Abstract
The mouse major urinary proteins are pheromone-binding proteins that function as carriers of volatile effectors of mouse physiology and behavior. Crystal structures of recombinant mouse major urinary protein-I (MUP-I) complexed with the synthetic pheromones, 2-sec-butyl-4,5-dihydrothiazole and 6-hydroxy-6-methyl-3-heptanone, have been determined at high resolution. The purification of MUP-I from mouse liver and a high-resolution structure of the natural isolate are also reported. These results show the binding of 6-hydroxy-6-methyl-3-heptanone to MUP-I, unambiguously define ligand orientations for two pheromones within the MUP-I binding site, and suggest how different chemical classes of pheromones can be accommodated within the MUP-I β-barrel.
Keywords: Pheromone, crystal structure, lipocalin, binding protein, X-ray crystallography
The mouse major urinary proteins (MUPs) define a highly homologous family of pheromone-binding proteins (for review, see Cavaggioni and Mucignat-Caretta 2000) encoded by approximately 30 genes (Bishop et al. 1982). The expression of MUPs varies in a sex- and strain-dependent manner. The family can be divided into two groups based on tissue distribution and nucleic acid sequence identity (Bishop et al. 1982). MUP-I is a member of the group 1 gene products that are expressed in the liver under hormonal control (al-Shawi et al. 1992) and are excreted in the urine of male mice at high levels (Finlayson et al. 1965).
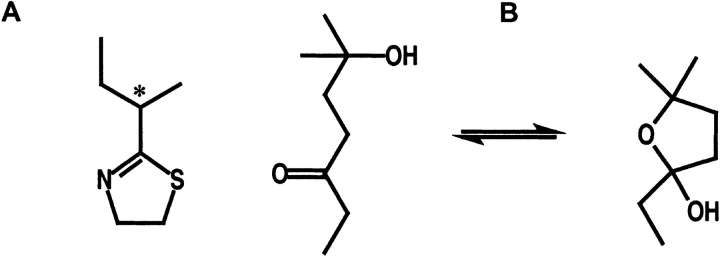
MUPs bind a variety of volatile pheromones (Bacchini et al. 1992; Robertson et al. 1993; Novotny et al. 1999a) that affect aspects of mouse physiology and behavior, including estrus (Marsden and Bronson 1964; Whitten et al. 1968; Jemiolo et al. 1986), puberty (Novotny et al. 1986; Novotny et al. 1999b; Mucignant-Careta et al. 1995) and inter-male aggression (Novotny et al. 1985). Although initially characterized as a binding protein for a five-membered thiazoline ring (Fig. 1 ▶), MUP-I should also be capable of binding additional pheromones, such as the recently identified 6-hydroxy-6-methyl-3-heptanone (Novotny et al. 1999b). This substance is the most abundant volatile constituent of male mouse urine that binds to MUPs and induces puberty acceleration in female mice (Novotny et al. 1999b). Thus, MUPs function is to carry small hydrophobic pheromones through an aqueous environment, and may protect pheromones from decomposition, control pheromone excretion, and control the release of volatile pheromones from urine. It has also been suggested that MUPs may be involved in the excretion and metabolism of lipophilic xenobiotic compounds (Larsen et al. 1990).
Fig. 1.
Chemical structures for (A) SBT (2-sec-butyl-4,5-dihydrothiazole) and (B) HMH (6-hydroxy-6-methyl-3-heptanone) are shown.
Structurally, MUPs have molecular weights of about 19 kD and acidic isoelectric points in the range of 4.2 to 4.7 (Duncan et al. 1988). A 2.4 Å crystal structure for MUP-I isolated from mouse urine (Böcskei et al. 1992) confirmed that MUPs are members of the lipocalin family of protein structures characterized by a conserved β-barrel structure consisting of eight β-strands and an α-helix (for review, see Flower 1996). Crystal structures have also been determined for several other lipocalins, including plasma retinol-binding protein (Cowan et al. 1990), β-lactoglobulin (Brownlow et al. 1997), bilin-binding protein (Huber et al. 1987), and α2U-globulin (Böcskei et al. 1992). The interiors of these β-barrel proteins form hydrophobic-binding sites for small lipophilic ligands.
Because of the number of highly homologous MUP isoforms and different pheromones present in mouse urine, the original crystallographic studies of MUP-I may have suffered from molecular heterogeneity in both the proteins and pheromones present in the isolate. Although ligand occupancy of the pheromone-binding site was suggested in these studies (Böcskei et al. 1992), pheromone was not included in refinement, thus leaving ambiguity in the structural basis of ligand binding. To eliminate problems of MUP heterogeneity, we have developed an expression system for producing recombinant MUP-I (Zídek et al. 1999a), a major constituent of the natural MUP complex. Methods have also been developed for synthesizing several pheromones of known biological activity (Novotny et al. 1990; Novotny et al. 1999b). Recombinant MUP-I was shown to be competent using nuclear magnetic resonance (NMR) studies of the structure and measuring pheromone-binding affinities (Zídek et al. 1999a, b). These studies also suggested an orientation for 2-sec-butyl-4,5-dihydrothiazole that differs from that proposed in the original report of the MUP-I crystal structure (Böcskei et al. 1992).
Here we report high-resolution crystallographic studies of the interactions of recombinant MUP-I with the synthetically derived pheromones 2-sec-butyl-4,5-dihydrothiazole (SBT) and 6-hydroxy-6-methyl-3-heptanone (HMH). Our results permit unequivocal assignment of the interacting functional groups. We also report on the isolation of MUP-I from mouse liver and on investigations of this natural protein after it was crystallized in a space group that differs from the previously reported crystallographic studies (Böcskei et al. 1991; Böcskei et al. 1992).
