Abstract
Although adenovirus can infect a wide range of cell types, lymphocytes are not generally susceptible to adenovirus infection, in part because of the absence of the expression of the cellular receptor for the adenoviral fiber protein. The cellular receptor for adenovirus and coxsackievirus (CAR) recently was cloned and shown to mediate adenoviral entry by interaction with the viral fiber protein. We show that the ectopic expression of CAR in various lymphocyte cell lines, which are almost completely resistant to adenovirus infection, is sufficient to facilitate the efficient transduction of these cells by recombinant adenoviruses. Furthermore, this property of CAR does not require its cytoplasmic domain, consistent with the idea that CAR primarily serves as a high affinity binding site for the adenoviral fiber protein, and that viral entry is mediated by interaction of the viral penton base proteins with cellular integrins. As a demonstration of their functional utility, we used CAR-expressing lymphocytes transduced with an adenovirus expressing Fas ligand to efficiently kill Fas receptor-expressing tumor cells. The ability to efficiently manipulate gene expression in lymphocyte cells by using adenovirus vectors should facilitate the functional characterization of pathways affecting lymphocyte physiology.
The inability to easily and efficiently introduce genes into lymphocytes and examine the consequences of such expression soon after gene delivery limits the characterization of lymphocyte pathways that control cell growth, differentiation, and death. Although retrovirus vectors have been used successfully to transduce cells of hematopoietic origins, experiments involving retroviral transduction of lymphocytes have been limited by the difficulty in transducing the entire population of cells, the requirement that the cells be proliferating for viral integration, and the time required for the transduction and expression of the introduced gene. Other approaches to manipulate gene expression in lymphocyte cell lines often require either the generation of cell lines that express the desired gene product or the transient transfection of the gene of interest into a fraction of the cells.
As an alternative approach to gene transfer in lymphocytes, we sought to develop methods to allow the introduction of genes by using adenovirus vectors. Adenoviral vectors are attractive in that either proliferating or quiescent cells can be transduced, the expression of the introduced gene is evident by 5 hr, the vectors can accommodate large insert sizes (7–9 kb), and high titer stocks are easily generated (1). A particular advantage of the adenovirus vectors lies in their ability to transduce the entire cell population, which allows detailed biochemical analyses of endogenous activities. Group C adenovirus (e.g., Ad2 and Ad5) infection requires the high affinity attachment of the viral fiber capsid protein to a cellular receptor and viral penton base binding to certain cellular integrins, followed by cell entry via receptor-mediated endocytosis (2). Unfortunately, although adenovirus can infect a wide range of cell types, lymphocytes are not very susceptible to adenovirus infection, apparently as a result of the failure of adenovirus to efficiently bind the cell surface and be internalized (3–5). In part, the inability of adenovirus to enter T cells is caused by very low expression levels of the cellular fiber receptor (6, 7). In addition, T cells express limiting levels of αV containing integrins, and indeed mitogen-mediated up-regulation of αV integrin expression confers limited infection by adenovirus vectors (6).
The cellular receptor for the adenoviral fiber protein, CAR (for coxsackievirus and adenovirus receptor), was recently cloned (8–10), and the expression of CAR in Chinese hamster ovary cells increased their susceptibility to adenovirus infection approximately 100-fold (8). We predicted that the expression of CAR in T cells would augment virus attachment to the cells and facilitate virus entry by increasing the frequency of viral penton base association with the integrins present on T cells. Indeed, we demonstrate that the expression of CAR in several lymphocyte cell lines confers full susceptibility to adenovirus transduction, and furthermore that this property does not require the cytoplasmic domain of CAR. Last, we demonstrate a functional application of this technique. CAR-expressing lymphocytes were transduced with an adenoviral construct encoding Fas ligand (FasL), and these cells then were able to specifically induce apoptosis of cells expressing the Fas receptor (FasR). Lymphocyte that express CAR will be valuable for the analysis of pathways, via gene transfer, which control various aspects of cellular physiology either in vitro or in vivo.
MATERIALS AND METHODS
Construction of Plasmids.
LXSN-human CAR (hCAR) and LXSN-mouse CAR (mCAR) plasmids were generated by ligation of EcoRI/XhoI cDNA fragments from either hCAR-pcDNA1-strider or pCMV-Sport2-mCAR (9) into EcoRI/XhoI-digested LXSN.Vec1 (11). Deletion mutations of hCAR were created by 25 cycles of PCR amplification of LXSN-hCAR with the following primers: GCGGAATTCCCAGGAGCGAGAGCC with either CGCAGCTCGAGCTATTTACGACAGCAAAAGATGAT (Δ1), CGCAGCTCGAGCTACACATCTTCCCTGATATCGTG (Δ2), or CGCAGCTCGAGCTATCCTTCCATGTTGGAAGG (Δ3). The resulting PCR products were digested with EcoRI/XhoI and cloned into EcoRI/XhoI-digested LXSN.Vec1.
