Abstract
We previously demonstrated that a β-hairpin peptide, termed BH9–10, derived from a single-layer β-sheet of Borrelia OspA protein, formed a native-like β-turn in trifluoroethanol (TFE) solution, and it assembled into amyloid-like fibrils at higher TFE concentrations. This peptide is highly charged, and fibrillization of such a hydrophilic peptide is quite unusual. In this study, we designed a circularly permutated peptide of BH9–10, termed BH10–9. When folded into their respective β-hairpin structures found in OspA, these peptides would have identical cross-strand interactions but different turns connecting the strands. NMR study revealed that BH10–9 had little propensity to form a turn structure both in aqueous and TFE solutions. At higher TFE concentration, BH10–9 precipitated with a concomitant α-to-β conformational conversion, in a similar manner to the BH9–10 fibrillization. However, the BH10–9 precipitates were nonfibrillar aggregation. The precipitation kinetics of BH10–9 was exponential, consistent with a first-order molecular assembly reaction, while the fibrillization of BH9–10 showed sigmoidal kinetics, indicative of a two-step reaction consisting of nucleation and molecular assembly. The correlation between native-like turn formation and fibrillization of our peptide system strongly suggests that BH9–10 adopts a native-like β-hairpin conformation in the fibrils. Remarkably, seeding with the preformed BH10–9 precipitates changed the two-step BH9–10 fibrillization to a one-step molecular assembly reaction, and disrupted the BH9–10 fibril structure, indicating interactions between the BH10–9 aggregates and the BH9–10 peptide. Our results suggest that, in these peptides, cross-strand interactions are the driving force for molecular assembly, and turn formation limits modes of peptide assembly.
Keywords: β-Sheet, β-hairpin, peptide design, folding, fibril formation
The mechanism underlying the formation of insoluble fibrils by polypeptides has drawn intense interest of the protein science community in recent years (Kelly 1996,1997; Dobson 1999). Amyloid fibrils are observed in pathogenic deposits for a range of human diseases, whereas recent studies demonstrated that nonpathogenic proteins or peptides also form amyloid-like fibrils under appropriate solution conditions (Mihara and Takahashi 1997; Guijarro et al. 1998; Chiti et al. 1999; Ohnishi et al. 2000). These findings suggest that the ability to form fibrils may be a common feature of polypeptides. Regardless of their native conformation, these fibrils share a morphology of unbranched fibers, in which continuous arrays of β-strands are thought to propagate along the long axis of the fibril with the β-strands perpendicular to the axis (Sunde et al. 1997; Dobson 1999). However, it is difficult to characterize the structure of amyloid fibrils with high-resolution techniques, because amyloid fibrils are neither crystalline nor soluble. Thus, the detailed mechanisms underlying polypeptide assembly into continuous β-sheets in fibrils are still unclear.
We previously demonstrated that a 23-residue peptide, named BH9–10 (Fig. 1B ▶), derived from the strand 9–loop–strand 10 segment of Borrelia burgdorferi outer surface protein A (OspA), formed amyloid-like fibrils (Ohnishi et al. 2000). OspA is a predominantly β-sheet protein (Li et al. 1997), and it contains a unique "single-layer" β-sheet segment (β-strands 8–10), which connects the N- and C-terminal globular domains. Both faces of this single-layer β-sheet are exposed to the solvent (Fig. 1A ▶), and this single-layer β-sheet contains many charged and polar amino acid residues. Thus, this hydrophilic segment does not follow the amphipathic pattern that is commonly found in natural β-sheets. Despite the lack of a hydrophobic core in this region, the single-layer β-sheet segment is highly stable (Pham et al. 1998), and it was possible to stably extend the single-layer β-sheet by inserting copies of a β-hairpin unit equivalent to BH9–10 (Fig. 1A ▶; Koide et al. 2000). These results clearly demonstrated that the interactions within the β-hairpin unit and those between adjacent units are sufficient to stabilize the single-layer β-sheet structure. The architecture of the extended single-layer β-sheet where a series of β-strands propagate perpendicular to the strands (Fig. 1A ▶; Koide et al. 2000) is reminiscent of the model structures of amyloid fibrils (Sunde et al. 1997; Dobson 1999).
Fig. 1.