Results and Discussion
The structure of MUP-I
Crystal structures of recombinant MUP-I complexed with the synthetic pheromones were determined in the space group P43212 using Protein Data Bank coordinates 1MUP (Böcskei et al. 1992). The MUP-I/SBT and MUP-I/HMH complexes have been refined at 1.9 Å and 2.0 Å resolution, respectively. Details of the crystallographic data and refinement are given in Table 1. The overall structure of MUP-I does not differ substantially from the structure in solution (Zídek et al. 1999a, b; Lücke et al. 1999) or the original crystal structure refined at 2.4 Å resolution (Böcskei et al. 1992). MUP-I contains a nine-stranded antiparallel β-sheet (Fig. 2 ▶), which forms an eight-stranded β-barrel structure. The β-strands will be referred to as βa through βi with intervening loops named according to adjacent strands (i.e., loop a/b joins βa and βb). Continuous hydrogen bonding between strands in the β-barrel is maintained by the two portions of the first β-strand separated by a β-bulge to interact with both βb and βh. Two 310 helices occur in the N-terminal third of the molecule, and a single α-helix and a short 310 helix occur near the C-terminal.
Table 1.
Crystallographic data and refinement
| SBT | HMH | TRIG | |
| Diffraction data | |||
| Resolution (Å) | 30 − 1.9 | 30 − 2.0 | 18 − 2.0 |
| Total observations | 62,479 | 40,377 | 48,342 |
| Unique observations | 14,794 | 12,397 | 14,762 |
| Completeness (%) | 99.6 (98.6) | 97.7 (98.6) | 97.9 (85.9) |
| Rmerge | 0.068 (0.376) | 0.082 (0.329) | 0.060 (0.276) |
| I/σ | 15.3 (3.7) | 12.3 (3.1) | 17.2 (3.3) |
| Wilson B-factor (Å2) | 23.8 | 21.5 | 25.8 |
| Refinement | |||
| Protein atomsa | 1236 | 1228 | 1279 |
| Solvent atomsa | 60 | 91 | 153 |
| Ligand atomsa | 9 | 10 | 0 |
| Ionsa | 4 | 7 | 0 |
| Rcryst | 0.217 | 0.194 | 0.219 |
| Rfree | 0.247 | 0.230 | 0.250 |
| rmsd bonds (Å) | 0.008 | 0.009 | 0.005 |
| rmsd angles (°) | 1.684 | 1.668 | 1.355 |
| Ramachandran | |||
| Most favored (%) | 88.7 | 85.3 | 84.2 |
| Allowed (%) | 10.6 | 14.0 | 15.1 |