Cells and Virus.
EL-4 (CD4+, CD3+ mouse lymphoma cells) (12) and DPK.C7 (13) mouse thymoma cell lines were grown in RP10 [RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS), 0.1 mM 2-mercaptoethanol, and 1% penicillin-streptomycin (P/S) (GIBCO/BRL)]. FL5.12 cells were grown in RP10 supplemented with 20% filtered WEHI-3 conditioned medium. The Ref52 rat embryo fibroblasts (14) were grown in 5% FBS, 5% calf serum, and 1% P/S in DMEM, and the Phoenix 293-T amphotropic producer cell line (15) was grown in 10% FBS and 1% P/S in DMEM (DME10). The LXSN plasmids were transfected by standard calcium phosphate procedures into Phoenix 293 producer cell line (in DME10) as described (15), the media was changed after 16 hr to 10% FBS and 1% P/S in αMEM (MEM10), and the LXSN retrovirus containing supernatant was harvested after 2 days and filtered through a 0.2-μm filter. Cells (2 × 106) were infected with 4 ml of this supernatant for 4 hr (with 4 μg/ml Polybrene), and then 16 ml of MEM10 was added. After 16 hr, the cells were changed to RP10. One day later, the cells were changed to RP10 with 0.5 mg/ml of Geneticin (GIBCO/BRL), and pooled G418 resistant cells were obtained after about 1 week. For the FL5.12 cells, the media was supplemented with 20% WEHI throughout.
Except as described otherwise, cells were transduced with adenovirus in their standard growth medium containing 2% serum at 107 cells per ml for 30 min at room temperature. Complete medium then was added to the transduced cells (final density of 4 × 105 cells/ml), and the cells were incubated 24 hr at 37°C. The cells then were washed once with PBS and analyzed on a Coulter Epics XL flow cytometer. For the isolation of single cell clones, the cells were sorted into 96-well plates on the MoFlo flow cytometer from Cytomation (Fort Collins, CO). Adenoviruses were purified by CsCl gradient centrifugation, and virus titers were determined by an indirect immunofluorescence assay by using an antibody against E2A and defined as focus-forming units per ml as described (1). The construction of the adenovirus-expressing mouse FasL (Ad-FasL) (T.H., J.S., S.M., Gary Miller, Andrew Kraft, S. Srikanth, and R.D., unpublished work), the viruses expressing EGFP (CLONTECH) (J.S., B. Allen, I. Maxwell, and R. Smith, unpublished work), and the control recombinant adenovirus (Ad-CON) (16) is described elsewhere.
Flow Cytometric Analysis for CAR Expression.
Cells (106) were incubated with a 1:100 dilution in 5% FBS in PBS of RmcB monoclonal ascites fluid for 45 min on ice. The cells were washed twice with PBS and incubated with a 1:100 dilution of fluorescein isothiocyanate-labeled goat anti-mouse IgG1 (PharMingen 02234D) for 45 min on ice. The cells were washed twice with PBS and analyzed by flow cytometry.
Cell Killing Assays.
EL-4-CAR 13 or EL-4 parental cells were transduced as described above with either AdCMV-green fluorescent protein (GFP), Ad-FasL, or no virus (control). Eighteen hours posttransduction the cells were washed twice, and 100 μl containing 2.5 × 104 cells [5:1 effector/target (E/T) ratio] or 2,500 cells (0.5:1 E/T ratio) were added in triplicate to a 96-well V-bottom plate. The target cell line L1210-FasR was labeled with 100 μCi 51Cr per 2 × 106 cells as described (17). After washing three times, 5,000 labeled cells in 100 μl were added per well to the various effector cells. Maximum lysis (100%) was determined by adding 100 μl of 1% Triton X-100 to the target cells, and spontaneous lysis was estimated by adding 100 μl of medium. The 96-well plate was briefly centrifuged at 500 rpm and incubated for 24 hr. The plate was recentrifuged before removing 100 μl of supernatant from each well for gamma counting.
RESULTS
The Expression of CAR Is Sufficient to Confer Full Susceptibility of Lymphocytes to Adenovirus Transduction.
