Abstract
Fast axonal transport is characterized by the bidirectional, microtubule-based movement of membranous organelles. Cytoplasmic dynein is necessary but not sufficient for retrograde transport directed from the synapse to the cell body. Dynactin is a heteromultimeric protein complex, enriched in neurons, that binds to both microtubules and cytoplasmic dynein. To determine whether dynactin is required for retrograde axonal transport, we examined the effects of anti-dynactin antibodies on organelle transport in extruded axoplasm. Treatment of axoplasm with antibodies to the p150Glued subunit of dynactin resulted in a significant decrease in the velocity of microtubule-based organelle transport, with many organelles bound along microtubules. We examined the molecular mechanism of the observed inhibition of motility, and we demonstrated that antibodies to p150Glued disrupted the binding of cytoplasmic dynein to dynactin and also inhibited the association of cytoplasmic dynein with organelles. In contrast, the anti-p150Glued antibodies had no effect on the binding of dynactin to microtubules nor on cytoplasmic dynein-driven microtubule gliding. These results indicate that the interaction between cytoplasmic dynein and the dynactin complex is required for the axonal transport of membrane-bound vesicles and support the hypothesis that dynactin may function as a link between the organelle, the microtubule, and cytoplasmic dynein during vesicle transport.
Neurons possess a striking asymmetric morphology in which elongated axonal processes extend from the cell body. The growth and maintenance of the axon, as well as the movement of materials between the cell body and the distal tip of the axon, rely on the mechanism of axonal transport. The fast bidirectional transport of organelles along the polarized microtubule array of the axon has been well characterized (1–8). Many microtubule-based motors have been identified in neurons, including members of the kinesin superfamily that are implicated in anterograde transport (4, 5) and cytoplasmic dynein, which is required for retrograde transport (6–8).
The molecular mechanisms by which these motors are specified for particular types of organelle cargo, as well as the regulation of the interactions between motor proteins, organelles, and microtubules, are largely unknown. A soluble accessory factor, dynactin, was originally isolated via its copurification with cytoplasmic dynein and is one candidate for the activation of cytoplasmic dynein-based organelle motility (reviewed in ref. 9). Dynactin consists of at least seven different polypeptides that cosediment at 20 S (10, 11). These include p150Glued; the actin-related protein centractin (Arp-1); the α and β subunits of capping protein (CapZ); dynamitin; as well as uncharacterized polypeptides with molecular masses of 62 and 24 kDa (10–17). The largest dynactin polypeptide, p150Glued, is capable of multiple, specific binding interactions. p150Glued binds to the 74-kDa intermediate chain of cytoplasmic dynein via a domain in the central region of the molecule (18, 19) and also binds directly to microtubules via an N-terminal microtubule-binding motif (20).
Genetic studies have shown that both cytoplasmic dynein and dynactin participate in mitotic spindle orientation and nuclear migration (reviewed in ref. 9). In addition, results from an in vitro vesicle motility assay implicated dynactin in the cytoplasmic dynein-based transport of membranous vesicles (13). In this report, we examined the role of dynactin in fast axonal transport in axoplasm extruded from the giant axon of the squid, a system in which the requirement for cytoplasmic dynein as a retrograde motor has previously been established (6, 7). We report that anti-dynactin antibodies that block the dynein–dynactin interaction inhibit organelle transport along microtubules.
MATERIALS AND METHODS
Antibody Production and Immunochemistry.
Polyclonal rabbit antibodies UP235 and UP236 were raised against purified recombinant polypeptides corresponding to residues 39–1325 and 150–899 of rat p150Glued, respectively (17). Polyclonal rabbit antibodies UP384 and UP385 were raised to purified recombinant polypeptides corresponding to residues 216–376 and 3–376 of human centractin, respectively (11). These antibodies were affinity purified as described (17). Fab fragments were prepared by papain digestion and purified by protein-A agarose affinity chromatography (21). In control experiments, anti-p150Glued antibodies UP235 and UP236 were blocked by incubation overnight with a 50-fold molar excess of immunogen polypeptide, followed by protein-A agarose affinity purification. Western blots were performed as previously described (22).
Isolation and Characterization of Dynactin from Squid.
Squid optic lobe cytosol was generated by homogenization of frozen optic lobes (generously provided by R. Sloboda, Dartmouth College, Hanover, NH) in one volume of either PHEM (50 mM Na Pipes/50 mM Hepes/1 mM EDTA/2 mM MgCl2, pH 7.0) or axoplasmic motility buffer (400 mM potassium aspartate/20 mM Hepes/5 mM MgCl2⋅6H2O/0.5 mM CaCl2/1.5 mM EGTA/2 mM ATP/30 mM glycine/40 mM betaine/74 mM taurine/0.5 mM DTT, pH 7.2) with protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml N-tosyl l-arginine methylester, and 1 μg/ml pepstatin A) followed by sequential centrifugation at 39,000 × g and 150,000 × g. The 0.4 M NaCl extract of bovine brain microtubules was prepared as described (12).
