytes.
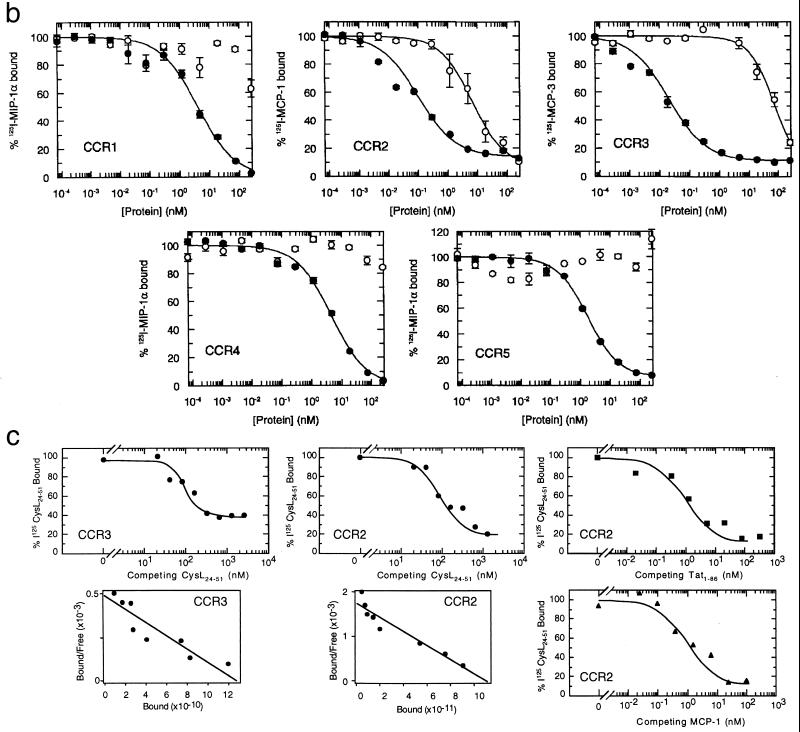
Receptor desensitization after chemokine stimulation can assess the usage of common receptors by different agonists (9). Desensitization with high concentrations of the CysL24–51 peptide inhibited the Ca2+ influx induced by Tat1–86; conversely, desensitization with high concentrations of Tat1–86 inhibited the Ca2+ influx induced by the Cys20–39 or the CysL24–51 peptides (Fig. 4a). These data indicate further that the chemokine-like properties of Tat map to the chemokine-like region within the molecule.
Figure 4.
(a) Cross-desensitization experiments with Tat1–86, the CysL24–51 peptide, and some β-chemokines on monocytes. The response to the ligand (indicated at the top of each panel) is given as the percent of maximal response to the same concentration of ligand without desensitization. The concentrations of the desensitization agent are indicated on the abscissa. −, indicates no desensitization agent added. (b) Equilibrium competition on cell membranes from Chinese hamster ovary cells expressing CCR1, CCR2, CCR3, or CCR5, and HEK 293 cells expressing CCR4, as indicated. The competition by Tat1–86 (○) or unlabeled chemokine (•) was determined by a scintillation proximity assay binding assay as described (22). The radiolabeled and unlabeled chemokines were MIP-1α for CCR1, CCR4, and CCR5; MCP-1 for CCR2; and MCP-3 for CCR3. (c) Competitive ligand binding of radiolabeled CysL24–51 peptide to transfected cells expressing CCR2 or CCR3, as indicated. The competition for 125I-CysL24–51 peptide binding by unlabeled CysL24–51 peptide (•), Tat (■), or MCP-1 (▴) was determined. Scatchard plot of unlabeled CysL24–51 peptide competition was calculated from the data and presented below binding curves.
The sequence similarities between Tat and chemokines, and their common mechanisms of signal transduction, strongly suggested that Tat could interact with either known or novel CC chemokine receptor(s). We therefore tested cross-desensitization between the CysL24–51 peptide, Tat1–86, and CC chemokines known to act on monocytes in Ca2+ mobilization experiments (Fig. 4a). The CysL24–51 peptide strongly desensitized the response to MCP-1 and vice versa, whereas the desensitization observed with MIP-1α was weaker. MCP-1 and MCP-3 effectively desensitized the response to Tat1–86, whereas eotaxin reduced the response to Tat by 20%. Tat1–86 partially desensitized the response to MCP-1 and could completely block the response to eotaxin by freshly isolated monocytes. Overlapping receptor sharing is a common feature of the chemokine family, where any one chemokine can activate a different array of receptors (26). The partial desensitization between Tat or the CysL24–51 peptide and the chemokines MCP-1, MCP-3, and eotaxin suggests that Tat interacts with both the same and different receptors as these chemokines.