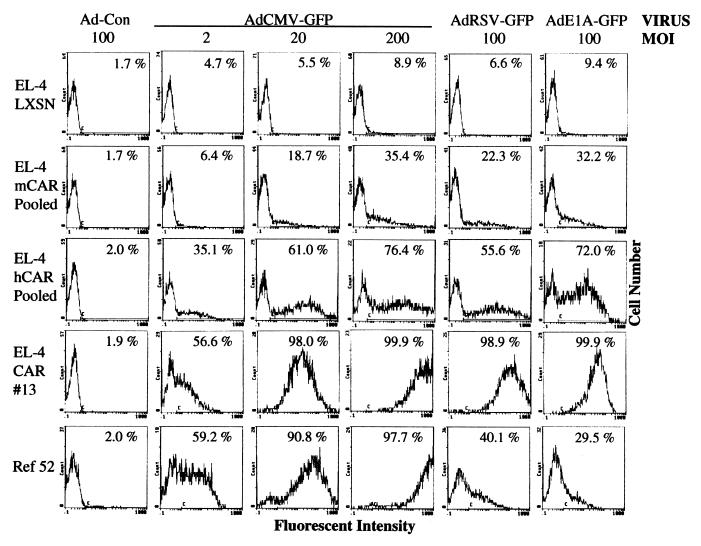
Previous studies have demonstrated the inefficient delivery of recombinant genes via adenoviruses to lymphocytes (4, 18). We examined the extent to which EL-4 mouse lymphoma cells could be transduced with adenovirus. EL-4 or EL-4 LXSN cells (vector control; see below) were transduced with either Ad-CON or recombinant viruses that express GFP from either the cytomegalovirus (CMV), Rous sarcoma virus, or E1A promoters (AdCMV-GFP, AdRSV-GFP or AdE1A-GFP, respectively). Exposure of EL-4 or EL-4 LXSN cells to high multiplicities of infection (MOI) of these viruses failed to result in expression of GFP, as determined by flow cytometry (Fig. 1 and data not shown). In contrast, transduction of the rat embryo fibroblast cell line (Ref52) with AdCMV-GFP at MOIs of 20 or 200 resulted in the transduction of the entire population of cells, as evidenced by their bright fluorescence. We then tested whether the expression of CAR would increase the susceptibility of EL-4 lymphocyte cells to adenovirus transduction. To facilitate the creation of lymphocyte cell lines that express CAR, we generated LXSN retrovirus vectors expressing either mCAR or hCAR cDNAs. EL-4 cells were transduced either with the CAR-expressing retroviruses or the control retrovirus (LXSN) and selected for neomycin resistance for 7–10 days. Pooled neomycin-resistant populations were transduced with Ad-CON or AdCMV-GFP at three different MOIs and analyzed by flow cytometry (Fig. 1). Seventy-six percent of the EL-4 cells expressing the hCAR cDNA and transduced with AdCMV-GFP (MOI of 200) were positive for GFP expression as demonstrated by increased fluorescence. Because the expression of the mCAR cDNA facilitated transduction by adenovirus to a lesser extent than hCAR (Fig. 1), most subsequent data presented are derived from hCAR-expressing cells.
Figure 1.
The expression of CAR in EL-4 cells confers susceptibility to adenovirus transduction. (A) Cells were transduced with the indicated MOI of either Ad-CON or with adenovirus expressing GFP from either a CMV, Rous sarcoma virus, or E1A promoter. After 24 hr, the live cells were analyzed by flow cytometry for the expression of GFP, as indicated by increased fluorescent intensity. The percentage of cells with fluorescence over background (denoted by C bar) is indicated.
EL-4 cells expressing hCAR subsequently were transduced with AdCMV-GFP (MOI of 20) and then single cell sorted for cells that displayed high fluorescence (fluorescent intensity ranging from 30 to 300). Fourteen single cell clones were expanded, and then retested for their susceptibility to adenoviral transduction. As seen in Fig. 1, transduction of the EL-4-CAR13 clone with various multiplicities of AdCMV-GFP resulted in nearly 100% of the cells that exhibited high fluorescence, indicating successful delivery of GFP to almost all of the cells. Similar results were observed for all tested clones (data not shown). In fact, the EL-4 CAR 13 cells were transduced as efficiently as the highly susceptible fibroblast cells over the range of tested MOIs. AdRSV-GFP and AdE1A-GFP, although poorly expressed in the fibroblast cells such that most cells did not exhibit significant fluorescence, efficiently transduced and were expressed in the EL-4 CAR 13 cells, demonstrating the utility of recombinant adenoviruses that use these promoters for the expression of transgenes in lymphocytes.
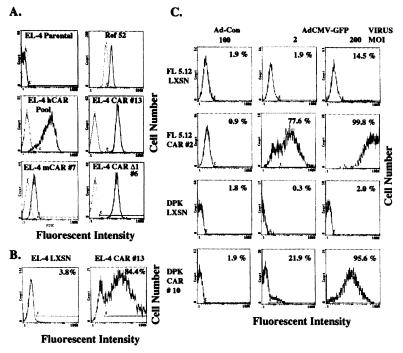
We directly measured the cell surface expression of CAR by flow cytometry of living cells after staining with the RmcB monoclonal (19) against hCAR (Fig. 2A). Although EL-4 cells do not express detectable CAR, Ref52 fibroblast cells exhibit significant CAR expression, consistent with their high susceptibility to adenovirus infection. Although the RmcB antibody appears to recognize mCAR (and presumably rat CAR) less efficiently than hCAR (10), we can detect ectopically expressed mCAR on EL-4 cells (Fig. 2A), although the staining is approximately 10-fold less than EL-4 cells expressing hCAR. The pooled neomycin-resistant EL-4 hCAR cells showed variable expression of CAR, over approximately two logs of fluorescence intensity, consistent with their intermediate susceptibility to adenovirus. In contrast, the cloned and highly infectable EL-4 CAR 13 cells exhibited high-level, uniform expression of cell surface hCAR.