The sedimentation coefficient of squid dynactin was determined by sucrose density gradient sedimentation of optic lobe cytosol as previously described (22). Dynactin was immunoprecipitated from optic lobe cytosol prepared in RIPA buffer without SDS (50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/1% Nonidet P-40/0.5% deoxycholate) using either UP235 or UP236 as described (22).
Antibody Inhibition of Organelle Motility in Extruded Axoplasm.
Squid were obtained fresh daily from the Department of Marine Resources (Woods Hole, MA). Giant axons were dissected as previously described (1) and cut in half. One half of the axoplasm was extruded into motility buffer with 0.8 mg/ml of affinity-purified antibodies and the other half was extruded into a control solution. Samples were observed within 1 hr after extrusion by video-enhanced contrast differential interference contrast (VEC-DIC) microscopy (23) on the Zeiss Axiophot video microscope system as previously described (24).
Motile activity (number of organelles moving/field per sec) was analyzed from videotapes by counting the total number of moving organelles in four areas (5 × 5 μm) over 15-sec intervals. Motion analysis of organelle movement along individual microtubules was performed as described using the software packages parti-movi or spss (23, 25).
Affinity Chromatography and Microtubule Cosedimentation Assays.
p150Glued affinity columns were prepared as described (18) using a 200-μl bed volume. The columns were preadsorbed with either preimmune serum or anti-p150Glued (UP235) serum and washed with 20 column volumes of PBS. The columns were further washed with 5-column volumes of 1 M LiCl/150 mM NaCl/10 mM Tris⋅HCl/0.5% Nonidet P-40, pH 8.0. The columns were equilibrated in axoplasmic motility buffer and loaded with 600 μl squid optic lobe cytosol prepared in motility buffer, then washed with 20 vol of motility buffer, and finally eluted with motility buffer with 500 mM NaCl. The final washes and the eluates were TCA-precipitated and analyzed by SDS/PAGE, followed by Western blotting using antibodies to heavy and intermediate chains of cytoplasmic dynein [generously provided by Drs. David Asai (Purdue University, West Lafayette, IN) and Walter Steffen (Vienna University, Austria)].
To assay the effects of antibodies on the interaction of dynactin with microtubules, cytosol was incubated with 1 mg/ml affinity-purified antibody for 45 min at room temperature. Taxol (40 μM, National Cancer Institute, Bethesda, MD) was added, and the incubation was continued for 30 min at 37°C. Taxol-stabilized microtubules (0.25 mg/ml) were added, and incubation continued for a final 30 min at 37°C. Alternatively, antibodies were added after microtubule polymerization. Microtubules and associated proteins were then isolated by microcentrifugation and washed once, and the supernatant and pellet fractions were analyzed by immunoblot.
Organelle Isolation and Analysis.
Axoplasmic organelles for immunoblot analysis were isolated by flotation on a sucrose step gradient as previously described (26, 27). Organelles were prepared from squid optic lobes by modification of the procedure of Niclas et al. (28). Briefly, optic lobes were homogenized in 1 vol of motility buffer with protease inhibitors; the homogenate was centrifuged for 30 min at 4°C at 25,000 × g. The resulting supernatant was subjected to ultracentrifugation at 80,000 × g to obtain a pellet fraction that was enriched in organelles and a high-speed supernatant (cytosol). The pellet fraction was either resuspended in motility buffer alone or in motility buffer with 0.8 mg/ml of anti-p150Glued antibody UP235. The organelle fractions were incubated at room temperature for 45 min followed by 15 min at 37°C. The organelle and cytosol fractions were then brought to a final sucrose concentration of 2 M and layered underneath steps of 1.5 M and 0.6 M sucrose in motility buffer. The gradients were centrifuged at 200,000 × g for 2 hr at 4°C. Samples were collected from the 1.5 M/0.6 M interface, the 1.5 M step, and the 2 M step from each gradient and were analyzed by SDS/PAGE and Western blotting. Results were analyzed using a UMAX PowerLook II scanner and nih image software.
In Vitro Microtubule Gliding Assays.
20S cytoplasmic dynein/dynactin and 9S kinesin fractions were isolated from optic lobe (16). Cytoplasmic dynein/dynactin with either (≈200 μg/ml protein) and kinesin (≈100 μg/ml protein) were incubated 40 min at 20°C with either 1 mg/ml affinity-purified antibody or 1% BSA in PEM (80 mM Pipes/2 mM MgCl2/1 mM EGTA, pH 6.9) and introduced into a slide/coverslip flow chamber coated with 10 mg/ml casein in PEM, and then rinsed with PEMT (PEM containing 10 μm Taxol and 0.1% 2-mercaptoethanol). Taxol-stabilized microtubules in PEMT with 10 mM ATP were introduced into the chamber, and microtubule gliding was observed by VEC-DIC as described (29). Analysis was performed using rtm custom software (30). The velocities of three to five microtubules were determined per 27 μm × 27 μm microscopic field examined, with at least three fields examined per preparation.