The ability of Tat to interact with individual known β-chemokine receptors expressed on freshly isolated monocytes was demonstrated by equilibrium competition binding assays with CCR transfected cells. Tat was able to specifically displace radiolabeled MCP-1 from membranes of CCR2 transfected cells and MCP-3 from membranes of CCR3 transfected cells (Fig. 4b). Tat had no effect on ligand binding to membranes from CCR1, CCR4, or CCR5 transfected cells (Fig. 4b), while clear competition with control ligands was seen in parallel experiments in all cases. Tat appeared to have a high affinity for CCR2 (IC50 = 5 nM), whereas the affinity of Tat for CCR3 was substantially lower (IC50 = 60 nM) than that of MCP-3.
To confirm specific interactions of the chemokine-like region of Tat with CCR2 and CCR3, the binding of radiolabeled CysL24–51 peptide to CCR2 or CCR3 transfected cells was examined. The CysL24–51 peptide does not contain the domains of Tat known to interact with integrins (RGD, ref. 1) or tyrosine kinase receptors (basic, ref. 31), thus binding to these receptors can be excluded. This peptide has been previously shown to bind specifically to monocyte cell surfaces (6) with an IC50 of 32 nM, and was displaced by unlabeled Tat with an IC50 of 1.25 nM. Radiolabeled CysL24–51 peptide specifically bound to both CCR2 and CCR3 transfected cells, as assessed by displacement with excess unlabeled peptide (Fig. 4c). No specific binding was found for untransfected parental cells (not shown). Scatchard analysis of the binding data of the CysL24–51 peptide to CCR2 and CCR3 transfected cells indicated single affinity binding sites (Fig. 4c). Dissociation constants (Kd) were 66.4 ± 8.4 nM for the CysL24–51 peptide binding to CCR3 transfected cells and 27.3 ± 5.2 nM for CysL24–51 peptide binding to CCR2 transfected cells. Further investigation of 125I-CysL24–51 peptide-CCR2 binding showed that the Kd for radiolabeled peptide displacement by Tat on CCR2 transfected cells was 1.4 ± 0.3 nM, while that for MCP-1 was 0.6 ± 0.1 nM. These values are in agreement with those obtained using radiolabeled chemokine displacement, with IC50s for CCR2 of 5 nM for Tat and 0.4 nM for MCP-1 (Fig. 4b).
The binding of Tat and the CysL24–51 peptide to CCR2 and CCR3 as well as other receptors is in agreement with the inhibition of Tat and CysL24–51 peptide-induced Ca2+ influxes in monocytes by desensitization with MCP-3, MCP-1, and eotaxin (Fig. 4a). The dissociation constant of Tat for CCR2 (1.4 ± 0.3 nM) is also close to that of the overall dissociation constant for Tat on monocyte cell surfaces (3.1 ± 0.2 nM) (6). In addition to CCR2 and CCR3, other Gi protein coupled receptors might be involved in Tat interactions with monocytes, as indicated by the incomplete inhibition of Tat1–86 or CysL24–51 peptide-induced Ca2+ fluxes by chemokines.
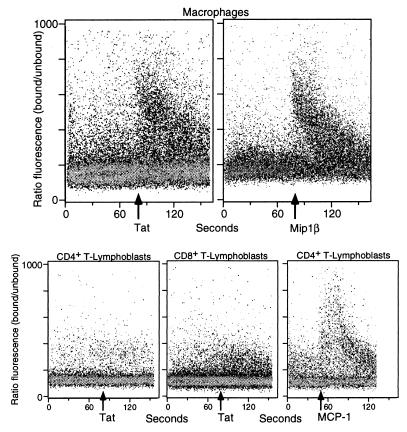
Monocyte-derived macrophages are readily infected by HIV. Tat is able to induce Ca2+ fluxes in monocyte-derived macrophages (Fig. 5), as determined by flow cytometry. This Ca2+ flux was comparable to that induced by Mip1β on the same cells (Fig. 5). No Ca2+ flux was induced by Tat in either CD4+ or CD8+ T-lymphoblasts, although these cells responded well to MCP-1 (Fig. 5) or MCP-3 (Fig. 2). HIV-envelope signaling through CCR5 and CXCR4 requires CD4 as well (29, 30). It is not known whether additional molecules may affect signaling through CCR receptors by Tat in lymphocytes.