Figure 2.
Analysis of cell surface CAR expression, in vivo transduction of EL-4 CAR by adenovirus, and CAR-mediated adenovirus transduction of other lymphocyte cell lines. (A) The expression of CAR was determined on the indicated cells by incubation of the cells with RmcB monoclonal against hCAR (or no primary antibody as a control, shown as a dotted line overlay) followed by a fluorescein isothiocyanate-linked goat α-mouse IgG1 antibody. EL-4 mCAR 7 is a single cell clone from the pooled EL-4 cells expressing mCAR. (B) EL-4 CAR 13 or EL-4 LXSN cells were injected i.p. into C57BL/6 mice (5 × 106 in 0.5 ml of PBS per mouse). Seventy-two hours later, the mice were injected i.p. with 2 × 107 focus-forming units of AdCMV-GFP in 0.4 ml of PBS. The mice were sacrificed 12 hr later. EL-4 cells were removed from the peritoneum by instilling then immediately withdrawing 5 ml of PBS injected into the peritoneum cavity with a 22-gauge needle. These cells were stained with Cy-chrome linked anti-CD3 (PharMingen 01088A), and then analyzed by flow cytometry. Cells gated for both size (as determined for uninjected EL-4 cells) and CD3 expression were analyzed for GFP expression as shown in B. The percentage of fluorescent cells (F bar) is indicated. (C) FL5.12, DPK.C7, and the hCAR expressing derivatives of either of these cell lines were transduced with either Ad-CON or AdCMV-GFP at the indicated MOIs and analyzed by flow cytometry, as described in Fig. 1. The percentage of fluorescent cells (C bar) is indicated.
Two of the advantages of adenovirus recombinants are the ability to generate high titer stocks of viruses and the high stability of the virus. These features facilitate the use of adenovirus for in vivo gene delivery (20). We therefore tested the ability of hCAR expressing EL-4 cells to be transduced in vivo by AdCMV-GFP. EL-4 CAR 13 or EL-4 LXSN cells were injected i.p. into syngeneic C57BL/6 mice. After 3 days, mice were injected i.p. with AdCMV-GFP, and the peritoneal cells were isolated 12 hr later for analysis by flow cytometry. EL-4 CAR 13 cells, but not the EL-4 LXSN cells, were efficiently transduced by AdCMV-GFP (Fig. 2B), demonstrating the utility of CAR-expressing lymphocytes for in vivo gene delivery.
Although the above results clearly show that the expression of CAR in the EL-4 lymphocyte cell line conferred susceptibility to adenoviral transduction both in vitro and in vivo, it was not clear whether this susceptibility also would be true for other lymphocyte cell lines. Several other clonal lymphocyte cell lines that express the hCAR cDNA were generated by the same procedure described above. Multiple cloned lines were generated for each cell line, and very similar results were obtained for all of the clones (data not shown). The cell lines used were the FL5.12 mouse interleukin (IL)-3-dependent pro-B cell line (21) and the DPK.C7 mouse thymoma line from the AND transgenic mouse (13, 22). As shown in Fig. 2C, the LXSN-transduced populations of FL5.12 and DPK.C7 cells were poorly transduced by AdCMV-GFP. In contrast, clones of these cells that express hCAR were very efficiently (ca. 100%) transduced by AdCMV-GFP. These results demonstrate that the expression of CAR facilitates the transduction of both B and T cell lines. Recent experiments indicate that CAR also can facilitate adenoviral gene transfer in the Ba/F3 IL-3-dependent pro-B cell and mouse 32Dcl3 premyeloid cell lines (data not shown).
Interaction with Integrins Is Required But the Cytoplasmic Domain of CAR Is Dispensable for Adenoviral Transduction.