RESULTS
Antibody Specificity and Characterization of the Squid Dynactin Complex.
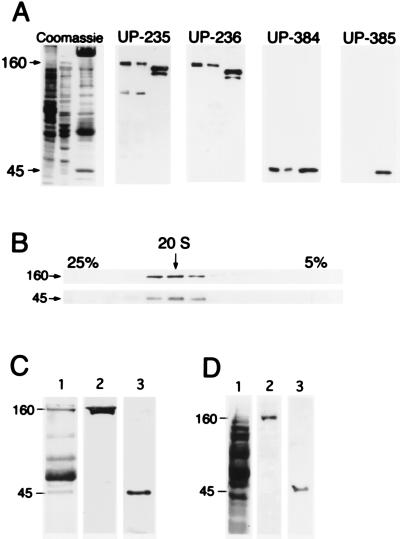
Polyclonal antibodies raised against mammalian forms of p150Glued and centractin were used to investigate the role of dynactin in axonal transport in the well-characterized extruded squid axoplasm system. Two p150Glued antibodies, UP235 and UP236, raised against overlapping regions of the protein expressed from a rat cDNA encoding p150Glued were found to react specifically with a polypeptide of 160 kDa in squid axoplasm and optic lobe cytosol (Fig. 1A). Antibody UP235 also demonstrated minor reactivity with a polypeptide of ≈80 kDa. However, blocking the antibody with excess immunogen completely inhibited the reactivity with the 160-kDa band while not affecting the reactivity with the 80-kDa polypeptide (data not shown). A polyclonal antibody raised against human centractin, UP384, specifically recognized a 45-kDa polypeptide in squid axoplasm (Fig. 1A), whereas a second anti-centractin antibody, UP385, did not react with the squid polypeptide and therefore was used as a control in the inhibition experiments described below.
Figure 1.
Characterization of the squid dynactin complex. (A) SDS/PAGE and immunoblot analysis of squid optic lobe cytosol (first lane), axoplasm (second lane), and a bovine brain fraction highly enriched in dynactin (third lane), stained for total protein, and probed with the anti-p150Glued antibodies UP235 and UP236 and with anti-centractin antibodies UP384 and UP385. (B) Squid optic lobe cytosol was sedimented on a 5–25% linear sucrose gradient, and gradient fractions were analyzed by SDS/PAGE and immunoblot with antibodies to p150Glued (Upper) and to centractin (Lower). The arrow indicates 20 S. (C) Anti-p150Glued antibody UP236 was used to immunoprecipitate dynactin from squid optic lobe cytosol. The immunoprecipitate was resolved by 8% SDS/PAGE and stained for total protein with Coomassie blue (lane 1). The band at 55 kDa is the IgG heavy chain, and the band at 66 kDa is BSA. Immunoblot analysis of the immunoprecipitate with anti-p150Glued antibody UP235 (lane 2) and anti-centractin antibody UP384 (lane 3). (D) Axoplasmic organelles were resolved by SDS/PAGE on a 10% gel and stained for total protein (lane 1). Immunoblotting was performed with anti-p150Glued antibody UP235 (lane 2) and anti-centractin antibody UP384 (lane 3).
Both the 160- and 45-kDa immunoreactive squid proteins cosedimented from whole optic lobe cytosol at 20 S on a 5–25% linear sucrose density, indicating that they are members of a cytosolic macromolecular complex (Fig. 1B). Both anti-p150Glued antibodies (UP236, Fig. 1C, or UP235, data not shown) coimmunoprecipitated five major polypeptides from optic lobe cytosol (168, 160, 82, 50, and 45 kDa) (Fig. 1C); these polypeptides are similar in size to those coprecipitated with anti-p150Glued antibodies from rat brain cytosol (150, 135, 62, 50, and 45 kDa; ref. 17). Immunoblot analysis of the squid immunoprecipitate showed that both the 168- and 160-kDa polypeptides reacted with anti-p150Glued antibodies, whereas the 45-kDa protein was recognized by anti-centractin antibody UP384. The 168-kDa polypeptide may represent a low-abundance isoform of the 160-kDa protein that is not detected in whole squid cytosol but that is concentrated in the immunoprecipitated sample. Isoform diversity due to alternative splicing of a single p150Glued gene has been characterized in other species (17). These results demonstrate the specificity of these antibodies for the squid dynactin complex.
Anti-p150Glued Antibodies Inhibit Microtubule-Based Fast Axonal Transport.