SBT, MUP-I with 2-sec-butyl-4,5-dihydrothiazole; HMH, MUP-I with 6-hydroxy-6-methyl-3-heptanone; TRIG, MUP isolated from liver; rmsd, root mean square deviation from ideal values. Numbers in parentheses are values for the highest resolution shell.
aNumbers of nonhydrogen atoms/ions represented in indicated structures.
Fig. 2.
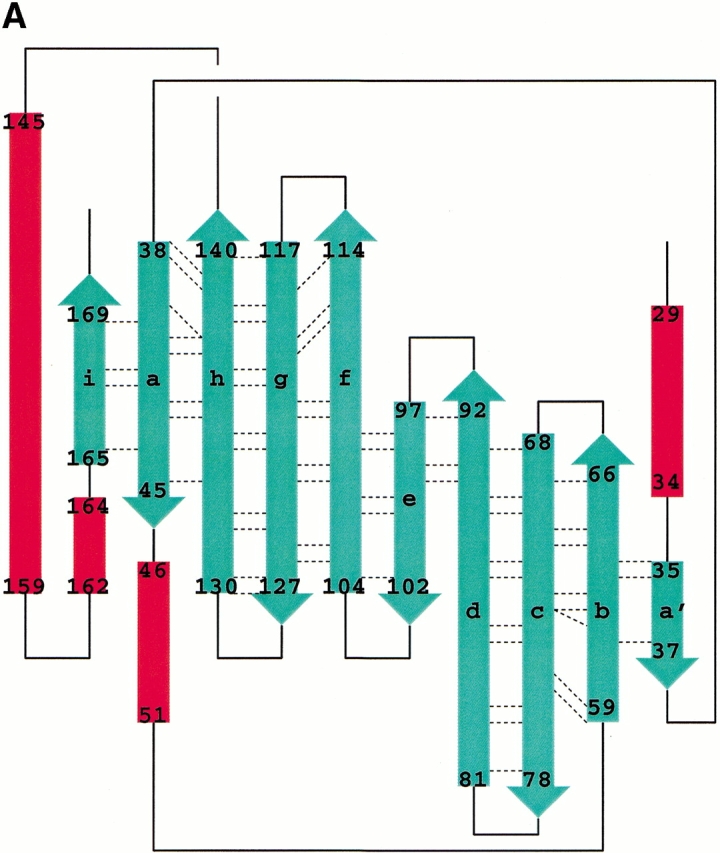
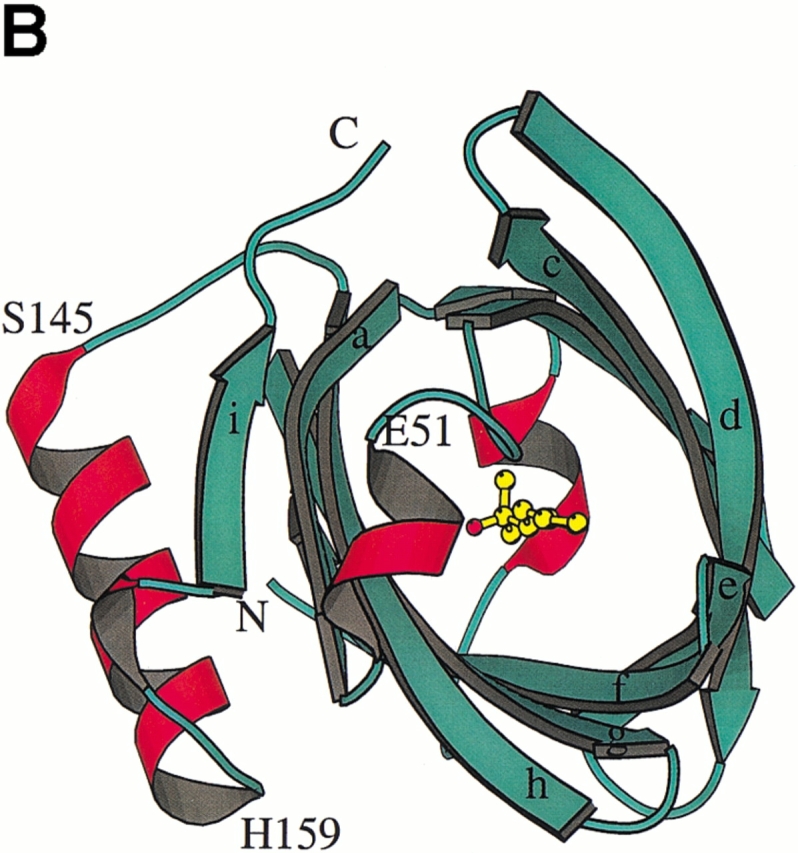
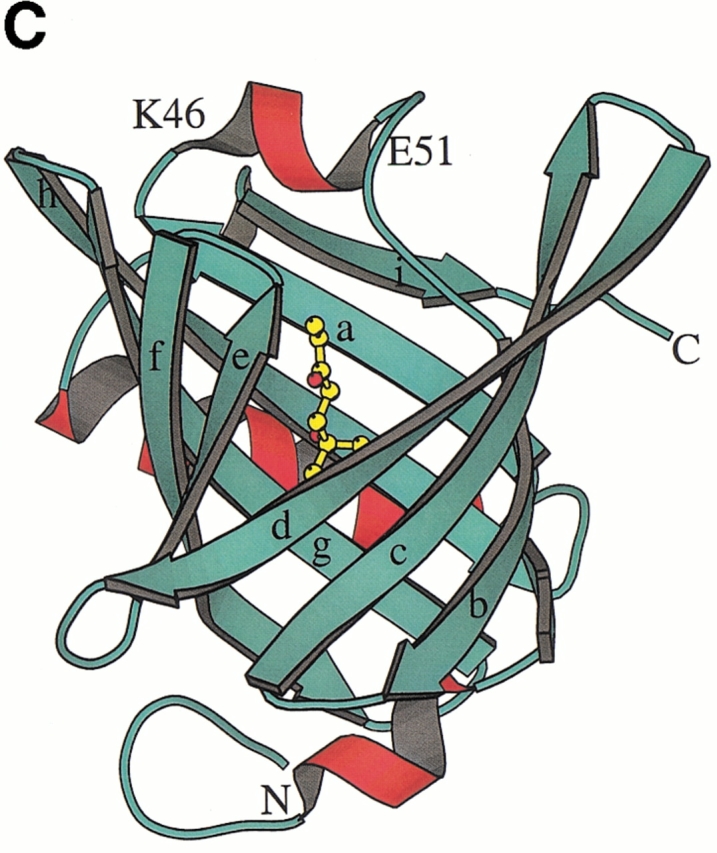
The MUP-I (major urinary protein-I) structure. (A) A topology diagram illustrates the main chain hydrogen bond pattern (dashed lines) between β-strands in the MUP-I β-barrel. (B) A ribbon diagram illustrates the position of HMH within the MUP-I pheromone binding site and highlights the proximity of pheromone to the point of separation between βd and βe. (C) A ribbon diagram illustrates HMH binding viewed from the entrance to the β-barrel.
Pheromone binding to MUP-I
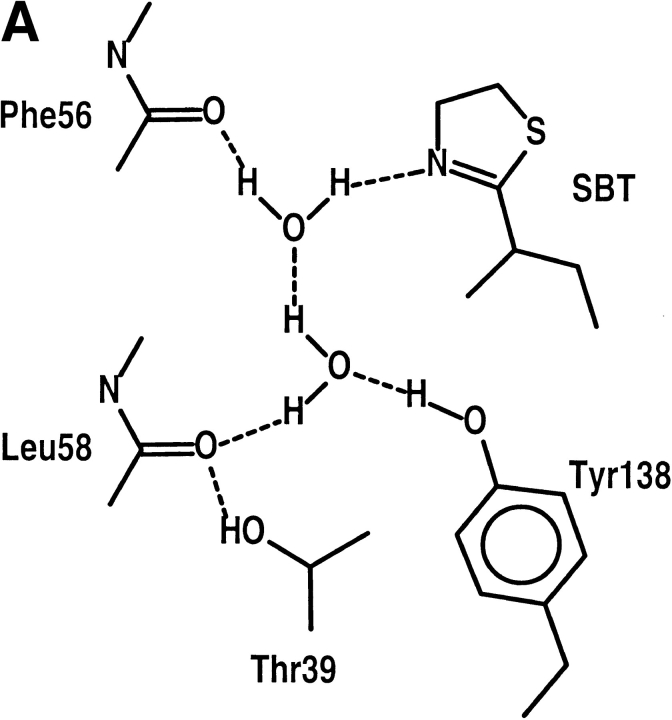
In contrast to the previous crystallographic studies, which indicated that several different ligands might be present within the crystals (Böcskei et al. 1992), the synthetic pheromone ligands were readily identified in electron density maps (Fig. 3 ▶) that clearly define the ligand orientations within the MUP-I β-barrel. Both pheromones are bound within the hydrophobic environment at one end of the β-barrel, formed by the side chains of Phe56, Leu58, Leu60, Ile63, Leu72, Phe 74, Met87, Val100, Tyr102, Phe108, Ala121, Leu123, Leu134, and Tyr138. MUP-I is translated as a precursor polypeptide of 180 residues with 18 N-terminal residues removed posttranslationally. Therefore, the polypeptide has been numbered starting with the mature N-terminal Glu19. A schematic view of SBT and HMH within the ligand binding site is presented in Figure 4 ▶. The outermost side chains of the ligand binding site (Met87, Phe56, and Leu58) are in van der Waals contact with the Tyr102 side chain, which caps the entrance to the interior of the β-barrel. Two water molecules are also present in the ligand binding site, interacting with the Tyr138 hydroxyl group and the carbonyl oxygen atoms of Phe56 and Leu58.