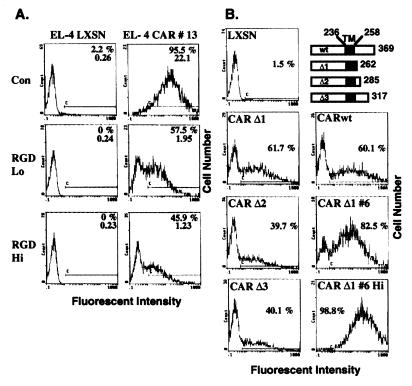
Although we cannot detect significant expression of either αV or αM integrins on EL-4 cells (data not shown), adenoviral transduction of hCAR expressing EL-4 cells is still integrin dependent. EL-4 CAR 13 or EL-4 LXSN control cells were incubated for 1 hr with either 0, 0.1, or 0.5 mg/ml of a GRGDS peptide. RGD-containing peptides inhibit integrin binding to fibronectin or vitronectin, preventing cell attachment to matrix proteins (23), and inhibit adenoviral infection of HeLa and M21 cells by inhibiting interaction of the viral penton base with αv integrins (2). The peptide-treated EL-4 cells were infected with AdCMV-GFP (MOI of 5) for 24 hr and analyzed by flow cytometry (Fig. 3A). Treatment of the EL-4 CAR 13 cells with 0.5 mg/ml of peptide substantially inhibited AdCMV-GFP transduction, indicating that interaction with cellular integrins is required for adenoviral transduction even when CAR is overexpressed.
Figure 3.
Adenoviral transduction is integrin dependent, but does not require the cytoplasmic domain of CAR. (A) Either EL-4 LXSN or EL-4 CAR 13 cells (105 cells per 0.1 ml in 96-well plates) were incubated for 1 hr at 37°C with 0 (Con), 0.1 (Lo), or 0.5 (Hi) mg/ml of GRGDS peptide (Sigma G4391) in 2% FBS in RPMI. AdCMV-GFP was added at an MOI of 5, and incubation continued for an additional hour at 37°C. Eight microliters of FBS then was added, and the cells were cultured at 37°C for 24 hr and analyzed on a flow cytometer. The percentage of fluorescent cells (C bar) is indicated, and the average fluorescent intensity of all of the cells is shown below the percentage. (B) Three different C-terminally truncated mutants of the hCAR receptor were generated by PCR amplification using internal primers, which resulted in a stop codon immediately following the indicated residue. The predicted transmembrane domain (TM) encompasses residues 236–258, as illustrated in the schemata (Upper Right). LXSN retroviruses encoding the CAR Δ mutants (or the LXSN and LXSN-hCAR as control viruses) were used to transduce EL-4 cells, and pooled NeoR cells were selected. The CAR Δ1 6 cell clone was derived from a single cell sorted from the highly fluorescent cells resulting from AdCMV-GFP transduction of the CAR Δ1 pool. The indicated cells were transduced with AdCMV-GFP at an MOI of either 200 (CAR Δ1 6 Hi) or 20 (all others) and analyzed by flow cytometry as described in Fig. 1. The percentage of fluorescent cells (C bar) is indicated.
Given that the expression of CAR facilitated adenoviral transduction of lymphoid cell lines, we next determined which regions of the hCAR protein are required for facilitating virus entry. We generated hCAR deletion mutants from the C-terminal cytoplasmic region and expressed them via retroviral vectors in EL-4 cells. As shown in Fig. 3B, all three hCAR constructs with deletions of C terminal sequence conferred adenoviral susceptibility to EL-4 cells. In particular, CAR Δ1, which encodes a protein with only four amino acids C terminal to the predicted transmembrane domain, and wild-type hCAR-expressing cells (both as pools of retroviral integrants) similarly were transduced by AdCMV-GFP (Fig. 3B, second row). CARΔ1 was expressed on the cell surface at levels similar to full-length hCAR (Fig. 2A). These data suggest that the cytoplasmic domain of hCAR is not required for adenovirus transduction and that the role of CAR is primarily as a docking protein for the adenovirus fiber protein. Nonetheless, our data indicate that a major limiting factor for group C adenoviral infection of lymphoid cell lines is insufficient expression of CAR.
Functional Expression of FasL in CAR-Expressing Lymphocytes.
A particular advantage of adenoviral-mediated gene delivery is the ability to efficiently express genes whose products have activities, such as the induction of apoptosis, that are incompatible with long-term expression. The binding of FasL to FasR (CD95) results in the recruitment of FADD via the death domain of FasR, followed by the activation of caspases culminating in apoptosis (24). Although expression of FasL together with FasR can contribute to the death of a cell, the expression of FasL alone can confer on a cell the license to kill, which contributes to target killing by cytotoxic T cells as well as immune privilege of certain tissues such as the eye and testis (25). To study the ability of FasL-expressing cells to kill FasR-expressing targets, we created a recombinant adenovirus that encodes mouse FasL (Ad-FasL).
