Abstract
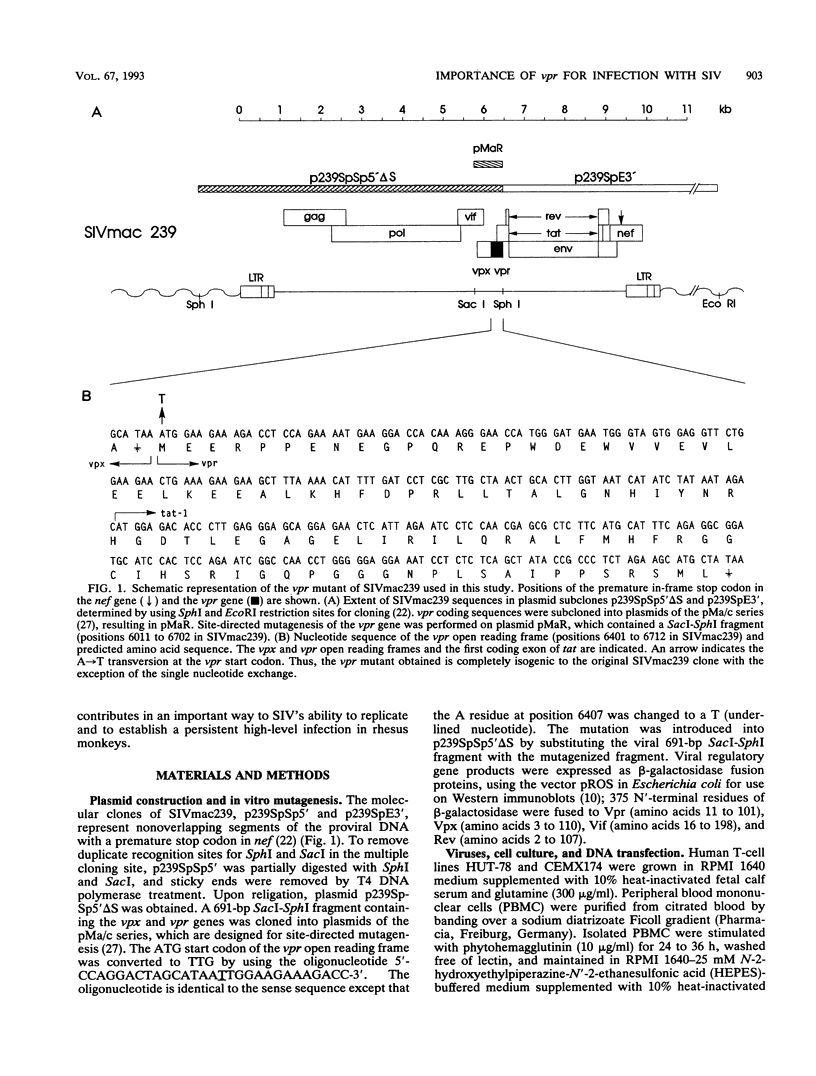
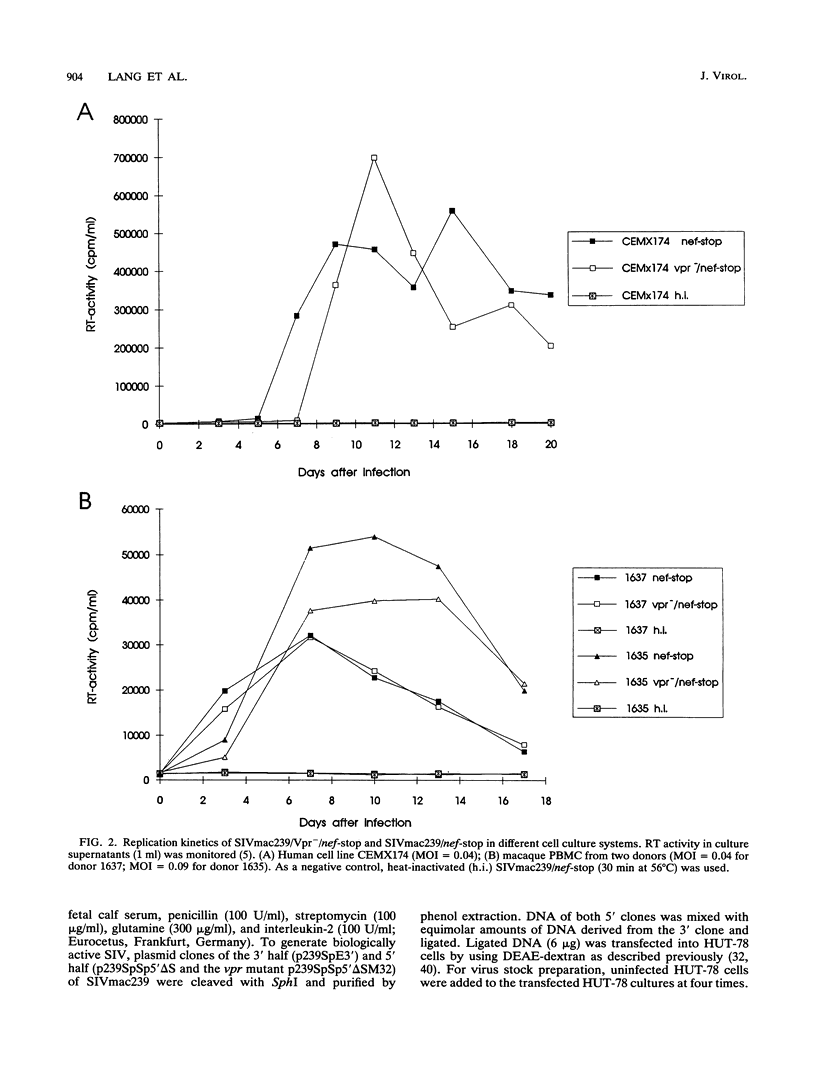
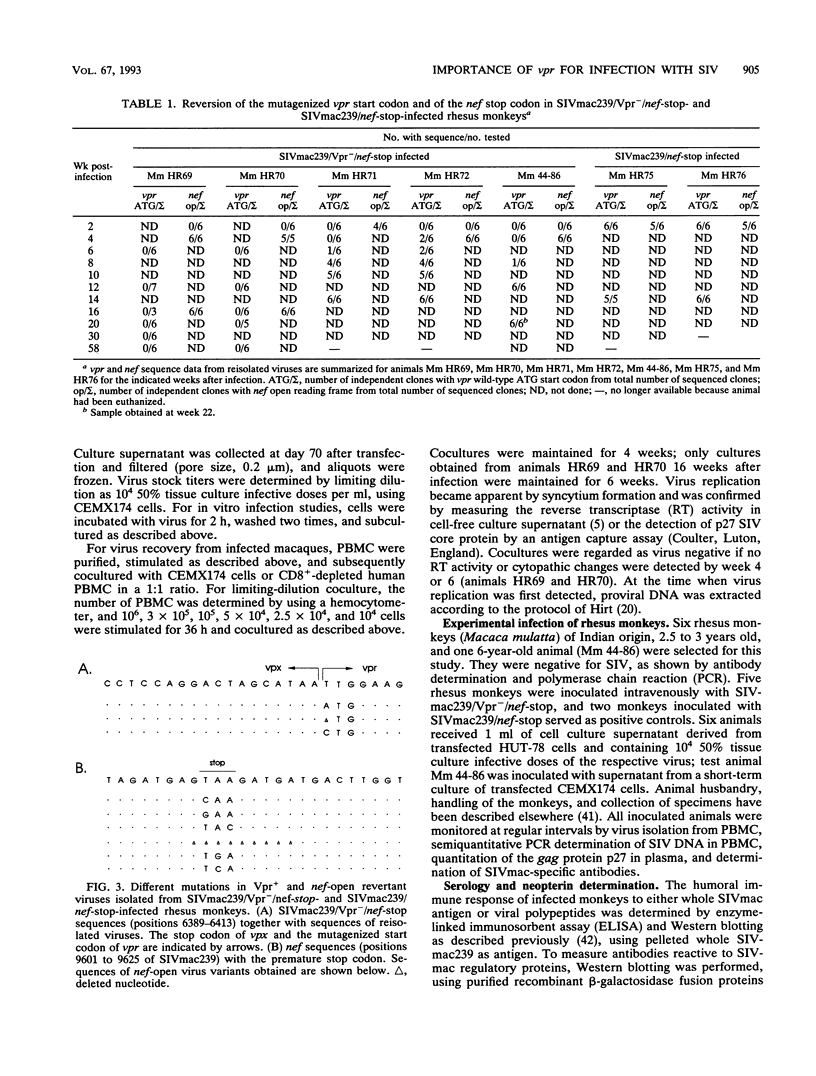
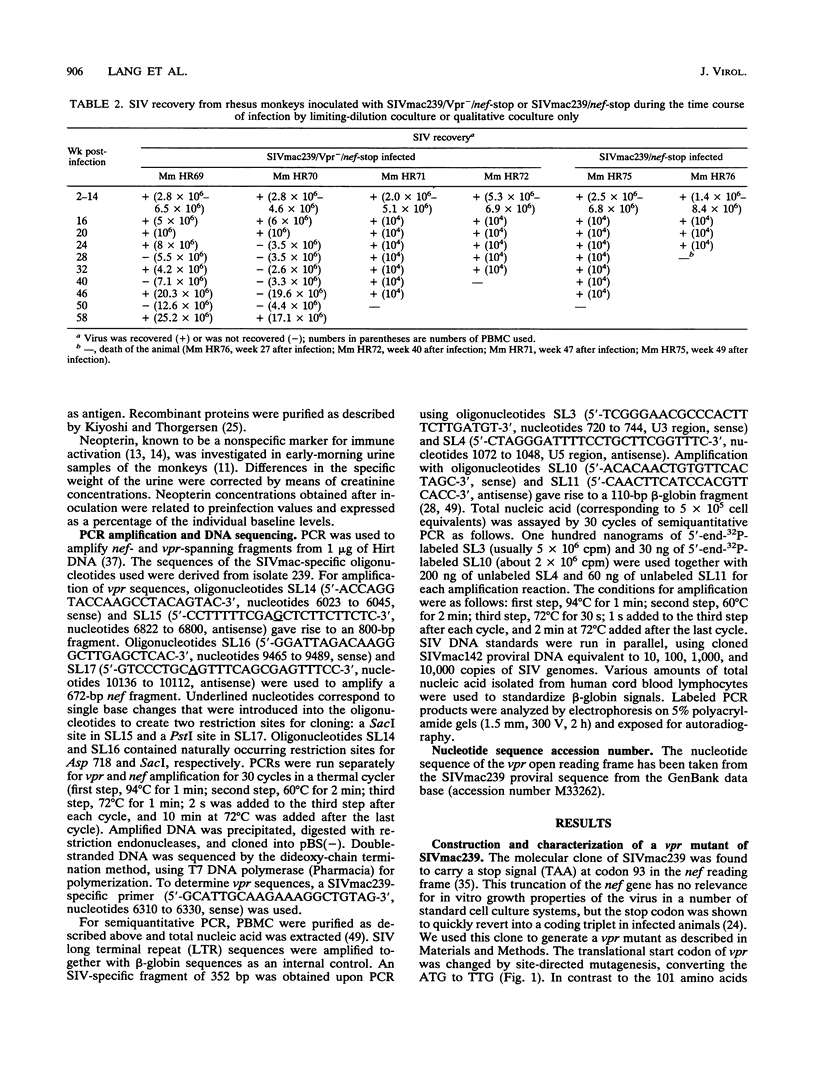
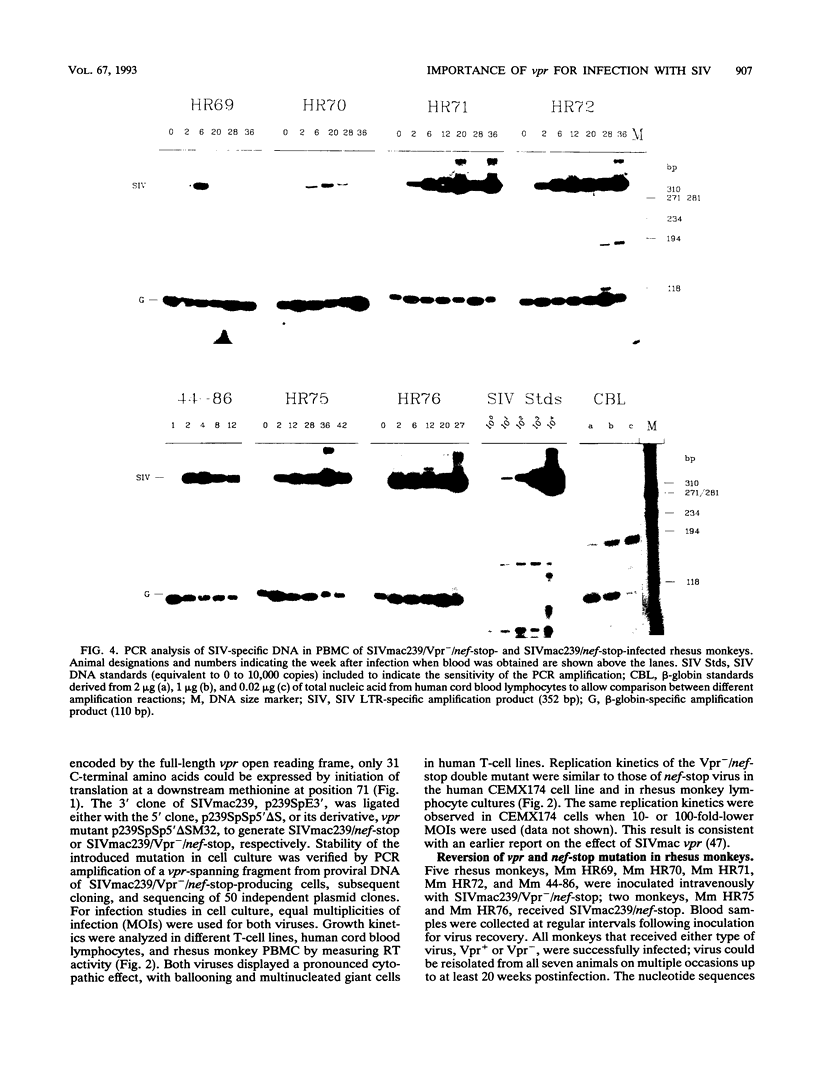
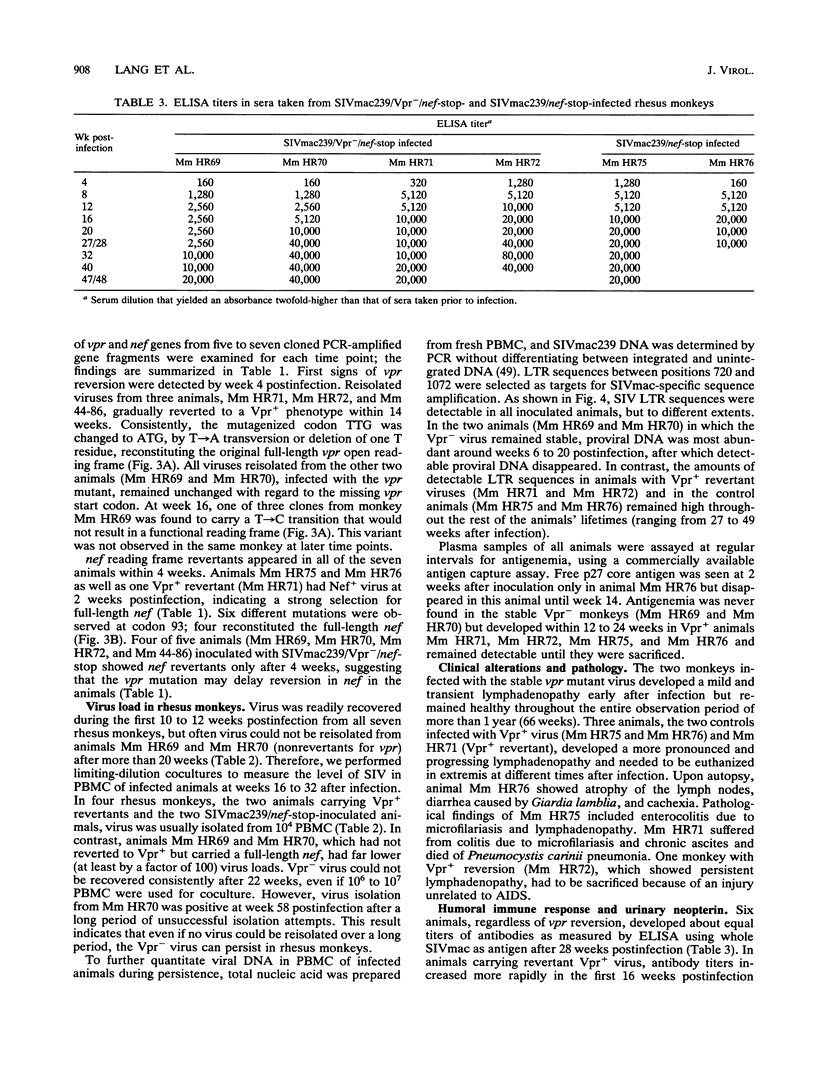
The importance of the vpr gene for simian immunodeficiency virus (SIV) replication, persistence, and disease progression was examined by using the infectious pathogenic molecular clone called SIVmac239. The ATG start codon of the vpr gene was converted to