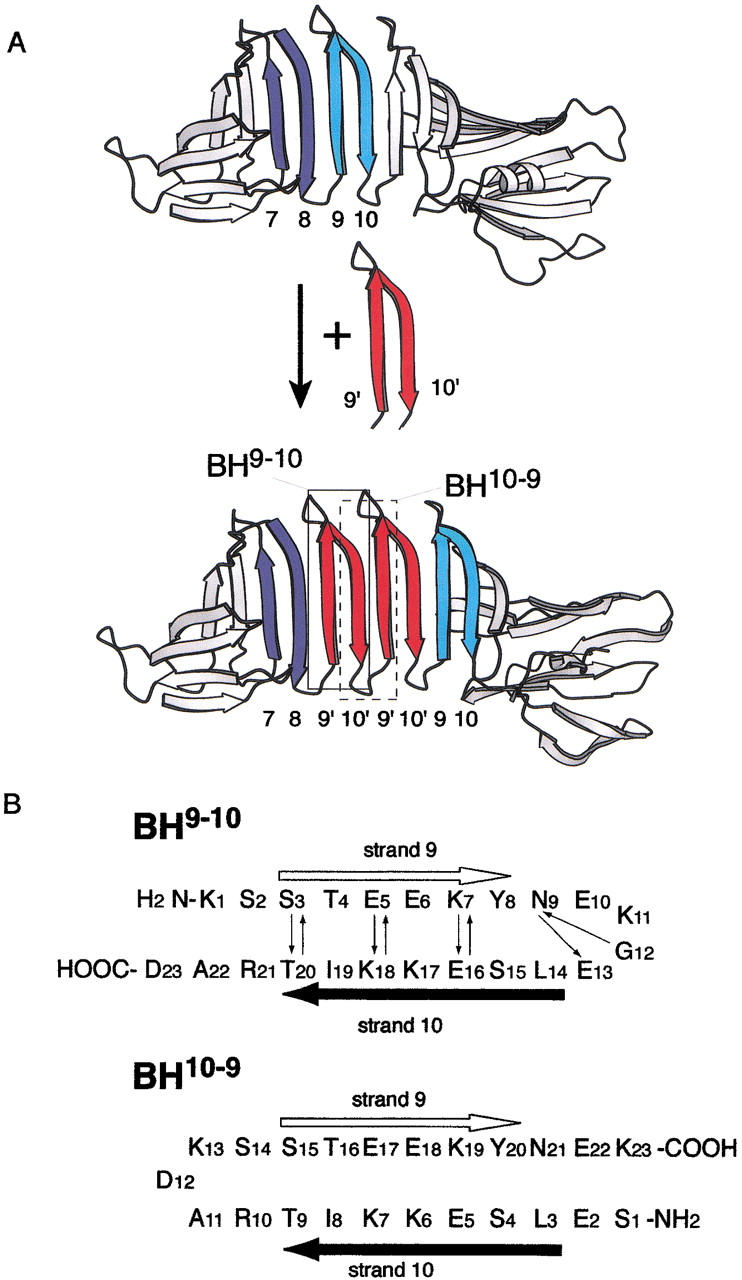
(A) Schematic representation of the extension of the single-layer β-sheet in OspA (Koide et al. 2000). In this design, two copies of a β-hairpin corresponding to the strand 9–loop–strand 10 were inserted between the eighth strand and ninth strands of wild-type OspA (top) to form OspA+2bh (bottom). β-Hairpins corresponding to peptides used in this study, BH9–10 and BH10–9, are shown enclosed. (B) Design of BH9–10 (top) and a circularly permutated peptide, BH10–9 (bottom). BH9–10 corresponds to a β-hairpin segment from K119 to D141 of OspA, which contains the ninth strand (S121–F126, white arrow) and tenth strand (V132–T138, black arrow), with three mutations of F126Y, V132L, and I136K. Small arrows represent backbone hydrogen bonds from the amide proton to the carbonyl oxygen found in the OspA crystal structure. The first residue of BH10–9 was mutated from Gly to Ser because of a PCR aact in the gene construction step (see text).
In the previous study, we found that the addition of trifluoroethanol (TFE) enhances the propensity of the BH9–10 peptide to form a native-like turn and to form amyloid-like fibrils (Ohnishi et al. 2000). This peptide contains large amounts of polar and charged amino acid residues, whereas most peptides known to form amyloid fibrils are highly hydrophobic or amphipathic (Jarvis et al. 1993; Serpell 2000; Broome and Hecht 2000). Our finding of amyloid-like fibril formation of BH9–10 may be significant for the understanding of the fibril formation mechanism, because studies of this hydrophilic peptide system may provide new insights into the mechanism of fibril formation that are complementary to those gained from hydrophobic peptides. However, like fibrils of other peptides, the conformation of BH9–10 in the fibrils is elusive. We also found that this peptide forms a native-like turn in solution in the presence of TFE, and it corresponds to the β-hairpin unit used in the extension of the single-layer β-sheet (Fig. 1A ▶). Thus, it is likely that the BH9–10 peptide forms a native-like β-hairpin in the fibrils. Therefore, the crystal structure of the single-layer β-sheet in OspA may be viewed as a high-resolution model for the continuous β-sheet structure in the fibrils, providing an advantage of studying the fibril formation mechanism using this system.
In the present study, we designed a permutated peptide of BH9–10, named BH10–9 (Fig. 1B ▶). These two peptides would have identical cross-strand interactions when folded into their respective β-hairpin structures in OspA. However, the location and the amino acid sequence of the turn connecting the two strands would be different between the two hairpins. Thus, comparative studies of these peptides should reveal the roles of the turn region in fibrillization of these peptides. We found that this permutation caused drastic changes in the solution structure and fibril formation. NMR results revealed little propensity of the new peptide to form a β-turn structure both in aqueous and TFE solutions. BH10–9 precipitated in the presence of a high concentration of TFE, in a similar manner to the original BH9–10 peptide (Ohnishi et al. 2000). However, we found that the BH10–9 precipitates were not amyloid-like fibrils but amorphous aggregates. Significantly, we found that cross-seeding of the amorphous BH10–9 aggregate disrupted the BH9–10 fibril formation. We will discuss implications on the roles of turn formation and cross-strand interactions in fibrillization of this peptide system.
Results
Peptide design
We designed the circularly permutated peptide, BH10–9, by keeping sequences in the β-strand regions (S3-Y8 and L14-T20) of BH9–10 identical and changing the position of the turn connecting the two strands (Fig. 1B ▶). Thus, in this design, BH10–9 has identical cross-strand interactions to the original peptide. The BH10–9 turn region between R10 and S14 does not exist in the wild-type OspA, but it does in a mutant OspA with an extended single-layer β-sheet (Fig. 1A ▶). This mutant protein containing the extended single-layer β-sheet was as stable as wild-type OspA (Koide et al. 2000). Therefore, the new peptide can also be considered as a building unit of the single-layer β-sheet, and one might expect that BH10–9 forms a β-turn and amyloid-like fibrils in a similar manner to that found for the original peptide. The first residue of BH10–9 was mutated from Gly to Ser due to a PCR aact in gene construction. Because this is a terminal residue and Ser has a higher β-sheet-forming propensity than Gly (Minor and Kim 1994; Smith and Regan 1995), we predicted that this mutation would not significantly disrupt β-turn and β-strand formation of the peptide. Thus, we did not correct this Gly-to-Ser mutation.