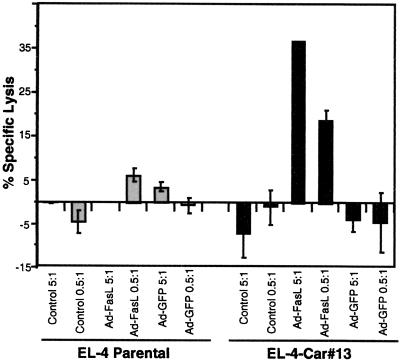
To test the ability of the hCAR-expressing EL-4 cells to express FasL encoded by an adenovirus construct, a standard cytotoxicity assay was used. The lymphocytic cell line L1210-FasR was chosen as a target cell line because it stably expresses high levels of FasR and readily undergoes apoptosis in response to FasL (26). L1210-FasR were cytoplasmically labeled with 51Cr, incubated with various numbers of adenovirus-transduced EL-4 cells, and cytolysis quantified based on the release of 51Cr from the cells into the supernatant. As shown in Fig. 4, parental EL-4 cells were unable to induce FasR-mediated death regardless of whether they were exposed to Ad-FasL. In contrast, EL-4 CAR 13 cells that were transduced with Ad-FasL killed L1210-FasR cells in a dose-dependent manner. This result indicates that the expression of FasL by EL-4 cells mediates cell killing of FasR-expressing target cells. This effect was specific for FasL because untransduced and AdCMV-GFP-transduced EL-4 hCAR cells failed to induce apoptosis. These data demonstrate a functional application of CAR expressing lymphocytes in the analysis of apoptotic pathways.
Figure 4.
EL-4-CAR cells transduced with an adenoviral FasL effectively kill FasR-expressing target cells. EL-4-CAR 13 cells were transduced (MOI of 100) with Ad-FasL, AdCMV-GFP, or no virus (control). The cells then were washed once and coincubated for 20 hr with 51Cr-labeled L1210-FasR cells at a 5:1 or 0.5:1 effector/target ratio. Each value represents the mean of triplicate measurements ± SD.
DISCUSSION
Implications for Gene Transfer in Lymphocytes.
Our data demonstrate that the expression of CAR in lymphocytes confers susceptibility to adenoviral transduction. The generation of CAR-expressing lymphocytes will facilitate a molecular analysis of pathways affecting lymphocyte physiology. In these cells, recombinant adenoviruses can be used to directly assess the involvement of suspected pathway components by introducing specific activators or inhibitors of these activities. Because the entire cell population can be transduced, the effect of the introduced gene product on endogenous biochemical activities can be assayed. A further advantage of adenoviral-mediated gene delivery is that multiple gene products can be simultaneously introduced by cotransduction with different recombinant viruses, and expression levels can be manipulated by varying the MOIs. Finally, this system avoids the need for clonal expansion and thus allows one to express and compare the activities of different gene products in genetically identical populations of cells.
Alternative approaches that increase adenoviral-mediated gene delivery to T cells include modification of the viral fiber protein to allow virus binding to cellular heparin sulfate receptors (27) or incorporation of a FLAG epitope into adenovirus together with the application of a bispecific antibody to FLAG and the T cell surface antigen CD3 (28). However, these approaches require the modification of the adenoviral vector and are less efficient than the approach presented here. Although retrovirus vectors also have been extensively used for gene delivery into lymphocyte cells (15, 29, 30), at times with efficiencies approaching 100% (31), experiments involving retroviral transduction of lymphocytes often are limited by the difficulty in transducing the majority of the cells (32) and the requirement, at least for nonlentivirus vectors, that the cells undergo mitosis for viral integration and expression (33). Other methods of gene transfer, such as transient transfection and stable cell lines, have been successfully used to study gene function in lymphocytes. However, transient transfection is relatively inefficient and endogenous activities affected by the introduced gene cannot be examined. Stable transfected cell lines entail the selection of cells that not only survive the antibiotic selection, but that also can tolerate the expression of the introduced gene. Even when inducible gene expression is used, it may be difficult to compare two different selected populations, either because of selection for cells that tolerate leaky transgene expression, or the inherent instability and heterogeneity of established lymphocyte cell lines. These problems are particularly relevant if the introduced gene product inhibits cell growth or promotes differentiation or apoptosis.
The cell lines generated in this paper should prove quite valuable, particularly for the study of apoptotic pathways. The FL5.12 and Ba/F3 cell lines have been used extensively as models for IL-3-dependent survival, as these cells rapidly undergo apoptosis after IL-3 withdrawal (34). Adenoviral-mediated gene delivery could be used to introduce inhibitors of suspected survival pathway components in the presence of IL-3, followed by assays for apoptosis as well as biochemical activities thought to be either upstream or downstream of the inhibited activity. Alternatively, viral gene delivery of survival pathway components (such as Bcl-2 and Akt) could determine the ability of these activities to abrogate cell death in the absence of IL-3. The DPK.C7 cell line is a CD4+CD8+ thymoma cell line that bears the AND T cell receptor, and undergoes apoptosis in response to presentation by I-Ek of a specific peptide from pigeon cytochrome c (22). This apoptosis is thought to mimic thymic negative selection, and adenoviral-mediated gene delivery will be useful in dissecting this cell death pathway.
