In this study, the MtxX protein from M. jannaschii has been cloned, expressed, purified and crystallized.
Keywords: MtxX, Methanococcus jannaschii
Abstract
Methanococcus jannaschii has an mtr gene cluster expressing N 5-methyltetrahydromethanopterin:coenzyme M methyltransferase, which generates methane by reducing CO2 with H2 with concomitant energy production under strictly anaerobic conditions. Some methanogenic archaea also have an mtr gene-cluster homologue, the mtxXAH gene cluster. M. jannaschii has both an entire mtr gene cluster and a single mtxX gene instead of the whole mtxXAH gene cluster. A PSI-BLAST search, secondary-structure prediction and the absence of phosphotransacetylase activity in M. jannaschii strongly support the possibility that the MtxX protein constitutes a unique methyltransferase family. In this study, the MtxX protein from M. jannaschii has been cloned, expressed, purified and crystallized. Synchrotron data were collected to 2.9 Å from a crystal of selenomethionine-substituted MtxX protein. The crystal belonged to the primitive hexagonal space group P6122, with unit-cell parameters a = 54.9, b = 54.9, c = 341.1 Å, β = 120.0°. A full structure determination is under way in order to provide insight into the structure–function relationship of this protein.
1. Introduction
Methanococcus jannaschii is strictly anaerobic and produces energy by reducing CO2 with H2 to generate methane (Jones et al., 1983 ▶). N 5-Methyltetrahydromethanopterin:coenzyme M methyltransferase is a membrane-associated corrinoid-containing enzyme complex which is involved in methanogenesis (Harms et al., 1995 ▶). It catalyzes the exergonic formation of methyl-coenzyme M and tetrahydroinethanopterin from N 5-methyltetrahydromethanopterin and coenzyme M with concomitant electrogenic translocation of sodium ions across the cytoplasmic membrane. This methyltransferase complex is composed of the eight different polypeptides clustered in the mtrEDCBAFGH genes first reported in the study of Methanobacterium thermoautotrophicum strain Marburg (Harms et al., 1995 ▶). In addition to the mtr gene cluster, the mtxXAH gene cluster, an mtr homologue, is also found in Methanosarcina barkeri (Harms & Thauer, 1997 ▶). The mtxA and mtxH genes encode proteins with high sequence similarity to mtrA and mtrH, respectively. In the MtrA–H complex, the MtrH subunit catalyzes the transfer of the methyl group of N 5-methyltetrahydromethanopterin to the prosthetic group of the corrinoid protein MtrA (Hippler & Thauer, 1999 ▶). The mtxX gene encodes a protein with unknown function.
M. jannaschii has the entire mtr gene cluster, although it does not have the mtxXAH gene cluster. However, it has a single gene encoding MJ1371 of 27.4 kDa (MjMtxX), which is known to be a homologue of MtxX from Methanosarcina barkeri (Harms & Thauer, 1997 ▶; Fig. 1 ▶). Since the molecular structure and function of the MtxX protein family have not been reported, the determination of its three-dimensional structure has been initiated. In the database of clusters of orthologous groups of proteins (COGs), MjMtxX belongs to COG4002, representing a unique phosphotransacetylase family (Tatusov et al., 2003 ▶). The major role of phosphotransacetylase is catalyzing the reversible transfer of the acetyl group between coenzyme A and orthophosphate in acetate metabolism. However, M. jannaschii has been reported to be devoid of detectable activity of the acetate-incorporating enzymes acetyl coenzyme A synthetase, acetate kinase and phosphotransacetylase (Sprott et al., 1993 ▶). Therefore, the annotation of MjMtxX as a phosphotransacetylase based on sequence homology in the COG database should be reconsidered. A PSI-BLAST search (http://www.ncbi.nlm.nih.gov) of this sequence revealed 54 proteins with full-length sequence similarity and an E value below 9 × 10−4 (iteration 2 with BLOSUM62 matrix). Most of the homologous sequences are annotated as methyltransferases, phosphotransacetylases or phosphate butyryltransferases. Secondary-structure prediction shows the approximate repeat of β-strands and α-helices (Fig. 1 ▶) usually found in methyltransferases (Banerjee & Ragsdale, 2003 ▶). Considering the prediction results, sequence comparison and the homology of the mtx genes to the mtr genes, MtxX may constitute a unique methyltransferase family. Here, the cloning, overexpression, purification, crystallization and preliminary X-ray study of MjMtxX are reported.
Figure 1.