Solution conformation of BH10–9
BH10–9 was highly soluble at least up to 11 mM in aqueous solution. The peptide was unstructured in 50 mM sodium phosphate buffer, pH 6.5, at 30°C, as judged by far-UV CD spectroscopy (Fig. 2A ▶). Adding 1 M NaCl, changing the pH ranging from 7 to 2, or decreasing the temperature to 4°C did not significantly affect the CD spectrum (data not shown). The addition of TFE increased the α-helical content of the peptide (Fig. 2A ▶). This helix-inducing effect of TFE is well known for many polypeptides (Nelson and Kallenbach 1986; Thomas and Dill 1993; Luo and Baldwin 1997), and it was similarly observed for BH9–10 (Ohnishi et al. 2000). α-Helix contents of BH9–10 and BH10–9 showed similar dependence on TFE concentration (Fig. 2B ▶). The highest helix contents observed for these two peptides were approximately 25%, corresponding to about six residues. Because these two peptides share identical amino acid sequences in the β-strand regions, these results suggest that the α-helical structure is induced in the strand regions.
Fig. 2.

(A) Far-UV CD spectra of BH10–9 at various concentrations of TFE recorded at 30°C. TFE concentrations are 0, 10, 20, 30, 40, 50, 60, and 70% (v/v) from the top to the bottom along the arrow. (B) Helix contents of BH9–10 (open circle) and BH10–9 (close square) plotted as a function of TFE concentration. The helix contents were calculated based on molar ellipticity at 222nm (Rohl and Baldwin 1998). (C) Far-UV CD spectra of 200 μM BH10–9 in 2 mM sodium phosphate (pH 6.5) containing 80% (v/v) TFE at 30°C. Spectra recorded immediately after the mixing and taken every 10 min up to 50 min after the mixing are shown from the bottom to the top along the arrow.
In 80% (v/v) TFE solution, BH10–9 gradually precipitated. The CD spectrum of the peptide recorded immediately after the addition of TFE exhibited characteristics of helical structure, and it gradually changed into a spectrum typical of the β-sheet with concomitant precipitation (Fig. 2C ▶). BH9–10 also precipitated under similar conditions, which was accompanied by an α-to-β conformational conversion (Ohnishi et al. 2000). We will describe the characterization of the precipitation reaction of the new peptide in the following section.
We then performed high-resolution NMR studies of BH10–9 to gain more detailed structural information. A uniformly 15N-labeled BH10–9 sample was prepared, and 1H, 15N heteronuclear NMR methods were used for the complete resonance assignments for the peptide. For comparison with our previous results of BH9–10 (Ohnishi et al. 2000), BH10–9 was examined under two sets of solvent conditions: 50 mM sodium phosphate buffer at pH 6.5, 5°C, and the same buffer containing 20% (v/v) TFE at 5°C. The data for NOE connectivities, chemical shift indices, and 3JHNHα coupling constants are summarized in Figure 3 ▶. In the absence of TFE, we did not find obvious features of a defined structure for BH10–9, such as characteristic 3JHNHα coupling constants and medium-range NOEs, while we found a small population of non-native turn for the original BH9–10 peptide in the aqueous buffer (Ohnishi et al. 2000). Although we found many nonsequential NOEs for the new peptide in the presence of 20% (v/v) TFE, these NOEs did not seem to support a unique conformation such as an α-helix or a β-hairpin. Furthermore, the Hα chemical shift index and the 3JHNHα coupling constants exhibited little derivatives from random-coil values, strongly suggesting the absence of a well-defined structure (Fig. 3B ▶). We also calculated model structures of BH10–9 in the TFE solution using these 3JHNHα coupling and NOE data, and found only the formation of a nascent helix around the region between T16 and Y20 (data not shown). Therefore, we concluded that BH10–9 is predominantly unstructured both in aqueous and TFE solutions, and that this peptide has little propensity to form a β-turn or a β-hairpin.
Fig. 3.

NOE connectivities, secondary Hα shifts and backbone JHNHα coupling constants observed for BH9–10 in 50 mM sodium phosphate buffer (pH 6.5) at 5°C (A) and those observed for BH10–9 in 50 mM sodium phosphate buffer (pH 6.5) containing 20% (v/v) deuterated TFE at 5°C (B). NOE designation is according to the scheme of Wüthrich (1986). NOE cross-peak intensities were classified into three categories (strong, medium, and weak), and the classification is indicated by the height of a line. Secondary shifts were determined by the method of Wishart et al. (1992). A positive value greater than 0.1 suggests a β-strand conformation, and a negative value smaller than −0.1 suggests an α-helix.
Nonfibrillar precipitation of BH10–9
As described above, BH10–9 gradually precipitated with a concomitant conformational conversion to a β-sheet-rich structure in 80% (v/v) TFE solution (Fig. 2C ▶). We have observed similar precipitation of BH9–10, and demonstrated that the BH9–10 precipitation contains amyloid-like fibrils (Ohnishi et al. 2000). We examined the precipitates of the new peptide using standard techniques for the investigation of amyloid fibrils (Kelly 1996, 1997; Dobson 1999; Lynn and Meredith 2000). The BH10–9 precipitates were stained with Congo red, and the difference spectrum showed a peak around 541 nm, but they did not show clear birefringence under polarized light (data not shown). We did not obtain a clear image of fibril-like structures by electron micrography. After repeated comparisons of electron micrographs of the BH10–9 precipitates with those for control samples containing no peptides, we concluded that the peptide precipitates have large amorphous structures shown in Figure 4A ▶. This amorphous structure is distinct from the BH9–10 fibrils (Fig. 6B ▶). Although the BH9–10 fibrils showed a clear X-ray diffraction maximum corresponding to a spacing of 0.47 nm, the BH10–9 precipitates did not show clear maxima (Fig. 4B ▶, D), indicating that the precipitates of the new peptide do not contain highly ordered β-sheets (Sunde et al. 1997). Taken together, these results demonstrate that BH10–9 precipitation is amorphous aggregation and, unlike the original BH9–10 peptide, the new peptide has little propensity to form amyloid-like fibrils.