In addition to allowing gene transfer into lymphocyte cell lines, the expression of CAR in mice should facilitate gene transfer either in vivo or ex vivo in primary cell culture. We currently are creating transgenic mice that express hCAR or CARΔ1 from the proximal Lck promoter, which directs expression primarily in T cells. CAR transgenic mice should facilitate either the in vivo delivery of genes into T cells by the inoculation of adenovirus recombinants into lymphoid organs or the ex vivo delivery of genes into transgenic T cells followed by their introduction into a recipient mouse. These mice also could be used to test the efficacy of proposed gene therapies, which could include the introduction of cytokine genes that promote tumor killing by cytotoxic T cells or the delivery of antiviral genes into virus-infected lymphocytes.
The Role of CAR in Virus Entry.
The fact that the expression of a hCAR mutant that lacks the entire cytoplasmic domain allows adenoviral entry supports a role for CAR as a primary binding site for interaction with the group C adenoviral fiber protein, which does not directly lead to endocytosis. The interaction of the fiber protein with CAR instead may facilitate the interaction of the RGD motif containing viral penton base proteins, surrounding the fiber protein, with particular cellular integrins (such as αVβ3, αVβ5, or αMβ2; refs. 2 and 7), which then leads to endocytosis via the formation of clathrin-coated pits (2, 35). In support of this model, virus entry mediated by the fiber protein has been shown to depend on penton base interaction with cellular integrin (2). In addition, mitogen-mediated up-regulated expression of αv integrin can allow some adenoviral entry (up to 15%) into human peripheral monocytes and T lymphocytes that is independent of the interaction of the fiber protein with CAR (6, 7). Although we cannot detect expression of αV or αM integrins on EL-4 cells, adenoviral transduction of hCAR-expressing EL-4 cells is inhibited by RGD containing peptides, indicating the requirement for viral penton base interaction with some RGD-recognizing integrin. Nonetheless, it appears that insufficient expression of CAR primarily accounts for the inefficient transduction of lymphocytes by adenovirus, because CAR expression enhances the susceptibility of either Chinese hamster ovary cells (8) or lymphocytes (this work) to adenoviral transduction much more dramatically than integrin expression in either cell type (6, 7). That the cytoplasmic domain of CAR is dispensable for virus transduction not only contributes to our understanding of virus entry, but from a practical standpoint, CARΔ1 may be attenuated in its normal function in the cell, and as such may have less of an effect on cell physiology when exogenously expressed. This observation is particularly important given the absence of any obvious catalytic domains in the cytoplasmic domain of CAR and our lack of understanding of the normal cellular role of the CAR protein.
Adenoviral-Mediated Expression of FasL in Lymphocytes Efficiently Promotes Apoptosis.
FasR and FasL are involved in the down-regulation of T cell-mediated immune responses (36). FasR is expressed at low levels on resting T cells but is rapidly up-regulated upon engagement of the T cell receptor (36, 37). Prolonged activation of the T cells results in FasR becoming functional, such that crosslinking by FasL results in apoptosis. Understanding of the role of FasL-mediated killing of activated lymphocytes in immune regulation should be greatly facilitated by the ability to efficiently transduce lymphocytes with recombinant adenovirus encoding FasL.
In addition to being expressed by activated lymphocytes, FasL is expressed in “immune-privileged” tissue, including testis, cornea, and many tumor cells (38). These observations have led to the desire to use FasL to generate artificial immune-privileged tissue for transplant purposes and to ameliorate T cell-mediated inflammatory disease (39). Adenovirus-encoding FasL has been used for this purpose with limited success (40, 41). The availability of transgenic tissue expressing CAR could lead to enhanced application of FasL-based immunosuppression.
Acknowledgments
This work was made possible with the excellent assistance of Karen Helm, Pat Schor, and Mike Ashton of the Cancer Center Flow Cytometry Core, which is supported by National Institutes of Health Grant 2 P30 CA 46934-09. We also thank J. Bergelson and R. Finberg for their generous gift of the CAR cDNAs and the RmcB monoclonal, Mark Boothby for the gift of the DPK.C7 cell line, and Gustavo Leone for his intellectual contribution to the genesis of this project. J.D. is supported by grants from the V Foundation, the Howard Hughes Medical Institute, and National Institutes of Health Grant RO1 CA77314-01; S.P.H. by a Professional Development Award from the Children’s Hospital Research Institute (Denver) and a grant from the Leukemia Research Foundation; R.C.D. by U.S. Public Health Service-National Institutes of Health Grants AI40394 and AI40607; J.S. by National Institutes of Health Grant HL58344; and T.H. by a grant from the Cancer League of Colorado and U.S. Public Health Service-National Institutes of Health Grant AI07405.