Sequence comparison of MtxX proteins. Abbreviations are as follows: MtMtxX, MtxX from Methanosaeta thermophila; MbMtxX, MtxX from Methanosarcina barkeri; MmMtxX, MtxX from Methanosarcina mazei. The secondary-structure prediction for MjMtxX was obtained using PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred). Black circles represent α-helices and grey diamonds represent β-strands. Gaps are represented by ‘-’, identical residues by ‘*’, highly conserved residues by ’:’ and and less highly conserved residues by ‘.’. The percentage sequence identities (ID) for MjMtxX are also shown.
2. Materials and methods
2.1. Cloning of MjMtxX in Escherichia coli
The sequence encoding MjMtxX was amplified by polymerase chain reaction (PCR) from M. jannaschii genomic DNA using Deep Vent DNA Polymerase (New England Biolabs, Beverly, Massachusetts, USA). The resulting PCR product was purified and prepared for ligation-independent cloning (LIC; Aslanidis & de Jong, 1990 ▶) by treatment with T4 DNA polymerase in the presence of 1 mM dTTP for 30 min at 310 K. The prepared DNA was then mixed with the pB4 vector, which is a construct consisting of an N-terminal His-tagged maltose-binding protein (MBP) fusion designed to be cleavable via a tobacco etch virus (TEV) protease site. After 5 min incubation at room temperature, the mixture was transformed into DH5α. Clones were screened by plasmid DNA analysis and transformed into BL21(DE3)/pSJS1244 for protein expression (Kim et al., 1998 ▶).
2.2. Protein expression, purification and crystallization
A selenomethionine derivative of the protein was expressed in a methionine auxotroph, E. coli strain B834(DE3)/pSJS1244, grown in PASM medium (Dr William Studier, Brookhaven National Laboratory, personal communication) supplied with selenomethionine. Cells were disrupted by microfluidization (Microfluidics, Newton, Massachusetts, USA) in 50 mM HEPES pH 7, 300 mM NaCl, 1.0 mM PMSF, 10 µg ml−1 DNAse, 0.1 µg ml−1 antipain, 1 µg ml−1 chymostatin, 0.5 µg ml−1 leupeptin and 0.7 µg ml−1 pepstatin A and cell debris was pelleted by centrifugation at 10 000 rev min−1 for 20 min in a Sorvall centrifuge. The supernatant was then spun in a Beckman Ti45 rotor at 35 000 rev min−1 for 30 min at 277 K.
The His6-MBP fusion protein was affinity-purified using two 5 ml HiTrap Chelating HP columns (GE Healthcare, Piscataway, New Jersey, USA). The fusion protein was bound to the column in 5% glycerol, 5 mM imidazole, 50 mM HEPES pH 7.0, 100 mM NaCl and was eluted with a gradient of 10–300 mM imidazole in 15 column volumes. Fractions were pooled and dialyzed overnight at room temperature against 20 mM Tris–HCl pH 8.0, 10 mM NaCl, 1 mM DTT and 1 mM EDTA in the presence of TEV protease. After centrifugation, the supernatant was applied onto a 5 ml HiTrap Chelating HP column again. The cleaved target protein was found in the flowthrough. Since the pI of MjMtxX is 5.3, further purification was performed using an anion-exchange column. The supernatant was applied onto a 5 ml HiTrap-Q column (GE Healthcare, Piscataway, New Jersey, USA). The protein was eluted in 20 mM Tris–HCl pH 8.0, 180 mM NaCl. The purity and identity of the target protein were confirmed by SDS–PAGE and MALDI–TOF mass spectrometry. The SDS–PAGE showed one band around 28 kDa, corresponding to the molecular weight of MjMtxX (Fig. 2 ▶). The protein was concentrated to 10 mg ml−1 for crystallization. Dynamic light scattering (DynaPro 99; Wyatt Technology Corporation, Santa Barbara, California, USA) showed a single monodisperse peak, indicating homogeneity of the protein. The purified protein contains six glycine residues at the N-terminus after TEV protease cleavage. Screening for crystallization conditions was performed using the sparse-matrix method (Jancarik & Kim, 1991 ▶) with several screens from Hampton Research (Laguna Niguel, California, USA) and Wizard Screen (deCODE Genetics, Bainbridge Island, Washington, USA). A Hydra Plus One crystallization robot (Matrix Technologies, Hudson, New Hampshire, USA) was used to set up the screens using the sitting-drop vapour-diffusion method at room temperature. The Additive Screen from Hampton Research was also used during refinement of the crystallization conditions. A 9:1 volume ratio of crystallization solution and Additive Screen solution was used in the refinement experiments.
Figure 2.
