The multidrug efflux transporter NorM from N. gonorrhoeae has been crystallized and X-ray diffraction data have been collected to a resolution of 6.5 Å.
Keywords: NorM, multidrug efflux transporter, drug resistance, Neisseria gonorrhoeae
Abstract
The crystallization and preliminary X-ray data analysis of the NorM multidrug efflux pump produced by Neisseria gonorrhoeae are reported. NorM is a cytoplasmic membrane protein that consists of 459 amino-acid residues. It is a member of the recently classified multidrug and toxic compound extrusion (MATE) family of transporters and recognizes a number of cationic toxic compounds such as ethidium bromide, acriflavin, 2-N-methylellipticinium and ciprofloxacin. Recombinant NorM protein was expressed in Escherichia coli and purified by metal-affinity and gel-filtration chromatography. The protein was crystallized using hanging-drop vapor diffusion. X-ray diffraction data were collected from cryocooled crystals at a synchrotron light source. The best crystal diffracted anisotropically to 3.8 Å and diffraction data were complete to 6.5 Å resolution. The space group was determined to be C2, with unit-cell parameters a = 81.5, b = 164.4, c = 111.5 Å.
1. Introduction
Gonorrhea is one of the most common sexually transmitted diseases in the world. It is estimated that 800 000 cases of gonorrhea occur annually in the USA at a cost of $1.1 billion (http://www.thebody.com/content/art6532.html). The disease is caused by the Gram-negative bacterium Neisseria gonorrhoeae, which is increasingly resistant to most inexpensive antibiotics, including penicillin, tetracycline, erythromycin and ciprofloxacin. In April 2007, the Centers for Disease Control and Prevention (CDC) officially added gonorrhea to the list of antibiotic-resistant ‘superbugs’ (http://www.cdc.gov/std/Gonorrhea/STDFact-gonorrhea.htm). Since N. gonorrhoeae is a strict human pathogen and can colonize male and female genital mucosal surfaces and other sites, it has developed mechanisms to overcome the host antimicrobial systems that are essential to innate host defense (Shafer et al., 2001 ▶). One important mechanism that N. gonorrhoeae uses to subvert antimicrobial agents is the expression of multidrug efflux transporters. These transporters recognize and actively export a wide variety of structurally unrelated toxic compounds, including antibacterial peptides, long-chain fatty acids and several clinically useful antibiotics, from the bacterial cell (Lee & Shafer, 1999 ▶; Shafer et al., 1998 ▶, 2001 ▶; Rouquette-Loughlin et al., 2003 ▶).
Four efflux transporters have been identified in N. gonorrhoeae. One such transporter is the MtrD inner membrane protein (Hagman et al., 1997 ▶), which exists as a component of the tripartite resistance nodulation cell division (RND) efflux pump (Tseng et al., 1999 ▶). This pump mediates the export of hydrophobic antimicrobial agents including antibiotics, nonionic detergents, certain antibacterial peptides, bile salts and gonadal steroid hormones from the bacterium (Shafer et al., 1998 ▶; Delahay et al., 1997 ▶; Hagman et al., 1995 ▶, 1997 ▶). The FarB transporter (Lee & Shafer, 1999 ▶), which belongs to the major facilitator (MF) family (Griffith et al., 1992 ▶; Marger & Saier, 1993 ▶; Pao et al., 1998 ▶), recognizes antibacterial long-chain fatty acids and exports them out of the cell. MacB has recently been described (Rouquette-Loughlin et al., 2005 ▶) and belongs to the ATP-binding cassette (ABC) transporter family (Higgins, 1992 ▶). It is poorly expressed in wild-type gonococci owing to a natural mutation in its promoter, but can recognize and export certain macrolide antibiotics. Finally, N. gonorrhoeae contains the NorM multidrug transporter (Rouquette-Loughlin et al., 2003 ▶), which is a member of the most recently classified multidrug and toxic compound extrusion (MATE) family of efflux pumps (Rouquette-Loughlin et al., 2003 ▶; Brown et al., 1999 ▶; Morita et al., 1998 ▶). As a multidrug efflux pump, the gonococcal NorM appears to recognize a number of cationic toxic compounds such as ethidium bromide, acriflavin, 2-N-methylellipticinium and ciprofloxacin (Rouquette-Loughlin et al., 2003 ▶). We recently determined that NorM binds a variety of structurally dissimilar agents in the micromolar range and behaves as an Na+-dependent transporter (Long et al., 2008 ▶). The capacity of NorM to export the antibiotic ciprofloxacin is recognized as being clinically relevant in the development of fluoroquinolone resistance in N. gonorrhoeae.