ABBREVIATIONS
- CAR
coxsackievirus and adenovirus receptor
- hCAR
human CAR
- mCAR
mouse CAR
- MOI
multiplicity of infection
- GFP
green fluorescent protein
- FasL
Fas ligand
- FasR
Fas receptor
- FBS
fetal bovine serum
- Ad-FasL
adenovirus-expressing mouse FasL
- Ad-CON
control recombinant adenovirus
- CMV
cytomegalovirus
- IL
interleukin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Nevins J R, DeGregori J, Jakoi L, Leone G. Methods Enzymol. 1997;283:205–219. doi: 10.1016/s0076-6879(97)83017-0. [DOI] [PubMed] [Google Scholar]
- 2.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 3.DeMatteo R P, Raper S E, Ahn M A, Fisher K J, Burke C, Radu A, Widera G, Claytor B R, Barker C F, Markmann J F. Ann Surg. 1997;222:229–242. doi: 10.1097/00000658-199509000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neering S J, Hardy S F, Minamoto D, Spratt S K, Jordan C T. Blood. 1996;88:1147–1155. [PubMed] [Google Scholar]
- 5.Chu Y, Sperber K, Mayer L, Hsu M T. Virology. 1992;188:793–800. doi: 10.1016/0042-6822(92)90534-v. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Endo R I, Nemerow G R. J Virol. 1997;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 9.Bergelson J M, Krithivas A, Celi L, Droguett G, Marshall H S, Wickham T J, Crowell R L, Finberg R W. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomko R, Ruliang X, Philipson L. Proc Natl Acad Sci USA. 1998;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller A D, Rosman G J. BioTechniques. 1989;7:980–988. [PMC free article] [PubMed] [Google Scholar]
- 12.Old L J, Boyse E A, Stockert E. Cancer Res. 1965;25:813–819. [PubMed] [Google Scholar]
- 13.Kaye J, Ellenberger D L. Cell. 1992;71:423–435. doi: 10.1016/0092-8674(92)90512-b. [DOI] [PubMed] [Google Scholar]
- 14.Logan J, Nicolaus J C, Topp W C, Giarad M, Shenk T, Levine A J. Virology. 1981;115:419–422. doi: 10.1016/0042-6822(81)90126-4. [DOI] [PubMed] [Google Scholar]
- 15.Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Lanfrancone L, Peschle C, Nolan G P, Pelicci P G. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- 16.Schwarz J K, Bassing C H, Kovesdi I, Datto M B, Blazing M, George S, Wang X-F, Nevins J R. Proc Natl Acad Sci USA. 1995;92:483–487. doi: 10.1073/pnas.92.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duke R, Chervenak R, Cohen J. Proc Natl Acad Sci USA. 1983;80:6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooke R, Waltzinger C, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 19.Hsu K-H L, Lonberg-Holm K, Alstein B, Crowell R L. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma I, Somia N. Nature (London) 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 21.McKearn J P, McCubrey J A, Fagg B. Proc Natl Acad Sci USA. 1985;82:7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalik J, Ansari B, Boothby M. J Immunol. 1996;157:5290–5299. [PubMed] [Google Scholar]
- 23.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 24.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 25.Fraser A, Evan G. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 26.Rouvier E, Luciani M F, Golstein P. J Exp Med. 1998;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham T J, Tzeng E, Shears L L, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staal F J, Bakker A Q, Verkuijlen M, van Oort E, Spits H. Cancer Gene Ther. 1996;3:345–351. [PubMed] [Google Scholar]
- 30.Bunnell B A, Muul L M, Donahue R E, Blaese R M, Morgan R A. Proc Natl Acad Sci USA. 1995;92:7739–7743. doi: 10.1073/pnas.92.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crompton T, Gilmour K C, Owen M J. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 32.Reimann J, Heeg K, Wagner H, Keller G, Wagner E F. J Immunol Methods. 1986;89:93–101. doi: 10.1016/0022-1759(86)90036-0. [DOI] [PubMed] [Google Scholar]
- 33.Miller D G, Adam M A, Miller A D. Mol Cell Biol. 1992;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunez G, London L, Hockenberry D, Alexander M, McKearn J P, Korsmeyer S J. J Immunol. 1990;144:3602–3610. [PubMed] [Google Scholar]
- 35.Greber U F, Willetts M, Webster P, Helenius A. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 36.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 37.Duke, R. C., Newell, E., Schleicher, M., Meech, S. & Bellgrau, D. (1998) Transplantation Proc., in press. [DOI] [PubMed]
- 38.Green D R, Ware C F. Proc Natl Acad Sci USA. 1997;94:5986–5990. doi: 10.1073/pnas.94.12.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Yang Y, Horton J L, Samoilova E B, Judge T A, Turka L A, Wilson J M, Chen Y. J Clin Invest. 1997;100:1951–1957. doi: 10.1172/JCI119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swenson K M, Ke B, Wang T, Markowitz J S, Maggard M A, Spear G S, Imagawa D K, Goss J A, Busuttil R W, Seu P. Transplantation. 1998;65:155–160. doi: 10.1097/00007890-199801270-00002. [DOI] [PubMed] [Google Scholar]