Fig. 4.

(A) Electron micrography of negatively stained BH10–9 precipitation in the presence of TFE. The gray and black areas represent the peptide precipitates, as determined by comparison with specimens prepared with the identical solvent but without the peptide. X-ray fiber diffraction of BH10–9 precipitates in the presence of TFE (B), a buffer solution containing TFE but not the peptide precipitates (C), and BH9–10 fibrils in the presence of TFE (D). A diametrical cross section of the diffraction image is shown under each diffraction pattern. The arrows indicate the diffraction maximum corresponding to a 0.47-nm spacing.
Fig. 6.

(A) Seeding effects on the kinetics of the BH9–10 fibril formation. The curve for "no seeds" was obtained for BH9–10 fibrillization reaction with the peptide concentration of 200 μM. A BH9–10 solution, TFE, and a defined amount of suspension of preformed peptide precipitates were mixed to initiate the precipitation reaction. Total peptide concentration of BH9–10 and BH10–9 was 200 μΜ for each measurement. (B) Electron micrography of BH9–10 fibrils formed without seeds. (C) Electron micrography of BH9–10 precipitates formed with 3% (v/v) BH10–9 seeds.
The BH10–9 precipitates formed in the presence of TFE were easily dissolved by decreasing TFE concentration (Fig. 5B ▶). Similar dissociation upon TFE dilution was observed for the BH9–10 fibrils (Ohnishi et al. 2000). The structure of BH10–9 dissociated from the precipitates was confirmed by 1H NMR spectroscopy to be identical to that of the peptide in the same solution conditions prepared without the precipitating step (data not shown), indicating that the precipitating process is reversible.
Fig. 5.

(A) Kinetics of the precipitation reactions of BH10–9 monitored by turbidity measurements with various peptide concentrations initiated by the addition of TFE. Final solvent conditions were 2 mM NaPB containing 80% (v/v) of TFE at pH 6.5, 30°C. (B) Dissociation kinetics of precipitation of BH10–9 initiated by the reduction of peptide and TFE concentrations. Turbid suspension of BH10–9 precipitation in 80% TFE solution was diluted to 80% (v/v) (downward triangle), 50% (v/v) (upward triangle), and 20% (v/v) (circle) TFE solutions. The final peptide concentration was 70 μM. White squares represent time course of 70 μM BH10–9 sample in the absence of TFE as a control.
Precipitation kinetics of BH10–9
Amyloid fibril formation typically exhibits sigmoidal kinetics, indicative of the presence of a slow nucleation step that is followed by a more rapid molecular assembly step (Mihara and Takahashi 1997). In contrast, a common protein aggregation process follows exponential kinetics. The precipitation reaction of BH10–9 in the presence of TFE showed exponential kinetics (Fig. 5A ▶), while the reaction of the BH9–10 fibril formation showed sigmoidal kinetics (Fig. 6A ▶). These results indicate that the BH10–9 precipitation reaction lacks a rate-limiting nucleation step, which is consistent with the observations that the BH10–9 precipitates were amorphous aggregations. The kinetics of the BH10–9 precipitation were very sensitive to both peptide and TFE concentrations, as expected for a molecular assembly reaction (Fig. 5A ▶). Under identical solvent conditions, the precipitation kinetics of the two peptides were similar, as judged by light scattering, except for the presence and absence of the lag phase (compare the data series for "200 μM" in Fig. 5A ▶ with those for "no seeds" in Fig. 6A ▶).
Seeding effects on the BH9–10 and BH10–9 precipitation reactions
We investigated the precipitation kinetics of the two peptides, initiated in the presence of small amounts of preformed precipitates ("seeds"). The exponential curve of BH10–9 was not affected by the addition of 10% (v/v) BH10–9 seeds (data not shown). In contrast, the sigmoidal kinetics of fibrillization of the original BH9–10 peptide were altered to a simple exponential curve by the addition of 10% (v/v) BH9–10 seeds (Fig. 6A ▶; Ohnishi et al. 2000). This change in the fibrillization kinetics of the original peptide indicates that the seeds eliminated a rate-limiting, nucleation step in the fibril formation (Krebs et al. 2000; Morozova-Roche et al. 2000; Ohnishi et al. 2000). The electron micrography images of the BH9–10 precipitates formed in the presence of the seeds of the same peptide were similar to those of the BH9–10 fibrils formed without the seeds (data not shown).
We then performed "cross-seeding" experiments, where we used pre-formed BH10–9 precipitates as seeds for the fibrillization reaction of the original BH9–10 peptide. The fibrillization kinetics of the original BH9–10 peptide were significantly altered by cross-seeding (Fig. 6A ▶). The amount of the BH10–9 seeds was critical to this effect, and the addition of 10% (v/v) BH10–9 seeds changed the sigmoidal fibrillization kinetics of the original peptide almost completely to an exponential curve. Electron micrography revealed that the BH9–10 precipitates with 3% (v/v) BH10–9 seeds had a significantly smaller amount of fibrils, and that the fibrils appeared shorter than the BH9–10 fibrils formed without seeds (Fig. 6B ▶, C). Furthermore, the BH9–10 precipitates formed with 10% (v/v) cross-seeding did not contain fibrils, and they showed an amorphous morphology similar to that of the BH10–9 precipitates shown in Figure 4A ▶ (data not shown). The conversion of the BH9–10 fibril formation to amorphous aggregation indicates that the BH10–9 aggregates can interact with the original BH9–10 peptide, and that the BH10–9 seeds disrupted the alignment of the original peptides that is necessary for fibril formation.