To investigate the role of the dynactin complex in fast transport in the squid axon, we treated extruded axoplasm with antibodies to p150Glued and centractin. Freshly dissected axons were divided in half; one half was extruded into motility buffer containing 0.8 mg/ml affinity-purified antibody while the other half was used as an internal control. Following extrusion into buffer alone, organelles moved bidirectionally and continuously at ≈2 μm/sec in both the central (bulk) regions of the axoplasm, where the native structure and polarity of the axon is retained (Table 1), and along individual microtubules in dissociated regions of axoplasm (Fig. 2), consistent with previous observations (1–3).
Table 1.
Effect of antibodies on organelle motility in bulk regions of extruded squid axoplasm.
| Organelle velocity, μm/sec | Motile activity | |
|---|---|---|
| Buffer | 2.0 ± 0.4, n = 28 | + |
| Anti-centractin | ||
| UP-385 | 2.1 ± 0.4, n = 21 | + |
| Anti-p150Glued | ||
| UP-235 | 0.6 ± 0.4, n = 5 | − |
| UP-235 (Fab) | 0.4 ± 0.1, n = 4 | − |
| UP-236 | 1.0 ± 0.2, n = 10 | −/+ |
| UP-236 (Fab) | 0.9 ± 0.4, n = 11 | −/+ |
The central axoplasm was examined by VEC-DIC 60 min after extrusion into either motility buffer alone, buffer containing 0.8 mg/ml of affinity-purified antibody, or 0.5 mg/ml affinity-purified Fab fragments. +, >50 organelles moving per microscopic field; −/+, <10 organelles moving per microscopic field; −, <1 organelle moving per microscopic field.
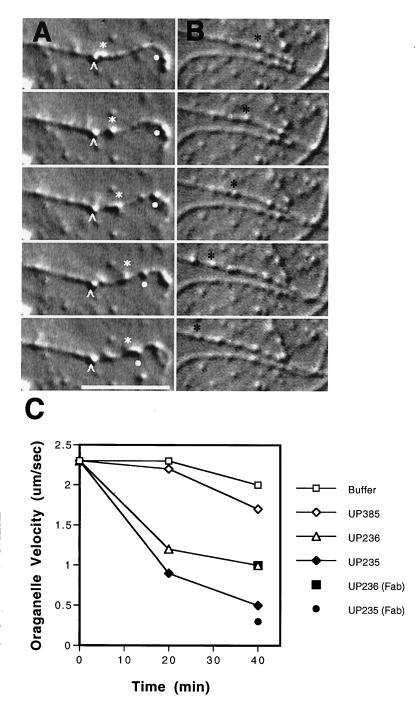
Figure 2.
Effects of antibodies on organelle motility along individual microtubules. VEC-DIC micrographs of axoplasm taken at 1S intervals following a ≈45-min incubation in 0.8 mg/ml anti-p150Glued antibody UP-235 (A) or after ≈45 min in motility buffer (B). (A) Organelle at white carat is moving at 0.0 μm/sec; organelle at white star is moving at 0.3 μm/sec; organelle at white dot is moving at 0.3 μm/sec in the opposite direction along the same microtubule. (B) Organelle at black star moving at 2.0 μm/sec. Bar = 10 μm. (C) Average velocities of organelle motility along individual microtubules were determined at 20 and 40 min. Affinity-purified antibodies to p150Glued (UP235 or UP236) or control antibodies (UP385) were at 0.8 mg/ml; Fab fragments of affinity-purified anti-p150Glued antibodies [UP235 (Fab) or UP236 (Fab)] were at 0.5 mg/ml. Average velocities for organelle motility [presented as mean (in μm/sec) +/− SEM] are as follows: buffer, 20 min: 2.3 ± 0.2, n = 40, 40 min: 2.0 ± 0.2, n = 36; UP385, 20 min: 2.2 ± 0.2, n = 20, 40 min: 1.7 ± 0.3, n = 22; UP236, 20 min: 1.2 ± 0.3, n = 10, 40 min: 1.0 ± 0.2, n = 10, UP235, 20 min: 0.9 ± 0.2, n = 36, 40 min: 0.5 ± 0.1, n = 18, UP236 Fab fragments, 40 min: 1.0 ± 0.5, n = 13, UP235 Fab fragments, 40 min: 0.3 ± 0.1, n = 6.
In contrast, after a 60-min incubation of axoplasm with 0.8 mg/ml of affinity-purified anti-p150Glued antibody we observed a significant reduction in organelle velocity in bulk axoplasm as compared with control values (UP235 induced a 70% inhibition compared with controls; UP236 induced a 50% inhibition) (Table 1). Both of the anti-p150Glued antibodies caused the bulk axoplasm to appear “frozen,” with organelles clearly stopped along microtubules and undergoing no Brownian motion (Table 1).