Fig. 3.
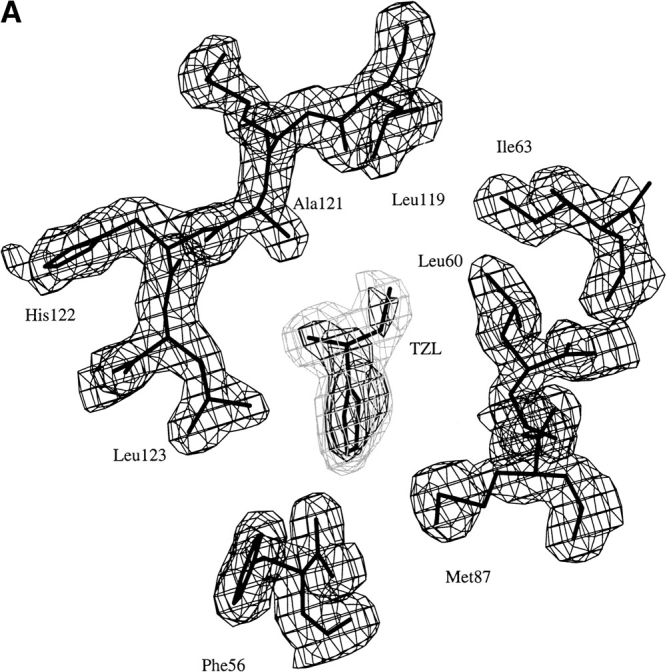
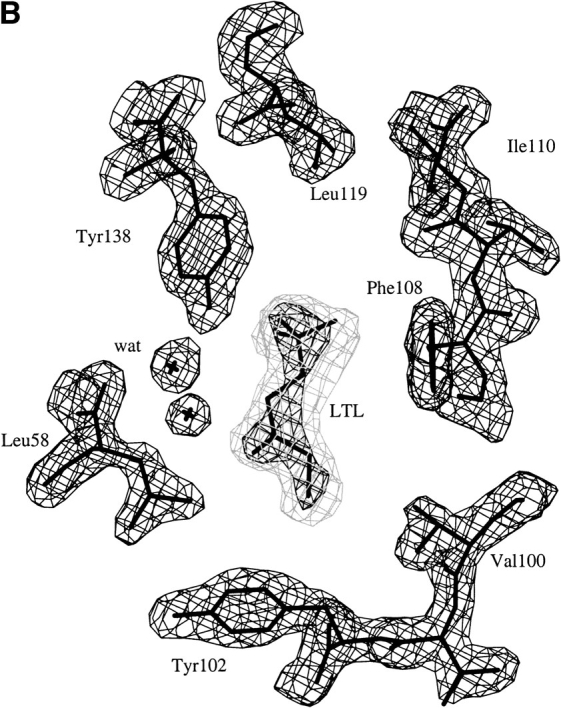
Electron density in the MUP-I ligand binding site. Omit maps calculated following simulated annealing refinement in the absence of ligand are shown for (A) the MUP-I/SBT and (B) the MUP-I/HMH structures. The two panels are approximately related by a 90° vertical rotation to emphasize branched groups of the ligands. The 2Fo-Fc and Fo-Fc maps are contoured at 1.5 σ (dark gray) and 3.0 σ (light gray), respectively.
Fig. 4.
Potential hydrogen bonds involved in (A) SBT and (B) HMH binding to MUP-I. Dashed lines indicate hydrogen bonds, which are drawn to account for the donor potential of the two water molecules present in the ligand binding site.
SBT binds in a specific orientation with the five-membered thiazoline ring proximal to the entrance to the active site and the branched sec-butyl group oriented toward the bottom of the β-barrel. This orientation is distinct from that for the ligand included in the coordinates deposited with the Protein Data Bank (1MUP), but is consistent with recent NMR studies of SBT binding to MUP-I (Zídek et al. 1999a). The branched electron density indicates that the SBT ethyl group is located on the side of the ligand-binding pocket formed by Leu60, Leu72, and Phe74. Whereas the methyl carbon atom makes potential van der Waals interactions with side-chain carbon atoms of Ala121, Leu123, and Leu134, the ethyl group is separated by at least 4.6 Å from the nearest carbon atom. The unoccupied space around the ethyl group could account for the reported binding of SBT analogs (i.e., iso-butyl-thiazole) to MUP-I (Novotny and Zídek, unpubl.).
The resolution of the diffraction data is not sufficient to determine the configuration of the chiral SBT C6 atom. A racemic mixture of SBT was isolated previously from urinary MUP preparations, and a racemic preparation of SBT was used in our experiments. Models of both enantiomers fit the electron density and could not be discriminated by refinement. Thus, it is likely that both enantiomers are present in the binding site, as indicated from NMR studies of SBT binding to MUP-I in solution (Zídek et al. 1999b).
Electron density peak heights and analysis of difference maps indicate that the thiazole ring also binds with a specific orientation. The thiazole S1 atom is located in potential van der Waals contact with the Leu72, Phe108, and Val100 side chains, whereas the N3 atom is located 2.9 Å from the water molecule bound to the Phe56 carbonyl oxygen atom. Thus, binding may involve a water-mediated hydrogen bond to the SBT N3 atom as part of a solvent-inaccessible hydrogen-bond network. This network is illustrated schematically in Figure 4 ▶, which has been interpreted to satisfy the hydrogen bond donor potential of the two water molecules clearly present in the hydrophobic interior of MUP-I. Additional interactions with the thiazole ring also include potential van der Waals contacts with the Phe74 and Leu123 side chains arranged approximately perpendicular to the plane of the pheromone ring. Thus, aliphatic and aromatic hydrogen atoms may contribute binding energy by interacting with π electrons on the thiazole ring. A comparison of the MUP-I binding site with that of rat α2U-globulin has been made in a recent report of the α2U-globulin complexed with the renal carcinogen, d-limonene 1,2-epoxide (Chaudhuri et al. 1999). The α2U-globulin complex shares many features in common with MUP-I pheromone binding, including the potential involvement of a Tyr hydroxyl group.