TTG by site-specific mutagenesis. The constructed Vpr- mutant virus is identical with the parental SIVmac239/nef-stop virus with the exception of this one nucleotide. These viruses replicated with similar kinetics and to similar extents in rhesus monkey lymphocyte cultures and in the human CEMX174 cell line. Five rhesus monkeys were inoculated with the Vpr- variant of SIVmac239/nef-stop, and two monkeys received SIVmac239/nef-stop as controls. Both controls showed reversion of the TAA stop signal in nef by 2 weeks postinfection, as has been observed previously. Reversion of the TAA stop codon in nef also occurred in the five monkeys that received the Vpr- variant, but reversion was delayed on average to about 4 weeks. Thus, the mutation in vpr appeared to delay the rapidity with which reversion occurred in the nef gene. Reversion of the TTG sequence in vpr to ATG was observed in three of the five test animals. Reversion in vpr was first observed in these three animals 4 to 8 weeks postinfection. No vpr revertants were found over the entire 66 weeks of observation in the other two test animals that received the vpr mutant. Antibodies to vpr developed in those three animals in which reversion of vpr was documented, but antibodies to vpr were not observed in the two animals in which reversion of vpr was not detected. Antibody responses to gag and to whole virus antigens were of similar strength in all seven animals. Both control animals and two of the test animals in which vpr reverted maintained high virus loads and developed progressive disease. Low virus burden and no disease have been observed in the two animals in which vpr did not revert and in the one animal in which vpr reversion was first detected only at 8 weeks. The reversion of vpr in three of the five test animals indicates that there is significant selective pressure for functional forms of vpr in vivo. Furthermore, the results suggest that both vpr and nef are important for maximal SIV replication and persistence in vivo and for disease progression.