Figure 5.
Ca2+ fluxes generated by Tat on monocyte-derived macrophages or CD4+ or CD8+ T-lymphoblasts (as indicated) assessed by multiparameter flow cytometry analysis. The response of monocytes to MIP-1β and of CD4+ T-lymphoblasts to MCP-1 is shown for comparison.
Tat has been found in the serum of HIV-1 infected patients in concentrations as high as 1 ng/ml (33), in the range of serum levels of many chemokines in HIV infected patients (34), suggesting that the chemokine-like activity of Tat may be biologically relevant. Comparable levels of Tat were found in the media of HIV infected H9 cells (33), and we have shown that substantial biologically active Tat inducing monocyte migration is released from Tat producing cells (6). Tat access to the extracellular space has been proposed to occur via an alternative secretion pathway (see ref. 35), but substantial levels of Tat may also come from the rapid turnover of the major HIV infected cellular compartment (for review see ref. 36). Local levels of Tat could exceed that found in the serum in the tissues where viral replication occurs (the lymph nodes), and the levels of Tat observed in the serum might reflect the level of viremia in the patient. Because Tat has been shown to bind to heparan sulfate (2), the concentration on cell surfaces in the tissue could even be higher, as has been hypothesized for the chemokine family (37).
We have shown here that extracellular Tat induces Ca2+ fluxes in monocytes and macrophages. Tat binds CCR2 with high affinity and with lower affinity to CCR3. The ability of Tat to signal through β-chemokine receptors would additionally attract monocytes toward virus producing cells, which may favor a rapid spread of infection. In addition, it has been postulated that HIV signaling through chemokine receptors may also enhance HIV infection (30). Our data indicate that the Tat protein actively participates in this stimulation. Our present findings underline a wider importance of the chemokine system in HIV pathogenesis.
Acknowledgments
We thank T. Cai (IST, Genoa, Italy) for plasmid preparation, Dr. G. Franchini (Istituto G. Gaslini, Genoa) for buffy coats, and Dr. Andrea Rubbert for the monocyte-derived macrophages and critical review of the manuscript. We are grateful to C. A. Power (Glaxo Wellcome) for receptor cloning and R. W. Barrett, E. Whitehorn, and E. Tate (Affymax) for construction of the Chinese hamster ovary tranfectants used in the binding assays. This work was partially supported by grants awarded by the Ministero della Sanità, X Progetto AIDS (A.A. and D.M.N.), the AIRC (Italian Association for Cancer Research, Milan) (A.A., S.F., and D.M.N.), and the EC Biomed II concerted action “HIV and Kaposi’s Sarcoma.” S.S. and M.G.A. are FIRC (Federazione Italiana Ricerca sul Cancro) fellows and D.G. is the recipient of a “Ministero della Sanità Progetto AIDS fellowship.”