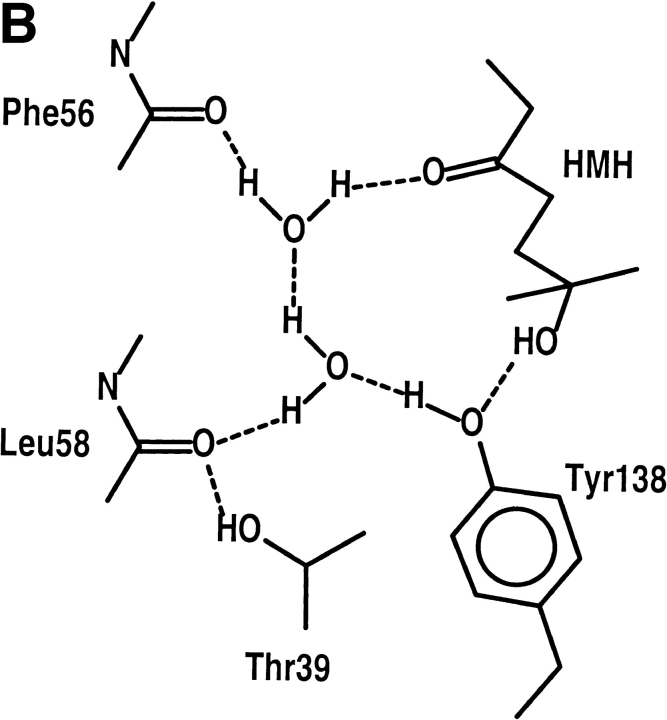
Another male mouse pheromone, HMH, binds to MUP-I as an open structure with a specific orientation that involves many features of SBT binding. Although studies are currently underway to determine the HMH dissociation constant, urinary HMH levels are reported to be about 1000-fold higher than SBT (Novotny et al. 1999b). HMH exists in solution in an equilibrium between a closed five-membered furan ring and an open hydroxyketone tautomer (Fig. 1 ▶). The closest contacts between nonhydrogen atoms in HMH and MUP-I again involve the side chains of Phe56, Leu58, Leu60, Phe74, Met87, Val100, Tyr102, Phe108, Ala121, Leu123, and Tyr138 at distances of 2.9–4.1 Å. HMH binding also involves the hydrogen bond network described above, with the ketone oxygen serving as a hydrogen bond acceptor in the place of the thiazole N3 atom. The hydroxyl oxygen atom serves as a potential hydrogen bond donor to the Tyr138 hydroxyl oxygen (Fig. 4B ▶). Thus, despite the chemical and structural differences between the two ligands, HMH binds in essentially the same position and makes many of the same interactions as SBT.
Given the earlier characterization of the MUP-I/SBT complex involving a five-membered ring structure, it was surprising to find that HMH binds in the open hydroxyketone structure with the ketone group near the entrance to the active site and the dimethyl/hydroxy moiety located near the center of the β-barrel. Binding of the open tautomeric structure would presumably stabilize the pheromone against dehydration to cyclic vinyl ethers. These biologically inactive products of the furan ring tautomer were readily detected in analyses of urinary fractions containing puberty-accelerating activity (Novotny et al. 1999b). The open hydroxyketone form will be less susceptible to these reactions. Therefore, this result provides support for the role of MUP-I in protecting pheromones against chemical decomposition.
Trigonal crystal structure of MUP-I isolated from mouse liver
MUP-I was found to be a major contaminant in preparations of protein disulfide isomerase (PDI) isolated from mouse liver, but was readily separated from PDI by gel filtration (data not shown). Although this isolate is referred to as MUP-I, the 20 cycles of N-terminal amino acid sequencing used to identify the contaminant are not sufficient to distinguish between the numerous MUP homologs potentially present in mouse liver. Therefore, as with MUP-I purified from urine, this preparation may represent a heterogeneous sample with respect to both protein and ligand.
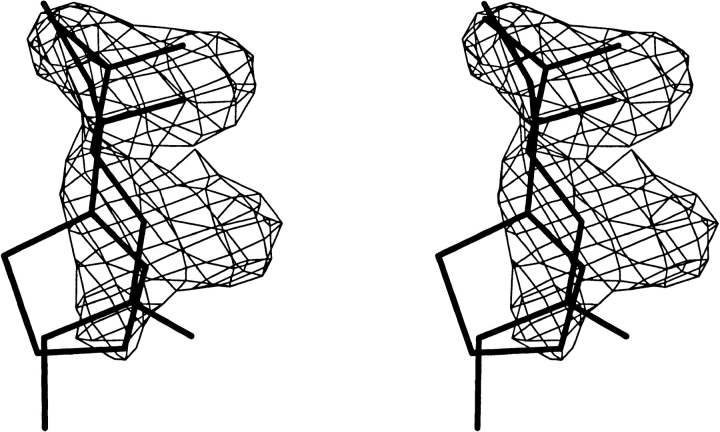
MUP-I isolated from liver was crystallized in the trigonal space group P3221, and the structure was determined by molecular replacement (see Materials and Methods). Although no exogenous pheromone was added during purification or crystallization, the ligand-binding site contains discrete, well-defined electron density that differs from that presented in the original crystallographic study of MUP-I (Böcskei et al. 1992). The position of these contiguous peaks is very similar to that observed in both pheromone complexes with MUP-I; however, the density is not consistent with SBT, HMH, or a mixture of the two pheromones (Fig. 5 ▶).
Fig. 5.