The MATE transporters characteristically possess 12 putative transmembrane domains. To date, the overall properties of the MATE family of proteins have not been completely determined and no structural models are available for this type of multidrug resistance-conferring protein. As an initial step to elucidate the structural basis of multidrug recognition and extrusion by NorM, we here report the crystallization and preliminary X-ray diffraction analysis of the NorM transporter.
2. Cloning, expression and purification
2.1. Cloning
Recombinant full-length NorM containing a 6×His tag directly attached to the C-terminal end (C-6×His) was produced in Escherichia coli TOP10 cells using the pBAD vector (Invitrogen). The cloning and expression procedures have been described previously (Long et al., 2008 ▶). This recombinant C-6×His NorM is fully functional in vivo and the purified protein has been demonstrated to bind antimicrobials in a detergent environment with dissociation constants spanning the micromolar range (Long et al., 2008 ▶).
To produce recombinant full-length NorM containing a 6×His tag at the N-terminus, the ORF for norM from the genomic DNA of N. gonorrhoeae strain FA19 was amplified by PCR using the primers 5′-AAACATATGCTGCTCGACCTCGACCGC-3′ and 5′-AAAGGATCCTCAGACGGCCTTGTGTGATTTGC-3′. The 1380 bp PCR fragment of the norM gene with flanking sequences was extracted from the agarose gel using a gel-extraction kit (Qiagen) and then digested with NdeI and BamHI (New England Biolabs). The digested products were ligated into the pET15b expression vector (Novagen) to generate a recombinant protein that contains a 6×His tag, a thrombin-cleavage site and a three-residue (GSH) N-terminal spacer attached to the N-terminal end of NorM (N-6×His). The sequence of this N-6×His NorM protein is MGSSHHHHHHSSGLVPRGSH-NorM. This recombinant plasmid (pET15bΩnorM) was transformed into DH5α cells and selected on LB plates containing 100 µg ml−1 ampicillin. The construction was verified by DNA sequencing.
2.2. Protein expression
The C-6×His NorM protein was overproduced in E. coli TOP10/pBADΩnorM cells as described elsewhere (Long et al., 2008 ▶). As these cells cannot be grown in M9 minimal media, recombinant selenomethionyl-NorM (SeMet-NorM) protein was overproduced using the N-6×His construct in E. coli B834/pET15ΩnorM cells. Briefly, a 10 ml overnight culture in Luria–Bertani (LB) broth was transferred into 120 ml LB broth containing 100 µg ml−1 ampicillin. The culture was grown with shaking (210 rev min−1) at 310 K. When the OD600 value reached 1.2, cells were harvested by centrifugation at 6000 rev min−1 for 10 min and then washed two times with 20 ml M9 minimal salts solution. The cells were resuspended in 120 ml M9 media and then transferred into 12 l pre-warmed M9 solution containing 100 µg ml−1 ampicillin. The cell culture was incubated at 310 K with shaking. When the OD600 reached 0.4, 60 mg l−1 l-selenomethionine was added. The culture was then induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) after 15 min. Cells were harvested within 3 h and were frozen and stored at 193 K.
2.3. Protein purification
The C-6×His NorM protein was purified using an Ni2+-affinity column as described in Long et al. (2008 ▶), followed by a G-200 sizing column to enhance purity. This step was also essential to exchange n-dodecyl-β-d-maltoside (β-DDM) with different detergents for crystallization attempts. The procedure for detergent exchange using gel-filtration chromatography was as follows: the purified C-6×His NorM protein in buffer containing 20 mM Na HEPES pH 7.5 and 0.1% β-DDM was concentrated to a volume of 800 µl (∼10 mg ml−1) using a YM-100 concentrator (Millipore, 100 kDa molecular-weight cutoff). The concentrated protein was then loaded onto a Superdex 200 (G-200) 16/60 column (Amersham Pharmacia Biotech) pre-equilibrated with buffer containing 20 mM Na HEPES pH 7.5 and the relevant detergent at the described concentration. The volume and length of the G-200 sizing column were 120 ml and 60 cm, respectively. A flow rate of 0.5 ml min−1 was used and 2 ml fractions were collected and analyzed by 10% SDS–PAGE.