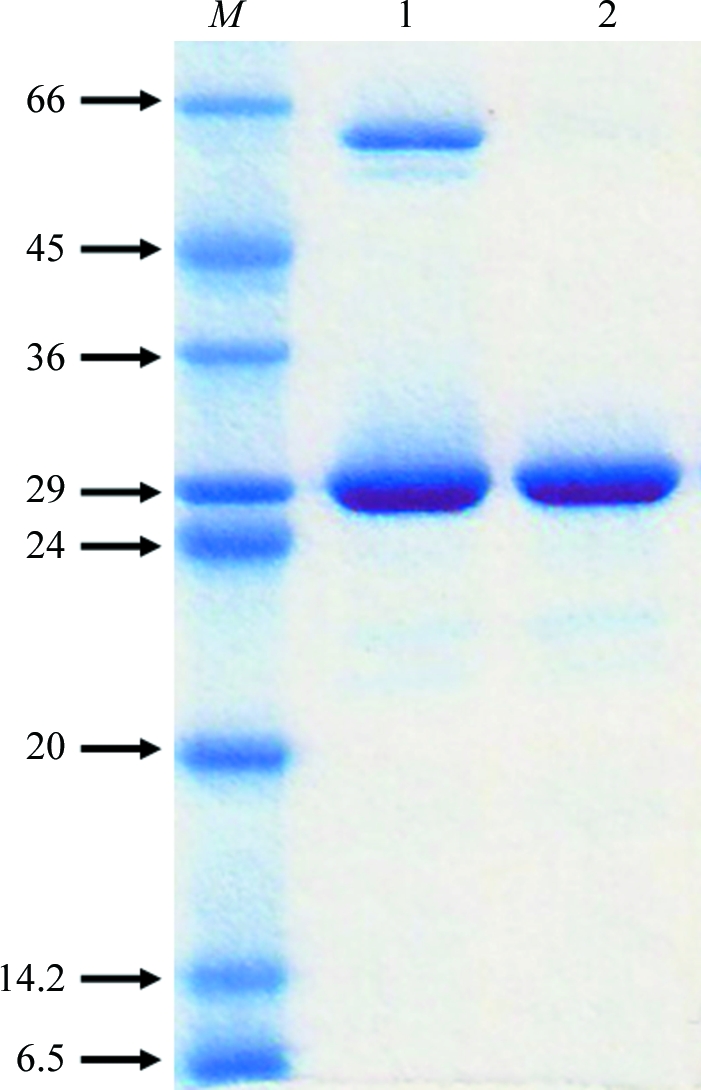
Coomassie-stained SDS–PAGE of MjMtxX. Lane 1, molecular-weight markers (kDa); lane 2, flowthrough of the second HiTrap Chelating HP column after TEV protease cleavage; lane 3, the purified protein after the HiTrap-Q column.
2.3. Data collection and reduction
Sodium formate was used as a cryoprotectant solution. Crystals were soaked in mother liquor containing 1.4 M sodium formate for 10 min. 1 µl of the equilibrated reservoir solution was added to the hanging drop before the crystal was flash-frozen in liquid nitrogen and exposed to X-rays. X-ray diffraction data sets were collected at a single wavelength on Macromolecular Crystallography Facility beamline 8.2.2 at the Advanced Light Source, Lawrence Berkeley National Laboratory using an Area Detector System Co. (Poway, California, USA) Quantum 4 CCD detector placed 400 mm from the sample. The oscillation range per image was 0.5°, with no overlap between contiguous images. X-ray diffraction data were processed and scaled using DENZO and SCALEPACK from the HKL program suite (Otwinowski & Minor, 1997 ▶). Synchrotron data were collected to 2.9 Å resolution. Data statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| X-ray source | Advanced Light Source beamline 8.2.2 |
| X-ray wavelength (Å) | 0.9793 |
| Temperature (K) | 100 |
| Space group | P6122 |
| Unit-cell parameters (Å, °) | a = b = 54.9, c = 341.1, α = β = 90.0, γ = 120 |
| Resolution range (Å) | 99.0–2.90 (2.95–2.90) |
| Total/unique reflections | 12649 (596) |
| Rmerge† (%) | 7.0 (47.6) |
| Data completeness (%) | 98.6 (99.3) |
| Average I/σ(I) | 19.8 (3.5) |
R
merge = 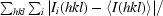
 .
.