Examination of motility in the peripheral regions of the extruded axoplasm demonstrated a significant reduction in organelle velocity along individual microtubules after a 40-min incubation with antibody (75% inhibition with UP235; 50% inhibition with UP236; Fig. 2). Individual organelles that were bound to microtubules either continued to move very slowly or were stopped completely, remaining bound to microtubules, often at one end, and undergoing neither Brownian movement nor one-dimensional diffusion along the microtubule lattice (Fig. 2A). There was no obvious increase or decrease in the number of organelles associated with microtubules.
In contrast to the inhibitory effects of the anti-p150Glued antibodies, treatment with 0.8 mg/ml of the immunoreactive UP384 anti-centractin antibody had little effect on the velocity of organelle motility in either the bulk or peripheral axoplasm (bulk, 20% reduction after 60 min, Table 1; peripheral, 35% reduction after 40 min, Fig. 2).
As controls for the specificity of inhibition by anti-p150Glued antibodies, extruded axoplasm was treated either with 1 mg/ml of the non-cross-reactive anti-centractin antibody or with immunogen-blocked anti-p150Glued antibodies (either UP235 or UP236). Neither of the control treatments had any significant effects on organelle motility either in bulk or in dissociated axoplasm (Table 1, Fig. 2, and data not shown). To investigate whether the inhibition of motility was due to the cross-linking of dynactin complexes by bivalent anti-p150Glued antibody molecules, Fab fragments were prepared and tested for their effect on organelle transport in the axoplasm. Fab fragments (0.5 mg/ml) of either UP235 or UP236 had a similar inhibitory effect on organelle motility as that observed for intact antibodies (Table 1 and Fig. 2).
Anti-Dynactin Antibodies Do Not Inhibit Dynein Motor Activity.
In the experiments performed in extruded axoplasm described above, we noted that free microtubules continued to undergo gliding movements along the coverslip surface in the presence of the antibodies that inhibited organelle transport. This observation supports previous results indicating that dynactin is not required for the generation of sliding force by cytoplasmic dynein (13). To examine the effects of the anti-p150Glued antibodies on force production, sucrose gradient-purified fractions of optic lobe cytoplasmic dynein and dynactin or kinesin were preincubated with 1 mg/ml of the anti-p150Glued antibody UP235, and rates of microtubule gliding were assayed by VEC-DIC. No significant differences were observed from control measurements performed in the absence of antibody (dynein/dynactin alone, 0.40 ± 0.04 μm/sec; dynein/dynactin with UP235, 0.41 ± 0.11 μm/sec; kinesin alone, 0.15 ± 0.21 μm/sec; kinesin with UP235, 0.11 ± 0.09 μm/sec).
Anti-p150Glued Antibodies Block the Binding of Cytoplasmic Dynein to Dynactin.
p150Glued is a multifunctional protein that is capable of binding both to cytoplasmic dynein (18, 19) and to microtubules (20). Thus, we investigated whether antibodies to p150Glued inhibited organelle transport in axoplasm by specifically disrupting either of these interactions.
We used an affinity column binding assay (18) to assess the effects of anti-p150Glued antibodies on the interaction between dynein and dynactin. First, we demonstrated that a bacterially expressed fragment of rat p150Glued covalently bound to a Sepharose matrix was capable of specifically retaining intact cytoplasmic dynein holoenzyme from squid optic lobe cytosol. Analysis of the high-ionic-strength column eluate by SDS/PAGE and immunoblotting revealed that both the heavy and intermediate chains of cytoplasmic dynein were retained by the affinity column. To determine the effect of anti-p150Glued antibodies on the p150Glued-cytoplasmic dynein interaction, columns were preabsorbed either with antibodies to p150Glued or with nonspecific rabbit antisera. Optic lobe cytosol was loaded onto each column. The columns were washed extensively in motility buffer and then eluted with motility buffer containing an additional 0.5 M NaCl. Analysis of the eluates by immunoblotting revealed that no dynein was retained by the UP235-blocked column, whereas blocking with serum (Fig. 3) or BSA (not shown) had no effect on the retention of dynein by the affinity matrix. In parallel experiments, antiserum UP236 was also found to inhibit the binding of squid dynein to the affinity matrix, although the inhibition of binding was less complete (data not shown).
Figure 3.
Effects of anti-p150Glued antibodies on the dynein–dynactin interaction. Optic lobe cytosol was loaded onto p150Glued affinity columns that were preadsorbed with either preimmune serum or UP235 serum. The columns were eluted with 0.5 M NaCl in motility buffer. The resulting load, flowthrough, wash, and eluate fractions were analyzed by SDS/PAGE stained for total protein with Coomassie blue (A) and by immunoblotting (B) using antibodies to the dynein heavy chain (DHC, a generous gift of David Asai) or the dynein intermediate chain (DIC, a generous gift of Walter Steffen).