The purification procedures for the N-6×His SeMet-NorM protein were similar to those used for C-6×His NorM. In brief, the N-6×His SeMet protein was purified using an Ni2+-affinity column as described in Long et al. (2008 ▶). The purified protein was extensively dialyzed against buffer containing 20 mM Na HEPES pH 7.5 and 0.1% β-DDM, concentrated to 2 mg ml−1 and then incubated for 24 h at 298 K in the presence of one unit of thrombin per 2 mg protein to cleave the hexahistidine tag. After thrombin cleavage, the newly formed SeMet-NorM protein, which contained a three-residue spacer, GSH, directly attached to the N-terminus (GSH-SeMet-NorM), was loaded onto a G-200 sizing column pre-equilibrated with buffer containing 20 mM Na HEPES pH 7.5 and 0.1% β-DDM for further purification. The purity of the purified GSH-SeMet-NorM protein was judged using 10% SDS–PAGE stained with Coomassie Brilliant Blue. NorM is a 459-amino-acid protein that contains 19 methionines. Replacement of these methionine sulfurs with seleniums in the GSH-SeMet-NorM protein was confirmed by MALDI time-of-flight mass spectrometry.
Both the C-6×His NorM and GSH-SeMet-NorM proteins were concentrated to 20 mg ml−1 in solution containing 20 mM Na HEPES pH 7.5 and 0.1% β-DDM. Typical starting and ending volumes were 10 ml and 300 µl, respectively. To avoid concentrating β-DDM in the protein solution, a YM-100 Centriprep concentrator (Millipore, 100 kDa molecular-weight cutoff) was used for protein concentration. The 100 kDa molecular-weight cutoff concentrators have been shown to be efficient enough to avoid concentrating the β-DDM micelles (Urbani & Warne, 2005 ▶).
3. Crystallization
3.1. Detergents
The full-length C-6×His NorM protein containing six histidines at the C-terminus was crystallized in 24-well plates using the hanging-drop vapor-diffusion method. Initial crystallization trials using commercially available screening kits, including Hampton Crystal Screens I, II and Lite, PEG/Ion and MembFac, failed. Accordingly, we performed crystallization screening by mixing different polyethylene glycols [PEGs; ranging from PEG 200 to PEG 20 000 and PEG 550 MME (monomethyl ether) to PEG 5500 MME] with different buffers (between pH 3.5 and pH 9.5), salts, additives and detergents. The experiments were performed at 277 or 298 K.
Initially, five different detergents, β-DDM, n-dodecyl-α-d-maltoside (α-DDM), polyoxyethylene(8)dodecyl ether (C12E8), n-undecyl-β-d-maltoside (UDM), n-tetradecyl-β-d-maltoside (TDM) and n-octyl-β-d-glucoside (OG), were used as primary detergents in our crystallization trials. The experiments suggested that protein solution containing 0.1% β-DDM or 0.8% OG resulted in the formation of protein crystals. Crystals grown in the presence of β-DDM diffracted to 8 Å; however, those grown in the presence of OG did not diffract X-rays. Thus, β-DDM was chosen as the primary detergent for further crystallization trials.
We attempted to improve the quality of the crystals by searching for a suitable secondary detergent using Detergent Screens 1, 2 and 3 (Hampton Research). After the initial screen, eight different secondary detergents, C12E8, UDM, polyoxyethylene(9)dodecyl ether (C12E9), 6-cyclohexyl-1-hexyl-β-d-maltoside (Cymal-6), n-decyl-β-d-maltoside (DM), n-dodecylphosphocholine (Fos-choline-12), n-nonyl-β-d-glucoside (NG) and 3-(3-butyl-3-phenylheptanamido)-N,N-dimethylpropan-1-amine oxide (TRIPAO), were chosen for further trials. However, it appeared that the addition of these secondary detergents did not improve the diffraction limit of the crystals.
3.2. Removal of hexahistidine tags
We then created a thrombin-cleavage site at position 454 of the protein in order to remove the hexahistidine tag at the C-terminus of C-6×His NorM. After removing the 6×His tag, this NorM protein (NorM454, amino acids 1–454) was purified in β-DDM and subjected to a broad screen with various PEGs, salts, buffers, additives and detergents as described above. Unfortunately, crystals of the NorM454 protein diffracted X-rays in a similar manner to those of C-6×His NorM and did not appear to enhance the resolution limit.
We also attempted to improve the crystal quality by crystallizing N-6×His NorM. After the removal of the 6×His tag using thrombin, this NorM protein, GSH-NorM, containing a three-residue spacer (GSH) at the N-terminal end, was screened with various PEGs, salts, buffers, additives and detergents as above. However, the best crystal obtained was no better than that obtained for C-6×His NorM. We thus focused our subsequent crystallization efforts on the C-6×His NorM protein.