3. Results and discussion
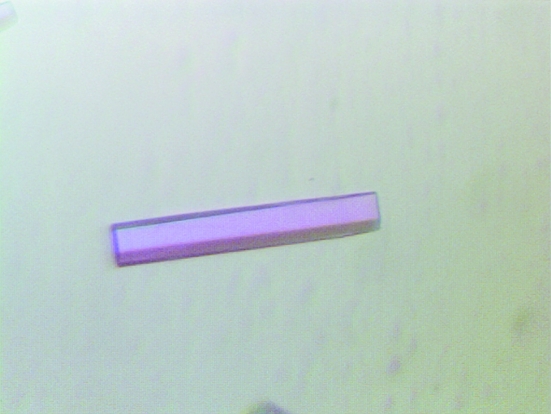
The yield of purified protein was ∼20 mg MjMtxX per litre of E. coli culture. After anion-exchange chromatography, MjMtxX appeared to be approximately 99% pure, with a prominent protein band at around 28 kDa on SDS–PAGE (Fig. 2 ▶). The first crystallization trial produced low-quality crystals from conditions using salt precipitants such as sodium malonate, trisodium citrate, diammonium hydrogen phosphate and ammonium sulfate. However, promising crystals grew using a well solution containing 0.1 M bis-tris propane pH 7.0 and 1.2 M trisodium citrate dehydrate. To obtain diffraction-quality crystals, further refinements were performed using Additive Screen. When 5% polyethylene glycol 400 was included in the above crystallization condition, hexagonal rod-shaped crystals appeared in 1 d with approximate dimensions of 0.1 × 0.02 × 0.02 mm. These crystals diffracted to 3.0 Å resolution. However, the crystal decayed rapidly on X-ray exposure during data collection. Therefore, further conditions were surveyed and the best crystals were obtained with well solution containing 0.1 M bis-tris propane pH 7.0, 1.2 M trisodium citrate dehydrate and 10 mM trimethylamine–HCl. Thick hexagonal rod-shaped crystals appeared in a week, with approximate dimensions of 0.5 × 0.1 × 0.1 mm (Fig. 3 ▶).
Figure 3.
Thick hexagonal rod-shaped crystal. These crystals appeared using trisodium citrate as a precipitant in the presence of 10 mM trimethylamine–HCl. The crystals grew in a week to approximate dimensions of 0.5 × 0.1 × 0.1 mm.
Synchrotron data were collected to 2.9 Å resolution. The X-ray diffraction data were processed and scaled using HKL-2000 (Otwinowski & Minor, 1997 ▶). The crystal belongs to the primitive hexagonal space group P6122, with unit-cell parameters a = 54.9, b = 54.9, c = 341.1 Å, β = 120.0°. A Matthews coefficient V M of 2.71 Å3 Da−1 and a solvent content of 52.9% (Matthews, 1968 ▶) were consistent with the presence of one MjMtxX molecule per asymmetric unit. Details of the data-collection statistics are presented in Table 1 ▶. Two Se-atom positions were located using the new substructure-searching procedure HySS included in the PHENIX software (Adams et al., 2004 ▶). Several heavy-atom data sets have also been collected in order to determine the complete structure of MjMtxX using combined phases from single-wavelength anomalous dispersion and multiple isomorphous replacement methods.
Acknowledgments
I am grateful to Dr Sung-Hou Kim and Dr Rosalind Kim for their kind advice during this project. The work described here was supported by grant No. R15-2006-020 from the NCRC program of MOST and KOSEF through the CCS and DDR at Ewha Womans University and by a Protein Structure Initiative grant from the National Institutes of Health GM 62412.
References
- Adams, P. D., Gopal, K., Grosse-Kunstleve, R. W., Hung, L.-W., Ioerger, T. R., McCoy, A. J., Moriarty, N. W., Pai, R. K., Read, R. J., Romo, T. D., Sacchettini, J. C., Sauter, N. K., Storoni, L. C. & Terwilliger, T. C. (2004). J. Synchrotron Rad.11, 53–55. [DOI] [PubMed]
- Aslanidis, C. & de Jong, P. J. (1990). Nucleic Acids Res.20, 6069–6074. [DOI] [PMC free article] [PubMed]
- Banerjee, R. & Ragsdale, S. W. (2003). Annu. Rev. Biochem.72, 209–247. [DOI] [PubMed]
- Harms, U. & Thauer, R. K. (1997). Eur. J. Biochem.250, 783–788. [DOI] [PubMed]
- Harms, U., Weiss, D. S., Gartner, P., Linder, D. & Thauer, R. K. (1995). Eur. J. Biochem.228, 640–648. [DOI] [PubMed]
- Hippler, B. & Thauer, R. K. (1999). FEBS Lett.23, 165–168. [DOI] [PubMed]
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411.
- Jones, W. J., Leigh, J. A., Mayer, F., Woese, C. R. & Wolfe, R. S. (1983). Arch. Microbiol.136, 254–261.
- Kim, R., Sandler, S. J, Goldman, S., Yokota, H., Clark, A. J. & Kim, S.-H. (1998). Biotechnol. Lett.20, 207–210.
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Sprott, G. D., Ekiel, I. & Patel, G. B. (1993). Appl. Environ. Microbiol.59, 1092–1098. [DOI] [PMC free article] [PubMed]
- Tatusov, R. L. et al. (2003). BMC Bioinformatics, 4, 41–54. [DOI] [PMC free article] [PubMed]