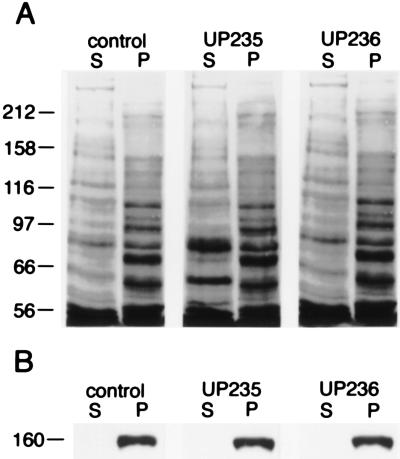
We then tested the effect of the anti-p150Glued antibodies on the microtubule-binding function of the dynactin complex. Squid optic lobe cytosol was incubated with 1 mg/ml of either of the anti-p150Glued antibodies, UP235 or UP236, for 45 min either before or after induction of microtubule polymerization in the cytosol by the addition of Taxol. Microtubules and associated proteins were isolated by sedimentation, and the resulting supernatant and pellet fractions were analyzed by immunoblotting (Fig. 4). Both in the presence and absence of antibodies, cytosolic p150Glued was found to cosediment exclusively with microtubules in the pellet.
Figure 4.
Effects of anti-p150Glued antibodies on the microtubule–dynactin interaction. Optic lobe cytosol was incubated for 45 min in the absence (control lanes) or in the presence of 1 mg/ml of the affinity-purified anti-p150Glued antibody UP235 or UP236. Microtubules were then assembled in the cytosol and isolated by centrifugation. The postmicrotubule supernatants (S) and microtubule pellets (P) were then analyzed by 8% SDS/PAGE and Coomassie blue staining for total protein (A) followed by immunoblotting with an antibody to p150Glued (B). Molecular mass in kDa is indicated on the left.
Anti-p150Glued Antibodies Inhibit the Association of Cytoplasmic Dynein with Organelles.
Both cytoplasmic dynein and dynactin copurify with axoplasmic vesicles (Fig. 1D). To test the effects of the anti-dynactin antibodies on the association of cytoplasmic dynein with membranous organelles, we isolated a corresponding organelle fraction from squid optic lobe and then incubated this fraction at 37°C in the absence or in the presence of the affinity-purified anti-p150Glued antibody UP235. Organelles were further purified by flotation on a sucrose step gradient to separate the membrane fractions from either soluble or particulate dynein. Parallel fractionation of squid cytosol revealed that dynein was not found at the 1.5 M/0.6 M interface in the absence of a vesicular association (Fig. 5).
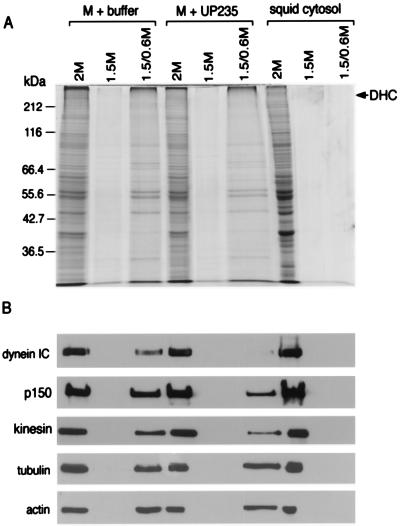
Figure 5.
Effects of anti-p150Glued antibodies on the dynein–organelle interaction. A membrane-enriched fraction from squid was divided in half and either resuspended in motility buffer alone (M + buffer) or in buffer with the anti-p150Glued antibody UP235 at 0.8 mg/ml final concentration (M + UP235). Squid cytosol was used as a control for these experiments. The samples were fractionated on step gradients, and samples from the 1.5/0.6-M interface (vesicular) and the 1.5- and 2-M (soluble) fractions were analyzed by SDS/PAGE (stained with Coomassie blue for total protein, A) and by immunoblot (B) with antibodies to cytoplasmic dynein, dynactin, kinesin, tubulin, and actin. (Anti-dynein intermediate chain antibody was provided by Walter Steffen, anti-kinesin antibody was provided by B. Schnapp, anti-tubulin antibody was from Sigma, and anti-actin antibody was from Boehringer Mannheim.) The gels and blots were analyzed by densitometry; when the results are normalized for equal loading per lane, there was no significant change detected in the amount of dynactin or kinesin associated with membranes, whereas the reduction in the amount of dynein associated with membranes was found to be significant.
Comparisons of vesicle fractions isolated following incubation either in the absence or in the presence of the anti-p150Glued antibody revealed no significant change in the amount of dynactin, kinesin, tubulin, or actin associated with membranes after normalization to account for differences in total protein loaded per lane. However, the anti-dynactin antibody was observed to induce nearly complete dissociation of cytoplasmic dynein from the membrane fraction (Fig. 5).