Discussion
The new, permutated peptide, BH10–9, did not form β-turn or β-hairpin in solution, and the peptide had little propensity to form amyloid-like fibrils. On the other hand, the parental peptide, BH9–10, had a tendency to form a turn structure in solution and a propensity to form amyloid-like fibrils (Ohnishi et al. 2000). In particular, the addition of TFE induced a native-like turn formation of BH9–10 in the monomeric form, and facilitated the fibril formation. These peptides share identical amino acid sequences in the strand regions, and differ only in the turn segment (Fig. 1B ▶). Thus, these results show a correlation between turn formation in the monomeric form and fibril formation of these peptides. This correlation strongly suggests that a native-like turn of BH9–10 exists in the fibrils, and that the peptide forms a native-like β-hairpin in the fibrils. However, no β-hairpin conformation of BH9–10 was detected in the solution. This suggests that the cross-strand interactions within monomeric BH9–10 are not sufficient to acquire a population of β-hairpin detectable by NMR, and that intermolecular cross-strand interactions are required to stabilize the β-hairpin conformation. This requirement of intermolecular interactions is satisfied in the context of OspA (Fig. 1A ▶) and also likely in the fibrils.
The difference in the β-turn propensity between these two peptides may be explained from a comparison of their model structures. As shown in Figure 1A ▶, the mutant OspA with an extended β-sheet contains a repetition of β-hairpin unit corresponding to BH9–10. In this architecture, one could also consider a segment corresponding to BH10–9 as a repeating unit. NMR studies revealed that the inserted β-hairpin unit takes on a conformation that is nearly identical to the original β-hairpin (Koide et al. 2000). Thus, we can use the structure of the strand 9–loop–strand 10 segment in the OspA crystal structure as a model for BH9–10, and that of the strand 8–loop–strand 9 segment as a model for BH10–9. As shown in Figure 7A ▶, the BH9–10 loop model contains a side-chain cluster of N127-K129-E131 with three hydrogen bonds (N127 Hδ–E131 Oɛ, N127 Oδ–E131 HN, and N127 Oδ–K129 HN) in addition to two backbone–backbone hydrogen bonds (N127 HN–E131 O, G130 HN–N127 O). In contrast, the BH10–9 loop model contains two hydrogen bonds between the side chain and backbone (S116 O>–D118 HN, D118 O>–S120 HN) and two backbone–backbone hydrogen bonds (S116 HN–S120 O, K119 HN–S116 O) (Fig. 7B ▶). However, the hydrogen bond between S116 O> and D118 HN in the original sequence does not exist in BH10–9, where Ser is replaced with Arg. Thus, the BH9–10 loop has a larger number of hydrogen bonds than the BH10–9 loop, which may result in stabilization of the native-like β-turn of BH9–10, particularly in concentrated TFE solution where electrostatic interactions are stronger (Thomas and Dill 1993).
Fig. 7.
Model structures for the BH9–10 loop (A) and BH10–9 loop (B). Loop regions of the strand 9–loop-strand 10 β-hairpin segment and the strand 8–loop-strand 9 β-hairpin segment in the OspA crystal are shown as models of the BH9–10 loop and the BH10–9 loop, respectively. Amino acid residues are labeled with the residue ideication in the OspA crystal structure with that in the corresponding peptide in parentheses. Dashed lines represent hydrogen bonds.
The CD experiment (Fig. 2C ▶) revealed an α-to-β conformational conversion of BH10–9 upon precipitation in the presence of TFE, indicating that the BH10–9 precipitates are rich in β-sheets. This conformational conversion upon precipitation by the addition of TFE was similarly observed to BH9–10 (Ohnishi et al. 2000). However, the structure of the precipitation of the new BH10–9 peptide was amorphous (Fig. 4A ▶), and drastically different from that of the BH9–10 fibrils made under the identical solution conditions (Fig. 6B ▶). Both peptides have identical sequences in the strand regions, but they have different β-turn propensities. Thus, our results strongly suggest that cross-strand interactions are responsible for the stabilization of β-sheet conformation and peptide assembly, and that the difference in the β-turn propensity causes the difference in the higher order structure of these peptides. Accordingly, we propose a possible mechanism for β-sheet fibril formation of these peptides in which cross-strand interactions are the driving force for molecular assembly, while turn structure regulates systematic molecular alignment into amyloid-like fibrils (Fig. 8 ▶). The lower polarity of TFE results in negative transfer energy from water to the alcohol for hydrophobic groups and positive for polar groups (Liu and Bolen 1995). Therefore, in TFE solution, polar interactions are strengthened (Thomas and Dill 1993; Liu and Bolen 1995; Shiraki et al. 1995). In addition to backbone amide and carboxyl groups, both BH9–10 and BH10–9 contain many polar side chains in their strand regions (Fig. 1B ▶). Therefore, the addition of TFE should facilitate cross-strand interactions, resulting in the molecular assembly of these peptides. Under these conditions, the original BH9–10 peptide with a modest β-turn propensity would prefer a β-hairpin conformation that provides two interfaces for molecular assembly (i.e., the two edges of a β-hairpin). In contrast, the BH10–9 peptide with a lower β-turn propensity has four potential interfaces (i.e., the two edges each for two β-strand regions). This model suggests that the turn formation may limit the modes of peptide assembly that are necessary for directional growth of fibrils. This model is consistent with our results from cross-seeding experiments, in which amorphous BH10–9 seeds disrupted the BH9–10 fibrils (Fig. 6C ▶) and changed the sigmoidal fibrillization kinetics of BH9–10 to exponential (Fig. 6A ▶). This model also suggests that β-hairpin formation is an important step in the nucleation of the BH9–10 fibrils. However, experimental and computational studies showed that β-hairpin formation occurs in a microsecond time scale (Munoz et al.. 1998; Klimov and Thirumalai 2000), which is orders of magnitude faster than the fibrillization of BH9–10. Also, β-hairpin conformation of BH9–10 is unstable in the monomeric state (Ohnishi et al. 2000). Therefore, oligomerization of a small number of β-hairpin units that serves as a stable scaffold for further assembly is most likely involved in the nucleation step, just like proto-fibril formation that is considered to be a major step in nucleation for Aβ fibril formation (Harper et al. 1999).