A stereo diagram shows the structures of SBT and HMH superimposed on electron density present in the active site of MUP-I isolated from liver following a 5000 K simulated annealing refinement in the absence of ligand. The HMH ketone oxygen and SBT nitrogen occupy similar positions in the ligand binding site. The Fo-Fc map is contoured at 3.0 σ. The closed ring form of HMH (Fig. 1 ▶) did not fit the electron density (data not shown).
Comparison of the trigonal MUP-I, SBT/MUP-I, and HMH/MUP-I structures
Despite different space groups, ligands, and crystallization conditions, differences among the three MUP-I structures presented here are limited to specific regions. The overall root mean square deviations (rmsds) between main chain atoms are 0.39 and 0.40 Å for superpositions of the trigonal MUP-I structure with the MUP-I/SBT and MUP-I/HMH structures, respectively. The MUP-I/SBT and MUP-I/HMH structures are more similar and superimpose with an rmsd for main chain atoms of 0.11 Å. The residues having the highest rmsds between main chain atoms in the three structures (data not shown) occur in loops a/b, c/d, and g/h at the calyx end of the β-barrel near the ligand binding site (Fig. 2 ▶). Consistent with thermal and/or conformational mobility, these loops also tend to have the highest refined B-factors in the three MUP-I structures (data not shown). However, with one exception (see Materials and Methods), the main chain atoms in these loops are clearly defined by electron density. Positional differences between core secondary structures of the trigonal and tetragonal structures also occur. The residues Val77 to Ala95, which include part of βc and all of βd and loop c/d have an overall average rmsd of 0.71 Å. The region between Leu144 and Glu164, which includes the C-terminal helix, also varies between the trigonal and tetragonal structures with an rmsd of about 0.63 Å for main chain atoms in this region. Lattice contacts that differ between the trigonal and tetragonal structures are likely to account for the positional differences involving loops a/b and c/d and the C-terminal helix. For example, Glu80 makes a Cd2+ -mediated lattice contact in the tetragonal crystals, whereas the c/b loop containing this residue makes no lattice contacts in the trigonal crystals.
Conformational mobility and the location of the pheromone within the β-barrel suggest that βc and βd and the intervening c/d loop may function as an entrance to the ligand-binding site. A previous report proposed that loop a/b might function as the entrance to the interior of the β-barrel (Flower 1996). The structures presented here indicate that the most direct route out of the ligand binding site would be for ligands to pass by Met87. The β-barrel splays open at residue Ala89 with only three interstrand hydrogen bonds forming between βd and βe (Fig. 2A ▶). Furthermore, no side-chain mediated contacts are made between the β-barrel and residues His75 through Glu84. Thus, conformational flexibility of βc and βd is consistent with the limited number of contacts with the β-barrel. Conformational mobility is also suggested for the Met87 side chain, which shows positional rmsds of 1.49 and 1.01 Å in superpositions of the trigonal structure with the MUP-I/HMH and MUP-I/SBT structures, respectively. A substantial rmsd of 0.95 Å is also found for this side chain in comparing the MUP-I/SBT and MUP-I/HMH complexes, largely the result of 120° and 130° rotations about the Chi 1 and Chi 3 side-chain torsion angles, respectively. This is by far the largest positional deviation for a residue in the ligand-binding site and is significantly greater than the average (0.28 ± 0.16 Å) of the rmsds from the pairwise superpositions of the Phe56, Leu58, and Leu60 side chains present in loop a/b. Mobility of Met87 and other residues in the vicinity of the c/d loop is also suggested by a MUP-I NMR structure ensemble (Lücke et al. 1999; PDB accession 1DF3), which shows deviations for Met87 and c/d loop residues that are greater than or equal to those for residues in the vicinity of the a/b loop. Thus, an entrance to the ligand-binding site near Met87 seems likely with βd functioning as a gate either independently or in concert with changes in the a/b loop conformation.
Summary
Three crystal structures of MUP-I have been determined in the presence of two distinct pheromones and in two distinct space groups. These results indicate that the ability of MUP-I to bind a variety of small lipophilic ligands derives from a limited degree of conformational flexibility and unoccupied space within the hydrophobic interior of the β-barrel. Furthermore, the two water molecules present in this hydrophobic environment make possible at least two separate sites for interactions between MUP-I and a limited number of polar groups present on the lipophilic pheromones. Finally, conformational mobility of loop regions at one end of the β-barrel is suggested by positional deviations between the three crystal structures and by crystallographic temperature factors, which indicate access to the ligand-binding site may be facilitated by the flexibility of residues on βc and βd.
Materials and methods
Expression and purification of recombinant MUP-I and pheromone synthesis
Recombinant His-tagged MUP-I was expressed and purified as described (Zídek et al. 1999b). Following removal of the His tag with Factor Xa to yield a native N terminus, MUP-I was dialyzed against 0.3 M NaCl and 10 mM TrisHCl at pH 7.9 and concentrated to 12 mg/mL by ultrafiltration (Centricon). Syntheses of SBT and HMH have also been described (Novotny et al. 1990; Novotny et al. 1999b).
Purification of MUP from liver
MUP was isolated as a contaminating protein present in protein disulfide isomerase purified from mouse liver, according to the method of Lundström and Holmgren (1990). Mouse livers were homogenized in 100 mM sodium phosphate at pH 7.5, 5 mM EDTA, 1% Triton X-100, and 0.5 mM PMSF at pH 7.5 and centrifuged at 15,000 rpm in a Sorvall SS34 rotor. The resulting supernatant was heated to 54°C for 15 min. Heat-precipitated material was removed by centrifugation. The resulting supernatant was fractionated with solid ammonium sulfate. The fraction precipitating between 55% and 85% saturation was dissolved in and dialyzed against 25 mM sodium citrate at pH 5.3. The sample was applied to a CM-Sephadex column equilibrated in 25 mM sodium citrate at pH 5.3. The column flow-through fractions were collected and precipitated by addition of ammonium sulfate to 100% saturation. This material was then dissolved in and dialyzed against 20 mM sodium phosphate at pH 6.3. This material was applied to a DE52 column and was eluted using a 0.0–0.7 M NaCl gradient in 20 mM sodium phosphate at pH 6.3. The second major peak of UV-absorbing material was pooled, concentrated, and applied to a G-75 column equilibrated in 5 mM EDTA, 200 mM NaCl, and 0.1% β-mercaptoethanol at pH 8.0. MUP was identified in the second peak by 20 cycles of N-terminal amino acid sequencing (data not shown) and was pooled and concentrated to 30 mg/mL by ultrafiltration.