DISCUSSION
Dynactin Is Required for Fast Axonal Transport.
We have observed that two different polyclonal antibodies to p150Glued specifically inhibit the microtubule-based motility of organelles in squid axoplasm, indicating that the function of dynactin is essential to fast transport in neurons. Previous studies have shown that cytoplasmic dynein is involved in the retrograde transport of axonal organelles (6–8) and that cytoplasmic dynein-based vesicle transport requires cytosolic factors other than the purified motor protein (13). In this study, we have shown that cytoplasmic dynein requires the function of the dynactin complex to engage in axonal transport.
Anti-p150Glued antibodies were observed to inhibit the bidirectional transport of organelles along microtubules. Previous studies on the inhibition of axonal transport using anti-kinesin antibodies have also noted a bidirectional inhibition (31), suggesting that the anterograde and retrograde organelle motors are closely linked either physically due to colocalization (8) or through a common regulatory mechanism. Although it has been suggested that kinesin and cytoplasmic dynein may share a common organelle-bound receptor (32), the data reported here do not specifically support this hypothesis. Instead, we suggest that the rigor-like binding of organelles to the microtubule that was induced by the anti-dynactin antibodies may have physically obstructed kinesin-mediated transport.
The Mechanism of Dynactin-Mediated, Cytoplasmic Dynein-Driven Motility.
The observed effect of anti-p150Glued antibodies on the microtubule-based motility of organelles in axoplasm, together with the results of our in vitro assays, provide insight into the function of dynactin at the molecular level. Because the anti-p150Glued antibodies that were observed to inhibit organelle transport in axoplasm were also shown to disrupt the binding interaction between cytoplasmic dynein and dynactin and to disrupt the ability of dynein to bind to membranous organelles, these results suggest that the observed inhibition in vesicle transport was due to the inability of cytoplasmic dynein to be recruited to organelles by the dynactin complex. Because the p150Glued polypeptide is not likely to be directly involved in anchoring dynactin to membranes (discussed below), it is not surprising that anti-p150Glued antibodies did not perturb the association of dynactin with vesicles. Instead, due to a microtubule-binding motif at the amino terminus of p150Glued (20), dynactin may link organelles to the microtubule independent of any association with cytoplasmic dynein. This model is consistent with our observation that antibody disruption of the dynein–dynactin interaction resulted in the rigor-like binding of vesicles along the microtubules of the dissociated axoplasm.
Although a direct interaction between cytoplasmic dynein and phospholipids has previously been observed (33), the physiological role of this interaction has not yet been determined. Instead, our observation that an antibody to dynactin effectively blocks the binding of dynein to membranes suggests that dynactin is required to mediate the dynein–vesicle interaction. We have recently demonstrated a direct interaction between dynactin and spectrin, mediated by the actin-like filament formed by centractin at the base of the dynactin complex (11). This observation suggests that the centractin filament in dynactin may act as a structural element linking dynein to a membrane-bound spectrin cytoskeleton on the organelle surface.
Our data thus support a model in which the dynactin complex acts as the hub in the formation of a supramolecular motile complex that: (i) links an organelle to a microtubule via its association with spectrin, (ii) recruits cytoplasmic dynein to the organelle to initiate transport, and (iii) is required during active dynein-based organelle transport. Cytoplasmic dynein may be recruited to the organelle due to its binding affinity for dynactin. Then, during active organelle transport dynactin may play a tethering role, preventing diffusion of the organelle away from the microtubule during the portion of the ATPase cycle when both heads of dynein may be dissociated from the microtubule, as we have suggested previously (20). Alternatively, dynactin may be required to regulate or “activate” dynein in some other fashion, which remains to be determined. However, the data described here clearly establish a critical role for the dynein intermediate chain in linking cytoplasmic dynein to its cargo. A similar observation recently has been made in the study of membrane extension along microtubules in Xenopus extracts by Steffen et al. (34).
The Dynein–Dynactin Interaction in Other Cellular Processes.
The results of our study provide direct evidence that dynactin is required for the microtubule-based motility of membranous organelles in neurons. Recent evidence suggests that the interaction between dynein and dynactin is also required for nuclear migration and for spindle orientation and assembly (9, 16, 35). Our results may thus provide insight into a conserved mechanism for cytoplasmic dynein-based motility as well as into the fundamental neuronal process of fast axonal transport at the molecular level.
Acknowledgments
We thank R. Sloboda, E. D. Salmon, G. Gries, M. Tokito, W. Steffen, E. Vaisberg, D. Asai, and B. Schnapp for their generous contributions to this work. Taxol was the gift of the National Cancer Institute. This work is supported by National Institutes of Health Grant GM48661 to E.L.F.H. During the tenure of this work, C.M.W.-S. and S. K. were supported by fellowships from the American Heart Association, Southeastern Affiliate; C.M.W.-S. was also supported by a predoctoral fellowship from the Pennsylvania Muscle Institute. E.L.F.H. is an Established Investigator of the American Heart Association.