Fig. 8.
Proposed molecular assembly mechanisms of BH9–10 and BH10–9. (A) A higher propensity of BH9–10 to form a native-like β-turn results in the formation of a native-like β-hairpin of the peptide with TFE-enhanced cross-strand interactions. The cross-strand interactions also drive molecular assembly through two hydrogen bonding interfaces of the β-hairpin into amyloid-like fibrils. (B) In the presence of TFE, BH10–9 with little β-turn propensity would prefer intermolecular cross-strand association rather than a β-hairpin formation by intramolecular cross-strand interactions. One BH10–9 molecule has two β-strand regions connected with a flexible loop, and each β-strand region can interact with two β-strand regions of other molecules at maximum. Therefore, the molecular assembly of this peptide results in aggregation rich in β-sheets, but lacking an ordered molecular orientation. Note that, in the monomeric state, conformations with an α-helical structure are also populated. However, such α-helical conformations may be an off-pathway intermediate state, because of their incompatibility with the β-sheet-rich assembly. Thus, we have omitted such α-helical conformations for clarity.
It is generally considered that amyloid fibril formation is caused by protein misfolding into a non-native conformation that is capable of self-assembly. Therefore, the protein conformation in fibrils may be drastically different from its native conformation. To understand the molecular mechanism for amyloid fibril formation by polypeptides, detailed structural information of amyloidogenic fibrils at an atomic resolution is essential. However, despite extensive efforts, the detailed molecular structure of polypeptides in noncrystalline and insoluble amyloid fibrils has not yet been determined. Our experimental system employing the peptides derived from the single-layer β-sheet of OspA may offer unique advantages, because the single-layer β-sheet in the native form of this protein shares a structural feature of continuous β-sheet with amyloid fibrils. We found a correlation between native-like β-turn formation and fibril formation, strongly suggesting that BH9–10 does not misfold but folds into the native β-hairpin structure to form fibrils. Thus, we can use the structure of a segment corresponding to BH9–10 in the OspA crystal structure as a model for fibrils. Further studies of the folding and fibril formation mechanisms of this system should provide significant insights into the molecular mechanism of amyloid fibril formation.
Materials and methods
Sample preparation
Oligonucleotides #1, TAATACGACTCACTATAGGG, and #2, TCCACCTCTCAATC-TCAAC, were used to amplify the ubiquitin gene from the vector of Kohno et al. (1998). Oligonucleotides #3, GTTGAGATTGAGAGGTGGAGGTGAGCTGAGCGAAAA GAAGA-TCACTCGTGCTGACAAGA, and #4, CGGCTCGA GTTACTTCTCGTTGTACTTCTCTTC - GGTAGAGCTCTTGT CAGCACGAGTGA, were annealed, and complementary strands were filled in using Taq polymerase to prepare a double-stranded DNA fragment coding for the BH10–9 sequence. These two PCR fragments were combined, and the entire gene coding for the ubiquitin–BH10–9 fusion protein was assembled by a PCR reaction using the primers #1 and #4, and inserted in pET15b (Novagen). BH10–9, with and without 15N labeling, was prepared as previously described (Ohnishi et al. 2000). The identity of the peptide was confirmed by mass spectroscopy. Peptide concentration was determined using ɛ280 of 0.474 mL mg−1 cm−1. The BH9–10 peptide was prepared as described previously (Ohnishi et al. 2000). All pH values reported are direct pH meter readings uncorrected for isotope effects or effects of TFE (Sigma) (Nelson and Kallenbach 1986).
Circular dichroism spectroscopy
CD measurements were performed on an AVIV 202 spectrophotometer. Helix content was calculated using molar ellipticity at 222 nm according to the method by Rohl and Baldwin (1998).
NMR spectroscopy
NMR spectra were acquired on a Varian Unity INOVA spectrometer operated at a 1H frequency of 600.013 MHz. The probe temperature was set at 5°C. Samples in the absence of TFE contained 1.1 mM of the 15N-BH10–9 peptide in 50 mM sodium phosphate buffer, pH 6.5 either in 95% (v/v) H2O/5% (v/v) D2O, or in 99.9% D2O. A sample in the presence of TFE contained 1.4 mM of the 15N labeled peptide in 50 mM sodium phosphate buffer, pH 6.5 containing 20% (v/v) 2,2,2-trifluoroethanol-1,1-D2 alcohol (Cambridge Isotope Laboratories). Two-dimensional 1H TOCSY (Braunschweiler and Ernst 1983; Cavanagh and Rance 1992), NOESY (Kumer et al. 1981) and 1H, 15N HSQC (Kay et al. 1992) spectra, and three-dimensional TOCSY-HSQC and NOESY-HSQC (Zhang et al. 1994) spectra were recorded and analyzed as reported previously (Ohnishi et al. 2000). The 3JHNHα coupling constants were determined by the J-modulated HSQC experiment (Billeter et al. 1992). Structural calculations based on NMR data were performed using the program CNS, version 0.5 (Brünger et al. 1998).
Kinetic measurements of fibril formation
Turbidity measurements were performed using an AVIV 202 CD spectrometer. Change in light scattering at 340 nm was followed by the reading of dynode voltage, which is proportional to the sample absorbance. A sealed 1-mm cuvette was used to avoid evaporation during measurements. The cuvette temperature was controlled at 30°C. Reactions were initiated by manual mixing, with a dead time of 20–45 sec.
Electron microscopy
A drop of peptide suspension formed in the presence of 80% (v/v) TFE was fixated on a carbon-coated electron microscopy grid, and stained with 2% (v/v) phosphotungstic acid, pH 7.0. The grid was examined with an Hitachi 7100 electron microscope. Buffer solutions with and without TFE were used as negative control in place of the peptide suspension.