Crystallization
Recombinant MUP-I was crystallized in the space group P43212 (a = b = 57.0 Å, c = 108.6 Å) at 4°C with minor modifications of the published method (Böcskei et al. 1991) using 200 mM malate at pH 5.0 and 100 mM CdCl2. Protein solutions contained a three-fold molar excess of pheromone to protein, and crystals were equilibrated by vapor diffusion against the precipitant solution saturated with pheromone for at least 3 d before data collection. MUP isolated from liver was crystallized in the space group P3221 (a = b = 61.2 Å, c = 100.6 Å) by the hanging drop vapor diffusion method using precipitant solutions of 50% to 70% ammonium sulfate containing 100 mM citrate/phophosphate buffer in the pH range of 5.0 to 7.0. The majority of the MUP formed twinned- or hair-like crystals under these conditions. A single individual cube-shaped crystal was obtained and was used to seed additional crystals of the same morphology.
Structure determinations
Data from capillary mounted MUP-I/SBT and MUP-I/HMH crystals were measured using a Rigaku RU200 rotating anode X-ray generator and a Rigaku R-Axis IIc detector at room temperature. The data were autoindexed, integrated, scaled, and merged using DENZO and SCALEPACK (Otwinowski and Minor 1997). The tetragonal crystals of recombinant MUP-I are sufficiently isomorphous with those used by Böcskei et al. (1992) to enable direct refinement of the 1MUP coordinates against the diffraction data (Table 1). Manual fitting of the model was performed using O (Jones et al. 1991), and the models were refined with REFMAC (Collaborative Computational Project No. 4 [CCP4]) and CNS (Brünger et al. 1998), using a bulk solvent correction (Table 1).
Crystals of MUP isolated from mouse liver were flash-cooled at 100°K using Paratone N and paper wicks to remove aqueous solution from the crystal surface. Diffraction data were collected using the Brandeis B1 detector at the Brookhaven National Laboratory NSLS beamline X12C. The data were autoindexed, integrated, scaled, and merged using DENZO and SCALEPACK (Otwinoski and Minor 1997). The space group P3221 was determined from autoindexing and scaling statistics and on the correlation coefficient and R-factor of the molecular replacement solution. The trigonal MUP crystal structure was determined by molecular replacement using AMORE (CCP4) with the coordinates 1MUP as a search model (Böcskei et al. 1992). The search model was centered at the origin of a P1 cell with cell dimensions of a = b = c = 65 Å and α = β = γ = 90°. A clear solution to the rotation function search was obtained using 12.0–3.5 Å data with a Patterson search radius of 25 Å, and the crystallographic R-factor following rigid body refinement of the solution to the translation function was 0.381 (R-free = 0.371). Manual fitting of the model was performed using O (Jones et al. 1991), and the model was refined with REFMAC (CCP4) and CNS (Brünger et al. 1998) using a bulk solvent correction (Table 1). Overall, the vast majority of residues are well defined by the electron density maps contoured between 1.0 and 1.5 σ (Fig. 3A,B ▶). Exceptions include a single break in the main chain electron density at residue Glu80 in the trigonal MUP-I structure and the solvent accessible side chains of Glu19, Asn53, Glu67, Arg78, Glu80, Glu93, Lys94, Glu164, and Asn171 in all three structures.
Acknowledgments
We thank Tom Hurley, Nic Steussy, Jean Hamilton, and Martin Stone for useful discussions, and Toni Poole, Joel Harp, and Gerry Bunick for technical assistance. D.E.T. was supported by NIH grant DK54738 and M.V.N. by NIH grants DC02418 and GM55055. Coordinates have been deposited in the Protein Data Bank under the accession numbers 1IO4, 1IO5, and 1IO6.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps52201.