References
- 1.Allen R D, Weiss D G, Hayden J H, Brown D T, Fujiwake H, Simpson M. J Cell Biol. 1985;100:1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnapp B J, Vale R D, Sheetz M P, Reese T S. Cell. 1985;40:455–462. doi: 10.1016/0092-8674(85)90160-6. [DOI] [PubMed] [Google Scholar]
- 3.Vale R D, Schnapp B J, Reese T S, Sheetz M P. Cell. 1985;40:449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- 4.Vale R D, Reese T S, Sheetz M P. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirokawa N. Neurosci Res. 1993;18:1–9. doi: 10.1016/0168-0102(93)90099-c. [DOI] [PubMed] [Google Scholar]
- 6.Schnapp B J, Reese T S. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroer T A, Steuer E R, Sheetz M P. Cell. 1989;56:937–946. doi: 10.1016/0092-8674(89)90627-2. [DOI] [PubMed] [Google Scholar]
- 8.Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney H L, Holzbaur E L F. Annu Rev Physiol. 1996;58:751–792. doi: 10.1146/annurev.ph.58.030196.003535. [DOI] [PubMed] [Google Scholar]
- 10.Schafer D A, Gill S R, Cooper J A, Heuser J E, Schroer T A. J Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holleran E A, Tokito M K, Karki S, Holzbaur E L F. J Cell Biol. 1996;135:1815–1830. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzbaur E L, Hammarback J A, Paschal B M, Kravit N G, Pfister K K, Vallee R B. Nature (London) 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- 13.Gill S R, Schroer T A, Szilak I, Steuer E R, Sheetz M P, Cleveland D W. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paschal B M, Holzbaur E L F, Pfister K K, Clark S, Meyer D I, Vallee R B. J Biol Chem. 1993;268:15318–15323. [PubMed] [Google Scholar]
- 15.Lees-Miller J P, Helfman D M, Schroer T A. Nature (London) 1992;359:244–246. doi: 10.1038/359244a0. [DOI] [PubMed] [Google Scholar]
- 16.Echeverri C J, Paschal B M, Vaughan K T, Vallee R B. J Cell Biol. 1996;4:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokito M K, Howland D S, Lee V M-Y, Holzbaur E L F. Mol Biol Cell. 1996;7:1167–1180. doi: 10.1091/mbc.7.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karki S, Holzbaur E L F. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan K T, Vallee R B. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterman-Storer C M, Karki S, Holzbaur E L F. Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1988. pp. 628–629. [Google Scholar]
- 22.Waterman-Storer C M, Holzbaur E L F. J Biol Chem. 1996;271:1153–1159. doi: 10.1074/jbc.271.2.1153. [DOI] [PubMed] [Google Scholar]
- 23.Weiss D G, Langford G M, Seitz-Tutter D, Maile W. Acta Histochem Supp. 1991;41:81–105. [PubMed] [Google Scholar]
- 24.Kuznetsov S A, Langford G M, Weiss D G. Nature (London) 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- 25.Weiss D G, Keller F, Gulden J, Maile W. Cell Motil Cytoskeleton. 1986;6:128–135. doi: 10.1002/cm.970060210. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert S P, Sloboda R D. J Cell Biol. 1984;99:445–452. doi: 10.1083/jcb.99.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert S P, Allen R D, Sloboda R D. Nature (London) 1985;315:245–248. doi: 10.1038/315245a0. [DOI] [PubMed] [Google Scholar]
- 28.Niclas J, Allan V J, Vale R D. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker R A, Gliksman N R, Salmon E D. In: Optical Microscopy for Biology. Herman B, Jacobson K, Lemasters J, editors. New York: Wiley; 1990. pp. 395–407. [Google Scholar]
- 30.Walker R A, O’Brien E T, Pryer N K, Soboeiro M, Voter W A, Erickson H P, Salmon E D. J Cell Biol. 1988;107:1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brady S T, Pfister K K, Bloom G S. Proc Natl Acad Sci USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallee R B, Sheetz M P. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- 33.Lacey M L, Haimo L T. Cell Motil Cytoskeleton. 1994;28:205–212. doi: 10.1002/cm.970280304. [DOI] [PubMed] [Google Scholar]
- 34.Steffen, W., Karki, S., Vaughan, K. T., Vallee, R. B., Holzbaur, E. L. F., Weiss, D. G. & Kuznetsov, S. A. (1997) Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 35.Gaglio T, Saredi A, Bingham J B, Hasbani M J, Gill S R, Schroer T R, Compton D A. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]