X-ray fiber diffraction
A suspension of the BH10–9 precipitation was placed in a 1-mm quartz capillary. Fiber diffraction data were collected using Cu Kα radiation from a Rigaku rotating anode operating at 50 kV and 100 mA. The diffraction pattern was recorded on an R-axis IV image plate (Rigaku) with the sample-to-detector distance of 80 mm, and the image was analyzed using the manufacturer's software and the Scion Image software, version 1.62 (Scion).
Dye binding assay
Precipitates of BH10–9 were suspended in Congo red saline solution with a final dye concentration of 10 μM (Klunk et al. 1999). Because the peptide precipitates dissolved upon the dilution of TFE, the Congo red solution contained 80% (v/v) of TFE. After a 30-min incubation at room temperature, the absorbance spectrum was recorded from 650 to 350 nm. For birefringence analysis, the stained materials were washed with 10 mM sodium phosphate buffer (pH 7.4) containing 80% (v/v) TFE, placed on a glass slide, and examined with a microscope under polarized light.
Seeding and cross-seeding experiments
For the standard seeding experiments, a precipitation reaction was initiated by simultaneously mixing either BH9–10 or BH10–9 solution in 10 mM sodium phosphate buffer (pH 6.5), TFE, and the suspension of preformed precipitation of the identical peptide. The final concentrations of the peptide, TFE, and sodium phosphate buffer were identical to those of the seed suspension. For the cross-seeding experiments, the procedure was identical to that described above except for seeding from the partner peptide precipitates. For example, the precipitation reaction of BH9–10 was initiated by the addition of TFE and a defined amount of suspension of the BH10–9 precipitate. The final concentrations of TFE and buffer were identical to those of the BH10–9 seed suspension, and the total peptide concentration of BH9–10 and BH10–9 was identical to the peptide concentration of the seed suspension.
Electronic supplemental material
NMR resonance assignments of BH10–9 in 50 mM sodium phosphate buffer (pH 6.5) at 5°C and those in 50 mM sodium phosphate buffer (pH6.5) containing 20% TFE (v/v) at 5°C are available as electronic supplemental material.
Acknowledgments
We thank Dr. T. Kohno for providing the plasmids for ubiquitin and ubiquitin hydrase; Dr. B.M. Goldstein and Y. Lin for collecting X-ray fiber diffraction data; K.L. Jensen for collecting electron micrography; Dr. G. Bedi for collecting mass spectra; Dr. S.D. Kennedy for assistance in NMR experiments; and F. Gruswitz for critically reading the manuscript. This work was supported by NIH Grants GM 57215 to S.K and RR14682 to the mass spectrometer facility.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
CD, circular dichroism
HSQC, heteronuclear single quantum coherence
NMR, nuclear magnetic resonance
NOE, nuclear Overhauser effect
NOESY, NOE spectroscopy
OspA, outer surface protein A
PCR, polymerase chain reaction
TFE, trifluoroethanol
TOCSY, total correlation spectroscopy
Article and publication are at www.proteinscience.org/cgi/doi/10.1101/ps.15901.
Supplemental material: See www.proteinscience.org
References
- Billeter, M., Neri, D., Otting, G., Qian, Y.Q., and Wüthrich, K. 1992. Precise vicinal coupling constants 3JHNHα in proteins from nonlinear fits of J-modulated [15N, 1H]-COSY experiments. J. Biomol. NMR 2 257–274. [DOI] [PubMed] [Google Scholar]
- Braunschweiler, L. and Ernst, R.R. 1983. Coherence transfer by isotropic mixing: Application to proton correlation spectroscopy. J. Magn. Reson. 53 521–528. [Google Scholar]
- Broome, B.M. and Hecht, M.H. 2000. Nature disfavors sequences of alternating polar and non-polar amino acids: Implications for amyloidogenesis. J. Mol. Biol. 296 961–968. [DOI] [PubMed] [Google Scholar]
- Brünger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S. et al. 1998. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D54 905–921. [DOI] [PubMed] [Google Scholar]
- Cavanagh, J. and Rance, M. 1992. Suppresion of cross-relaxation effects in TOCSY spectra via a modified DIPSI-2 mixing sequence. J. Magn. Reson. 96 670–678. [Google Scholar]
- Chiti, F., Webster, P., Taddei, N., Clark, A., Stefani, M., Ramponi, G., and Dobson, C.M. 1999. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl. Acad. Sci. 96 3590–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C.M. 1999. Protein misfolding, evolution and disease. Trends. Biochem. Sci. 24 329–32. [DOI] [PubMed] [Google Scholar]
- Guijarro, J.I., Sunde, M., Jones, J.A., Campbell, I.D., and Dobson, C.M. 1998. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. 95 4224–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J.D., Wong, S.S., Lieber, C.M., and Lansbury Jr., P.T. 1999. Assembly of Aβ amyloid protofibrils: An in vitro model for a possible early event in Alzheimer's disease. Biochemistry 38 8972–8980. [DOI] [PubMed] [Google Scholar]
- Jarvis, J.A., Craik, D.J., and Wilce, M.C. 1993. X-ray diffraction studies of fibrils formed from peptide fragments of transthyretin. Biochem. Biophys. Res. Commun. 192 991–998. [DOI] [PubMed] [Google Scholar]
- Kay, L.E., Keifer, P., and Saarinen, T. 1992. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 114 10663–10665. [Google Scholar]
- Kelly, J.W. 1996. Alternative conformations of amyloidogenic proteins govern their behavior. Curr. Opin. Struct. Biol. 6 11–17. [DOI] [PubMed] [Google Scholar]