3.3. Crystallization conditions
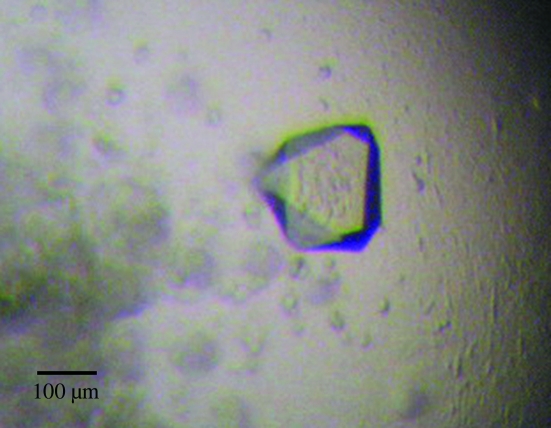
For hanging-drop vapor diffusion, a 2 µl drop consisting of 1 µl protein solution (20 mg ml−1 NorM in 20 mM Na HEPES pH 7.5 and 0.1% β-DDM) and 1 µl reservoir solution was equilibrated against 500 µl reservoir solution. The initial crystals of NorM were grown in 15–18% PEG 1000 and 20–100 mM Na HEPES pH 7.5. After optimization, the best C-6×His NorM crystals were obtained at 298 K from well solution containing 16% PEG 1000, 40 mM Na HEPES buffer pH 7.5 and 4% glycerol. Crystals appeared within one week and typically had dimensions of about 200 × 200 × 200 µm. Fig. 1 ▶ illustrates a typical native crystal of C-6×His NorM. Cryoprotection was achieved by raising the PEG 1000 concentration stepwise to 26% in 5% increments. Crystals of the GSH-SeMet-NorM protein (after removal of the 6×His tag at the N-terminus) were grown under the same conditions.
Figure 1.
N. gonorrhoeae C-6×His NorM crystal. The dimensions of the crystal are approximately 200 × 200 × 200 µm.
4. Data collection and processing
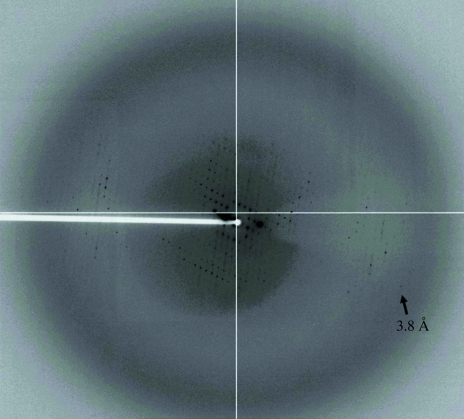
For data collection, a single native crystal of C-6×His NorM was flash-cooled in a cryoprotectant solution containing 26% PEG 1000, 40 mM Na HEPES buffer pH 7.5 and 4% glycerol at 100 K. The best crystal diffracted anisotropically to a resolution of 3.8 Å. Fig. 2 ▶ depicts one of the diffraction images of the native NorM crystal. Single-wavelength anomalous diffraction data were collected at the peak wavelength from a single GSH-SeMet-NorM crystal. The best native C-6×His NorM and GSH-SeMet-NorM diffraction data were obtained to resolutions of 6.5 and 7.2 Å, respectively (Table 1 ▶).
Figure 2.
X-ray diffraction pattern of the native C-6×His NorM crystal. The crystal diffracted anisotropically to a resolution beyond 3.8 Å.
Table 1. Data collection and crystallographic analysis of NorM.
Vaues in parentheses are for the highest resolution shell.
| Native C-6×His NorM | GSH-SeMet-NorM (peak) | |
|---|---|---|
| Wavelength (Å) | 0.9795 | 0.9795 |
| Space group | C2 | C2 |
| Unit-cell parameters (Å) | a = 81.5, b = 164.4, c = 111.5 | a = 82.5, b = 164.8, c = 113.7 |
| Resolution (Å) | 6.49 (8.18–6.49) | 7.15 (7.43–7.15) |
| Completeness (%) | 99.6 (90.8) | 99.4 (92.3) |
| Total No. of reflections | 305366 | 179056 |
| No. of unique reflections | 14522 | 11967 |
| Rmerge (%) | 7.8 (28.2) | 8.3 (34.7) |
| Average I/σ(I) | 14.1 (3.4) | 22.3 (4.9) |
Diffraction data sets were obtained from the native C-6×His NorM crystals at the Advanced Photon Source (APS, beamline 24IDC) at cryogenic temperature (100 K) using an ADSC Quantum 315 CCD-based detector. The beam size was 50 × 20 µm. Data for the GSH-SeMet-NorM crystals were obtained at the Advanced Light Source (ALS, beamline 8.2.2) at cryogenic temperature (100 K) using an ADSC Quantum 315 CCD-based detector. The beam size was 140 × 150 µm. Diffraction data sets were processed with DENZO and scaled with SCALEPACK (Otwinowski & Minor, 1997 ▶). The native C-6×His NorM crystal belonged to space group C2, with unit-cell parameters a = 81.5, b = 164.4, c = 111.5 Å. The GSH-SeMet-NorM crystal belonged to the same space group with very similar unit-cell parameters (Table 1 ▶). Based on the molecular weight of the C-6×His NorM protein (50.6 kDa, including the 6×His tag at the C-terminus) and the volume of the asymmetric unit, the Matthews parameters (Matthews, 1968 ▶, 1977 ▶) for one and two molecules of NorM in the asymmetric unit were found to be 7.0 and 3.5 Å3 Da−1, respectively. This suggests the presence of one or two NorM molecules per asymmetric unit, with solvent contents of 82.2% or 64.4%, respectively.