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Brake D, Debouck C, Biesecker G. J Cell Biol. 1990;111:1275–1281. doi: 10.1083/jcb.111.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F, Noonan D. Oncogene. 1996;12:289–297. [PubMed] [Google Scholar]
- 3.Lafrenie R M, Wahl L M, Epstein J S, Hewlett I K, Yamada K M, Dhawan S. J Immunol. 1996;157:974–977. [PubMed] [Google Scholar]
- 4.Mitola S, Sozzani S, Luini W, Primo L, Bosatti A, Weich H, Bussolino F. Blood. 1997;90:1365–1372. [PubMed] [Google Scholar]
- 5.Benelli R, Mortarini R, Anichini A, Giunciuglio D, Noonan D M, Montalti S, Tacchetti C, Albini A. AIDS. 1998;12:261–268. doi: 10.1097/00002030-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Albini A, Benelli R, Giunciuglio D, Cai T, Mariani G, Ferrini S, Noonan D M. J Biol Chem. 1998;273:15895–15900. doi: 10.1074/jbc.273.26.15895. [DOI] [PubMed] [Google Scholar]
- 7.Cairns J S, D’Souza M P. Nat Med. 1998;4:563–568. doi: 10.1038/nm0598-563. [DOI] [PubMed] [Google Scholar]
- 8.Berger E A. AIDS. 1997;11:S3–S16. [PubMed] [Google Scholar]
- 9.Uguccioni M, D’Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 10.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 11.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 12.Smith M W, Dean M, Carrington M, Winkler C, Huttley G A, Lomb D A, Goedert J J, O’Brien T R, Jacobson L P, Kaslow R, et al. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 13.Kostrikis L G, Huang Y, Moore J P, Wolinsky S M, Zhang L, Guo Y, Deutsch L, Phair J, Neumann A U, Ho D D. Nat Med. 1998;4:350–353. doi: 10.1038/nm0398-350. [DOI] [PubMed] [Google Scholar]
- 14.Kurihara T, Warr G, Loy J, Bravo R. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boring L, Gosling J, Chensue S W, Kunkel S L, Farese R J, Broxmeyer H E, Charo I F. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruvo M, Scarallo A, Vecchio G, Palombo G, Fassina G. In: Peptides 1996. Ramage R, Epton R, editors. Kingswilford, Westmidlands, U.K.: Mayflower Scientific; 1998. pp. 771–772. [Google Scholar]
- 17.Grynkiewicz G, Poenic M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 18.Neote K, DiGregorio D, Mak J Y, Horuk R, Schall T J. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 19.Charo I F, Myers S J, Herman A, Franci C, Connolly A J, Coughlin S R. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty B L, Siciliano S J, DeMartino J A, Malkowitz L, Sirotina A, Springer M S. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 22.Coulin F, Power C A, Alouani S, Peitsch M C, Schroeder J M, Moshizuki M, Clark L I, Wells T N. Eur J Biochem. 1997;248:507–515. doi: 10.1111/j.1432-1033.1997.00507.x. [DOI] [PubMed] [Google Scholar]
- 23.Power C A, Meyer A, Nemeth K, Bacon K B, Hoogewerf A J, Proudfoot A E I, Wells T N C. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 24.Lusti-Narasimham M, Power C A, Allet B, Alouani S, Bacon K B, Mermod J-J, Proudfoot A E I, Wells T N C. J Biol Chem. 1995;270:2716–2721. doi: 10.1074/jbc.270.6.2716. [DOI] [PubMed] [Google Scholar]
- 25.Jeang, K.-T. (1994) The Human Retroviruses and AIDS Compendium On Line. http://hiv-web.lanl.gov.
- 26.Premack B A, Schall T J. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 27.Sozzani S, Zhou D, Locati M, Pieppi M, Proost P, Magazin M, Vita N, van Damme J, Mantovani A. J Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- 28.Bayer P, Kraft M, Ejchart A, Westendorp M, Frank R, Rosch P. J Mol Biol. 1995;247:529–535. doi: 10.1006/jmbi.1995.0158. [DOI] [PubMed] [Google Scholar]
- 29.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissman D, Rabin R L, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber J M, Fauci A S. Nature (London) 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 31.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, et al. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 32.Sozzani S, Molino M, Locati M, Luini W, Cerletti C, Vecchi A, Mantovani A. J Immunol. 1993;150:1544–1553. [PubMed] [Google Scholar]
- 33.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 34.McKenzie S W, Dallalio G, North M, Frame P, Means R J. AIDS. 1996;10:F29–F33. doi: 10.1097/00002030-199610090-00001. [DOI] [PubMed] [Google Scholar]
- 35.Rubartelli A, Sitia R. In: Unusual Secretory Pathways: From Bacteria to Man. Kuchler K, Rubartelli A, Holland B, editors. Heidelberg: Springer; 1997. pp. 87–114. [Google Scholar]
- 36.Finzi D, Siliciano R F. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 37.Hoogewerf A J, Kuschert G S V, Proudfoot A E I, Borlat F, Clark-Lewis I, Power C A, Wells T N C. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]