- Kelly, J.W. 1997. Amyloid fibril formation and protein misassembly: A structural quest for insights into amyloid and prion diseases. Structure 5 595–600. [DOI] [PubMed] [Google Scholar]
- Klimov, D.K. and Thirumalai, D. 2000. Mechanisms and kinetics of β-hairpin formation. Proc. Natl. Acad. Sci. 97 2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk, W.E., Jacob, R.F., and Mason, R.P. 1999. Quaying amyloid beta-peptide (Abeta) aggregation using the Congo red-Abeta (CR-abeta) spectrophotometric assay. Anal. Biochem. 266 66–76. [DOI] [PubMed] [Google Scholar]
- Kohno, T., Kusunoki, H., Sato, K., and Wakamatsu, K. 1998. A new general method for the biosynthesis of stable isotope-enriched peptides using a decahistidine-tagged ubiquitin fusion system: An application to the production of mastoparan-X uniformly enriched with 15N and 15N/13C. J. Biomol. NMR 12 109–121. [DOI] [PubMed] [Google Scholar]
- Koide, S., Huang, X., Link, K., Koide, A., Bu, Z., and Engelman, D.M. 2000. Design of single-layer β-sheets without a hydrophobic core. Nature 403 456–460. [DOI] [PubMed] [Google Scholar]
- Krebs, M.R., Wilkins, D.K., Chung, E.W., Pitkeathly, M.C., Chamberlain, A.K., Zurdo, J., Robinson, C.V., and Dobson, C.M. 2000. Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the β-domain. J. Mol. Biol. 300 541–549. [DOI] [PubMed] [Google Scholar]
- Kumar, A., Wagner, G., Ernst, R., and Wüthrich, K.. 1981. Buildup rates of the nuclear Overhauser effect measured by two-dimensional proton magnetic resonance spectroscopy: Implications for studies of protein conformation. J. Am. Chem. Soc. 103 3654–3658. [Google Scholar]
- Li, H., Dunn, J.J., Luft, B.J., and Lawson, C.L. 1997. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc. Natl. Acad. Sci. 94 3584–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. and Bolen, D.W. 1995. The peptide backbone plays a dominant role in protein stabilization by naturally occurring osmolytes. Biochemistry 34 12884–12891. [DOI] [PubMed] [Google Scholar]
- Luo, P. and Baldwin, R.L. 1997. Mechanism of helix induction by trifluoroethanol: A framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 36 8413–8421. [DOI] [PubMed] [Google Scholar]
- Lynn, D.G. and Meredith, S.C. 2000. Review: Model peptides and the physicochemical approach to β-amyloids. J. Struct. Biol. 130 153–173. [DOI] [PubMed] [Google Scholar]
- Mihara, H. and Takahashi, Y. 1997. Engineering peptides and proteins that undergo alpha-to-beta transitions. Curr. Opin. Struct. Biol. 7 501–508. [DOI] [PubMed] [Google Scholar]
- Minor, D.L. and Kim, P.S. 1994. Measurement of the β-sheet-forming propensities of amino acids. Nature 367 660–663. [DOI] [PubMed] [Google Scholar]
- Morozova-Roche, L.A., Zurdo, J., Spencer, A., Noppe, W., Receveur, V., Archer, D.B., Joniau, M., and Dobson, C.M. 1999. Amyloid fibril formation and seeding by wild-type human lysozyme and its disease-related mutational variants. J. Struct. Biol. 130 339–351. [DOI] [PubMed] [Google Scholar]
- Munoz, V., Thompson, P.A., Hofrichter, J., and Eaton, W.A. 1997. Folding dynamics and mechanism of β-hairpin formation. Nature 390 196–199. [DOI] [PubMed] [Google Scholar]
- Nelson, J.W. and Kallenbach, N.R. 1986. Stabilization of the ribonuclease S-peptide α-helix by trifluoroethanol. Proteins Struct. Funct. Genet. 1 211–217. [DOI] [PubMed] [Google Scholar]
- Ohnishi, S., Koide, A., and Koide, S. 2000. Solution conformation and amyloid-like fibril formation of a polar peptide derived from a β-hairpin in the OspA single-layer β-sheet. J. Mol. Biol. 301 477–489. [DOI] [PubMed] [Google Scholar]
- Pham, T.N., Koide, A., and Koide, S. 1998. A stable single-layer β-sheet without a hydrophobic core. Nat. Struct. Biol. 5 115–119. [DOI] [PubMed] [Google Scholar]
- Rohl, C.A. and Baldwin, R.L. 1998. Deciphering rules of helix stability in peptides. Methods Enzymol. 295 1–26. [DOI] [PubMed] [Google Scholar]
- Serpell, L.C. 2000. Alzheimer's amyloid fibrils: Structure and assembly. Biochim. Biophys. Acta 1502 16–30. [DOI] [PubMed] [Google Scholar]
- Shiraki, K., Nishikawa, K., and Goto, Y. 1995. Trifluoroethanol-induced stabilization of the alpha-helical structure of beta-lactoglobulin: Implication for non-hierarchical protein folding. J. Mol. Biol. 245 180–194. [DOI] [PubMed] [Google Scholar]
- Smith, C.K. and Regan, L. 1995. Guidelines for protein design: the energetics of β-sheet side chain interactions. Science 270 980–982. [DOI] [PubMed] [Google Scholar]
- Sunde, M., Serpell, L.C., Bartlam, M., Fraser, P.E., Pepys, M.B., and Blake, C.C. 1997. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 273 729–739. [DOI] [PubMed] [Google Scholar]
- Thomas, P.D. and Dill, K.A. 1993. Local and nonlocal interactions in globular proteins and mechanisms of alcohol denaturation. Protein Sci. 2 2050–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart, D.S., Sykes, B.D., and Richards, F.M. 1992. The chemical shift index: A fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry 31 1647–1651. [DOI] [PubMed] [Google Scholar]
- Wüthrich, K. 1986. NMR of proteins and nucleic acids. John Wiley & Sons, New York.
- Zhang, O., Kay, L.E., Olivier, J.P., and Forman-Kay, J.D. 1994. Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J. Biomol. NMR 4 845–858. [DOI] [PubMed] [Google Scholar]