References
- al-Shawi, R., Wallace, H., Harrison, S., Jones, C., Johnson, D., and Bishop, J.O. 1992. Sexual dimorphism and growth hormone regulation of a hybrid gene in transgenic mice. Mol. Endocrinol. 6 181–190. [DOI] [PubMed] [Google Scholar]
- Bacchini, A., Gaetani, E., and Cavaggioni, A. 1992. Pheromone binding proteins of the mouse (Mus musculus). Experimentia 48 419–421. [DOI] [PubMed] [Google Scholar]
- Bishop, J.O., Clark, A.J., Clissold, P.M., Hainey, S., and Francke, U. 1982. Two main groups of mouse major urinary protein genes, both largely located on chromosome 4. EMBO J. 1 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcskei, Z., Findlay, J.B., North, A.C., Phillips, S.E., Somers, W.S., Wright, C.E., Lionetti, C., Tirindelli, R., and Cavaggioni, A. 1991. Crystallization of and preliminary X-ray data for the mouse major urinary protein and rat α2-globulin. J. Mol. Biol. 218 699–707. [DOI] [PubMed] [Google Scholar]
- Böcskei, Z., Groom, C.R., Flower, D.R., Wright, C.E., Phillips, S.E., Cavaggioni, A., Findlay, J.B., and North, A.C. 1992. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature 360 186–188. [DOI] [PubMed] [Google Scholar]
- Brownlow, S., Morais Cabral J.H., Cooper, R., Flower, D.R., Yewdall, S.J., Polikarpov, I., North, A.C., and Sawyer, L. 1997. Bovine beta-lactoglobulin at 1.8 Å resolution-still an enigmatic lipocalin. Structure 5 481–495. [DOI] [PubMed] [Google Scholar]
- Brünger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography and NMR system (CNS): A new software suite for macromolecular structure determination. Acta Crystallogr. D54 905–921. [DOI] [PubMed] [Google Scholar]
- Cavaggioni, A. and Mucignat-Caretta, C. 2000. Major urinary proteins, α2u-globulins and aphrodisin. Biochim. Biophys. Acta 1482 218–228. [DOI] [PubMed] [Google Scholar]
- CCP4 (Collaborative Computational Project No. 4). 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D54 905–921. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, B.N., Kleywegt, G.J., Björkman, J., Lehman-McKeeman, L.D., Oliver, J.D., and Jones, T.A. 1999. The structures of α2u-globulin and its complex with a hyaline droplet inducer. Acta Crystallogr. D55 743–762. [DOI] [PubMed] [Google Scholar]
- Cowan, S.W., Newcomer, M.E., and Jones, T.A. 1990. Crystallographic refinement of human serum retinol binding protein at 2 Å resolution. Proteins 8 44–61. [DOI] [PubMed] [Google Scholar]
- Duncan, R., Matthai, R., Huppi, K., Roderick, T., and Potter, M. 1988. Genes that modify expression of major urinary proteins in mice. Mol. Cell. Biol. 8 2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson, J.S., Asofsy, R., Potter, M., and Runner, C.C. 1965. Major urinary protein complex of normal mice: Origin. Science 149 981–982. [DOI] [PubMed] [Google Scholar]
- Flower, D.R. 1996. The lipocalin protein family: Structure and function. Biochem. J. 318 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, R., Schneider, M., Mayr, I., Muller, R., Deutzmann, R., Suter, F., Zuber, H., Falk, H., and Kayser, H. 1987. Molecular structure of the bilin binding protein (BBP) from Pieris brassicae after refinement at 2.0 Å resolution. J. Mol. Biol. 198 499–513. [DOI] [PubMed] [Google Scholar]
- Jemiolo, B., Harvey, S., and Novotny, M. 1986. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc. Natl. Acad. Sci. USA 83 4576–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.Y., Cowan, S.W., and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47 110–119. [DOI] [PubMed] [Google Scholar]
- Larsen, G.L., Bergman, A., and Klasson-Wehler, E. 1990. A methylsulphonyl metabolite of a polychlorinated biphenyl can serve as a ligand for alpha 2u-globulin in rat and major-urinary-protein in mice. Xenobiotica 20 1343–1352. [DOI] [PubMed] [Google Scholar]
- Lücke, C., Franzoni, L., Abbate, F., Löhr, F., Ferrari, E., Sorbi, R.T., Rüterjans, H., and Spisni, A. 1999. Solution structure of a recombinant mouse major urinary protein. Eur. J. Biochem. 266 1210–1218. [DOI] [PubMed] [Google Scholar]
- Lundström, J. and Holmgren, A. 1990. Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J. Biol. Chem. 265 9114–9120. [PubMed] [Google Scholar]
- Marsden, H.M. and Bronson, F.H. 1964. Estrous synchrony in mice: Alteration by exposure to male urine. Science 144 1469. [DOI] [PubMed] [Google Scholar]
- Mucignant-Careta, C., Caretta, A., and Cavaggioni, A. 1995. Acceleration of puberty onset in female mice by urinary proteins. J. Physiol. 486 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny, M., Harvey, S., Jemiolo, B., and Alberts, J. 1985. Synthetic pheromones that promote inter-male aggression in mice. Proc. Natl. Acad. Sci. USA 82 2059–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny, M., Jemiolo, B., Harvey, S., Wiesler, D., and Marchlewska-Koj, A. 1986. Adrenal-mediated endogenous metabolites inhibit puberty in female mice. Science 231 722–725. [DOI] [PubMed] [Google Scholar]
- Novotny, M.V., Jemiolo, B., and Harvey, S. 1990. In Chemical Signals in Vertebrates 5 (eds. D. Muller-Schwarze and R.M. Silverstein), pp. 1–22. Oxford University Press, Oxford.
- Novotny, M.V., Ma, W., Wiesler, D., and Zídek, L. 1999a. Positive identification of the puberty-accelerating pheromone of the house mouse: The volatile ligands associating with the major urinary protein. Proc. R. Soc. Lond. B. Biol. Sci. 266 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny, M.V., Jemiolo, B., Wiesler, D., Ma, W., Harvey, S., Xu, F., Xie, T.M., and Carmack, M. 1999b. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 6 377–383. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. and Minor, W. 1997. Processing of X-ray diffraction data collected in oscillation data reduction mode. Methods Enzymol. 276 307–326. [DOI] [PubMed] [Google Scholar]
- Robertson, D.H.L., Beynon, R.J., and Evershed, R.P. 1993. Extraction, characterization, and binding analysis of two pheromonally active ligands associated with major urinary protein of house mouse (Mus musculus). J. Chem. Ecol. 19 1405–1416. [DOI] [PubMed] [Google Scholar]
- Whitten, W.K., Bronson, F.H., and Greenstein, J.A. 1968. Estrus-inducing pheromone of male mice: Transport by movement of air. Science 161 584–585. [DOI] [PubMed] [Google Scholar]
- Zídek, L., Novotny, M.V., and Stone, M.J. 1999a. Increased protein backbone conformational entropy upon hydrophobic ligand binding. Nat. Struct. Biol. 6 1118–1121. [DOI] [PubMed] [Google Scholar]
- Zídek, L., Stone, M.J., Lato, S.M., Pagel, M.D., Miao, Z., Ellington, A.D., and Novotny, M.V. 1999b. NMR mapping of the recombinant mouse major urinary protein I binding site occupied by the pheromone 2-sec-butyl-4,5-dihydrothiazole. Biochemistry 38 9850–9861. [DOI] [PubMed] [Google Scholar]