Full text
PDF
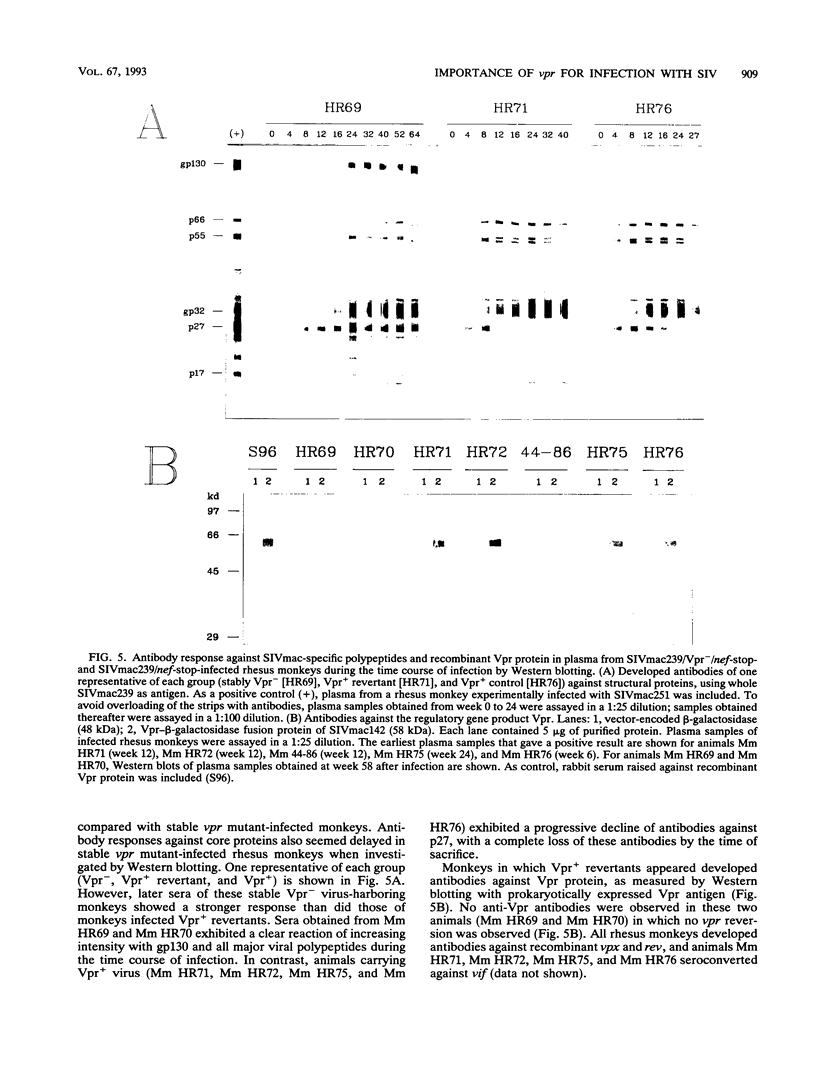
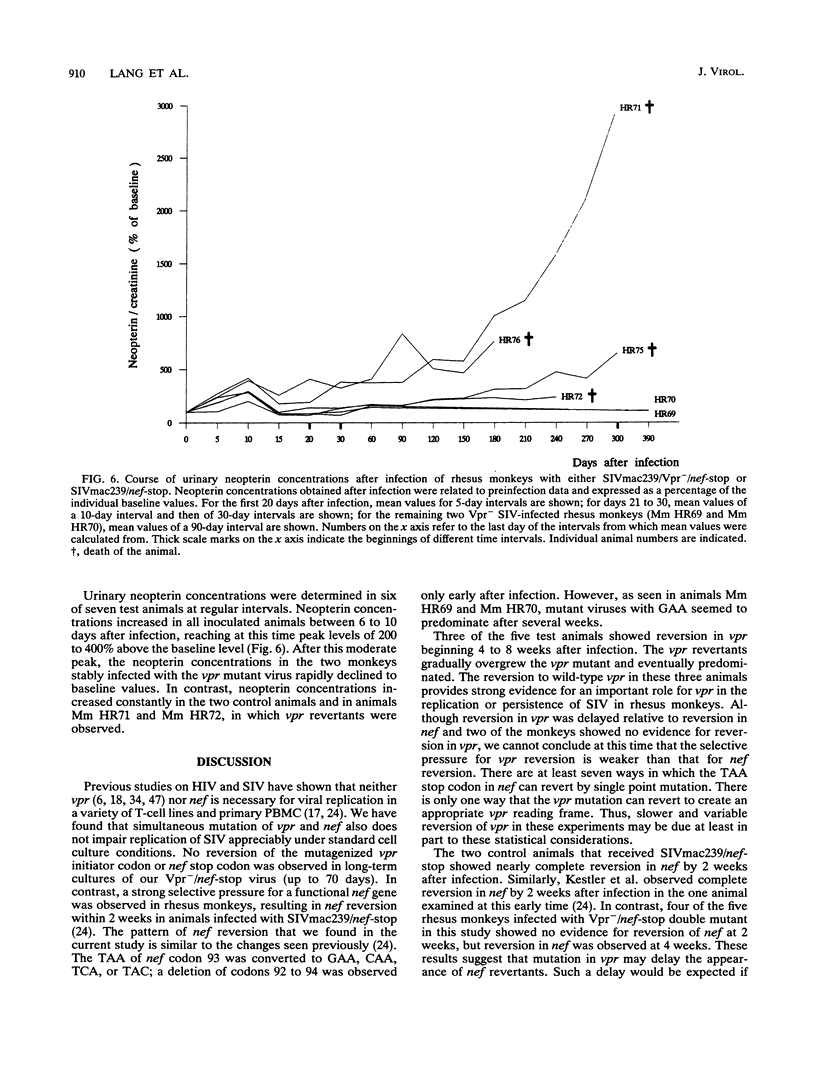


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Baskin G. B., Murphey-Corb M., Watson E. A., Martin L. N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol. 1988 Nov;25(6):456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Dehni G., Sodroski J. G., Haseltine W. A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990 Jun;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Greene W. C. Functions of the auxiliary gene products of the human immunodeficiency virus type 1. Virology. 1990 Sep;178(1):1–5. doi: 10.1016/0042-6822(90)90373-y. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Dedera D., Hu W., Vander Heyden N., Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989 Jul;63(7):3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Ringler D. J. Use of simian immunodeficiency viruses for AIDS research. Intervirology. 1989;30(6):301–312. doi: 10.1159/000150108. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Embretson J. E., Anderson D. C., Mullins J. I., Fultz P. N. Sequence analysis and acute pathogenicity of molecularly cloned SIVSMM-PBj14. Nature. 1990 Jun 14;345(6276):636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- Ellinger S., Glockshuber R., Jahn G., Plückthun A. Cleavage of procaryotically expressed human immunodeficiency virus fusion proteins by factor Xa and application in western blot (immunoblot) assays. J Clin Microbiol. 1989 May;27(5):971–976. doi: 10.1128/jcm.27.5.971-976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich C., Lüke W., Stahl-Hennig C., Herchenröder O., Fuchs D., Wachter H., Hunsmann G. Urinary neopterin concentrations in rhesus monkeys after infection with simian immunodeficiency virus (SIVmac 251). AIDS. 1989 May;3(5):305–307. doi: 10.1097/00002030-198905000-00010. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Albert J., Asjö B., Fenyö E. M., Reibnegger G., Wachter H. Association between serum neopterin concentrations and in vitro replicative capacity of HIV-1 isolates. J Infect Dis. 1989 Oct;160(4):724–725. doi: 10.1093/infdis/160.4.724. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Hausen A., Reibnegger G., Werner E. R., Dierich M. P., Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988 May;9(5):150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Montagnier L., Peden K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 1989 Apr;8(4):1169–1175. doi: 10.1002/j.1460-2075.1989.tb03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes S. R., Dixon E. P., Malim M. H., Cullen B. R., Greene W. C. Nef protein of human immunodeficiency virus type 1: evidence against its role as a transcriptional inhibitor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Olmsted R. A., Murphey-Corb M., Purcell R. H., Johnson P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989 Jun 1;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huet T., Cheynier R., Meyerhans A., Roelants G., Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990 May 24;345(6273):356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Li Y., Naidu Y. M., Butler C. V., Ochs M. F., Jaenel G., King N. W., Daniel M. D., Desrosiers R. C. Comparison of simian immunodeficiency virus isolates. Nature. 1988 Feb 18;331(6157):619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Klimkait T., Strebel K., Hoggan M. D., Martin M. A., Orenstein J. M. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol. 1990 Feb;64(2):621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Cheng-Mayer C., Levy J. A. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Milman G., Herzberg M. Efficient DNA transfection and rapid assay for thymidine kinase activity and viral antigenic determinants. Somatic Cell Genet. 1981 Mar;7(2):161–170. doi: 10.1007/BF01567655. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Shibata R., Kiyomasu T., Higuchi I., Kishida Y., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989 Sep;63(9):4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Reiss P., Lange J. M., de Ronde A., de Wolf F., Dekker J., Danner S. A., Debouck C., Goudsmit J. Antibody response to viral proteins U (vpu) and R (vpr) in HIV-1-infected individuals. J Acquir Immune Defic Syndr. 