Acknowledgments
This work was supported by PHS grants AI-21150 (WMS) and GM-074027 (EWY) from the NIH. WMS is the recipient of a Senior Research Career Scientist Award from the VA Medical Research Service.
References
- Brown, M. H., Paulsen, I. T. & Skurray, R. A. (1999). Mol. Microbiol.31, 394–395. [DOI] [PubMed]
- Delahay, R. M., Robertson, B. D., Balthazar, J. T. & Ison, C. A. (1997). Microbiology, 143, 2127–2133. [DOI] [PubMed]
- Griffith, J. K., Baker, M. E., Rouch, D. A., Page, M. G., Skurray, R. A., Paulsen, I. T., Chater, K. F., Baldwin, S. A. & Henderson, P. J. (1992). Curr. Opin. Cell Biol.4, 684–695. [DOI] [PubMed]
- Hagman, K. E., Lucas, C. E., Balthazar, J. T., Snyder, L. A., Nilles, M., Judd, R. C. & Shafer, W. M. (1997). Microbiology, 143, 2117–2125. [DOI] [PubMed]
- Hagman, K. E., Pan, W., Spratt, B. G., Balthazar, J. T., Judd, R. C. & Shafer, W. M. (1995). Microbiology, 141, 611–622. [DOI] [PubMed]
- Higgins, C. F. (1992). Annu. Rev. Cell Biol.8, 67–113. [DOI] [PubMed]
- Lee, E.-H. & Shafer, W. M. (1999). Mol. Microbiol.33, 839–845. [DOI] [PubMed]
- Long, F., Rouquette-Loughlin, C., Shafer, W. M. & Yu, E. W. (2008). Submitted. [DOI] [PMC free article] [PubMed]
- Marger, M. D. & Saier, M. H. Jr (1993). Trends Biochem. Sci.18, 13–20. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Matthews, B. W. (1977). The Proteins, edited by H. Neurath & R. L. Hill, Vol. 3, pp. 468–477. New York: Academic Press.
- Morita, Y., Kodama, K., Shiota, S., Mine, T., Kataoka, A., Mizushima, T. & Tsuchiya, T. (1998). Antimicrob. Agents Chemother.42, 1778–1782. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, M. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Pao, S. S., Paulsen, I. T. & Saier, M. H. Jr (1998). Mol. Biol. Rev.62, 1–34. [DOI] [PMC free article] [PubMed]
- Rouquette-Loughlin, C. E., Balthazar, J. T. & Shafer, W. M. (2005). J. Antimicrob. Chemother.56, 856–860. [DOI] [PubMed]
- Rouquette-Loughlin, C., Dunham, S. A., Kuhn, M., Balthazar, J. T. & Shafer, W. M. (2003). J. Bacteriol.185, 1101–1106. [DOI] [PMC free article] [PubMed]
- Shafer, W. M., Qu, X.-D., Waring, A. J. & Lehrer, R. I. (1998). Proc. Natl Acad. Sci. USA, 95, 1829–1833. [DOI] [PMC free article] [PubMed]
- Shafer, W. M., Veal, W. L., Lee, E.-H., Zarentonelli, L., Balthazar, J. T. & Rouquette, C. (2001). J. Mol. Microbiol. Biotechnol.3, 219–225. [PubMed]
- Tseng, T. T., Gratwick, K. S., Kollman, J., Park, D., Nies, D. H., Goffeau, A. & Saier, M. H. Jr (1999). J. Mol. Microbiol. Biotechnol.1, 107–125. [PubMed]
- Urbani, A. & Warne, T. (2005). Anal. Biochem.336, 117–124. [DOI] [PubMed]