1990;3(2):115–122. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sakai K., Ma X. Y., Gordienko I., Volsky D. J. Recombinational analysis of a natural noncytopathic human immunodeficiency virus type 1 (HIV-1) isolate: role of the vif gene in HIV-1 infection kinetics and cytopathicity. J Virol. 1991 Nov;65(11):5765–5773. doi: 10.1128/jvi.65.11.5765-5773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Hennig C., Herchenröder O., Nick S., Evers M., Stille-Siegener M., Jentsch K. D., Kirchhoff F., Tolle T., Gatesman T. J., Lüke W. Experimental infection of macaques with HIV-2ben, a novel HIV-2 isolate. AIDS. 1990 Jul;4(7):611–617. doi: 10.1097/00002030-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C., Voss G., Nick S., Petry H., Fuchs D., Wachter H., Coulibaly C., Lüke W., Hunsmann G. Immunization with tween-ether-treated SIV adsorbed onto aluminum hydroxide protects monkeys against experimental SIV infection. Virology. 1992 Feb;186(2):588–596. doi: 10.1016/0042-6822(92)90025-k. [DOI] [PubMed] [Google Scholar]
- Strebel K., Daugherty D., Clouse K., Cohen D., Folks T., Martin M. A. The HIV 'A' (sor) gene product is essential for virus infectivity. Nature. 1987 Aug 20;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- Terwilliger E., Sodroski J. G., Rosen C. A., Haseltine W. A. Effects of mutations within the 3' orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986 Nov;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H., Hasegawa A., Maki N., Fukasawa M., Miura T., Speidel S., Cooper R. W., Moriyama E. N., Gojobori T., Hayami M. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature. 1989 Oct 12;341(6242):539–541. doi: 10.1038/341539a0. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Chanda P. K., Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987 Spring;3(1):33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Yu X. F., Matsuda M., Essex M., Lee T. H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990 Nov;64(11):5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Matsuda Z., Matsuda M., Essex M., Lee T. H. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]