Abstract
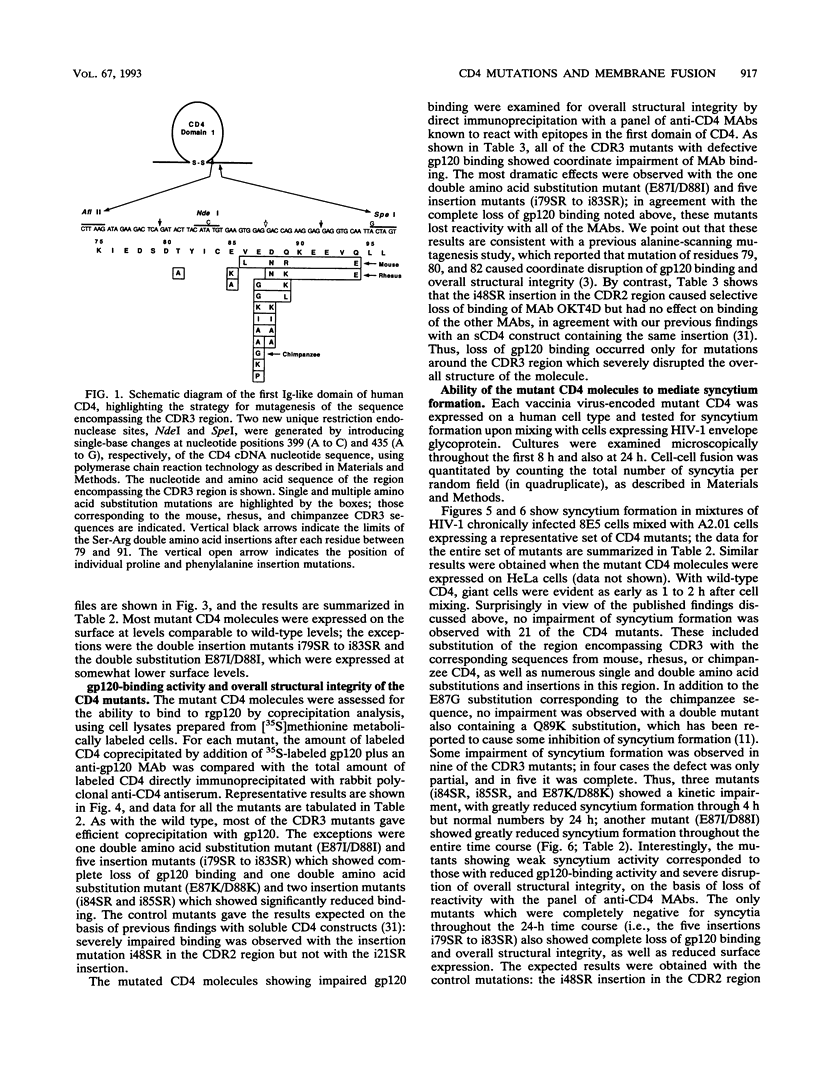
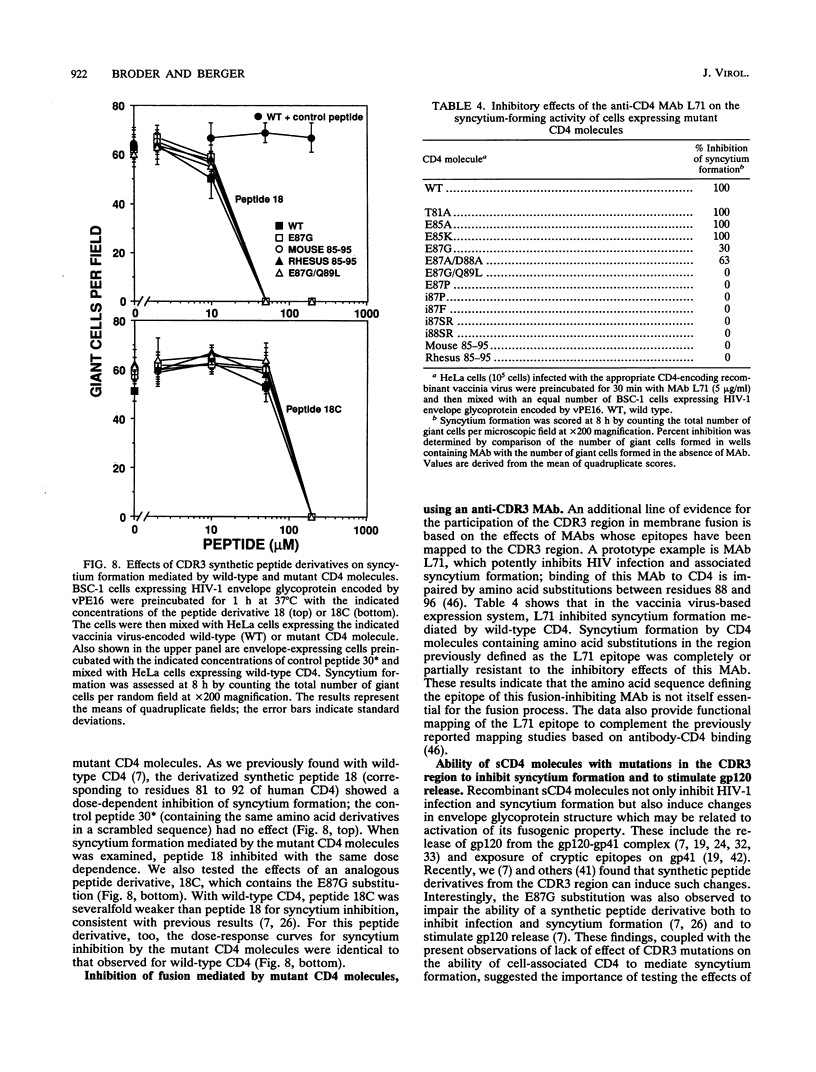
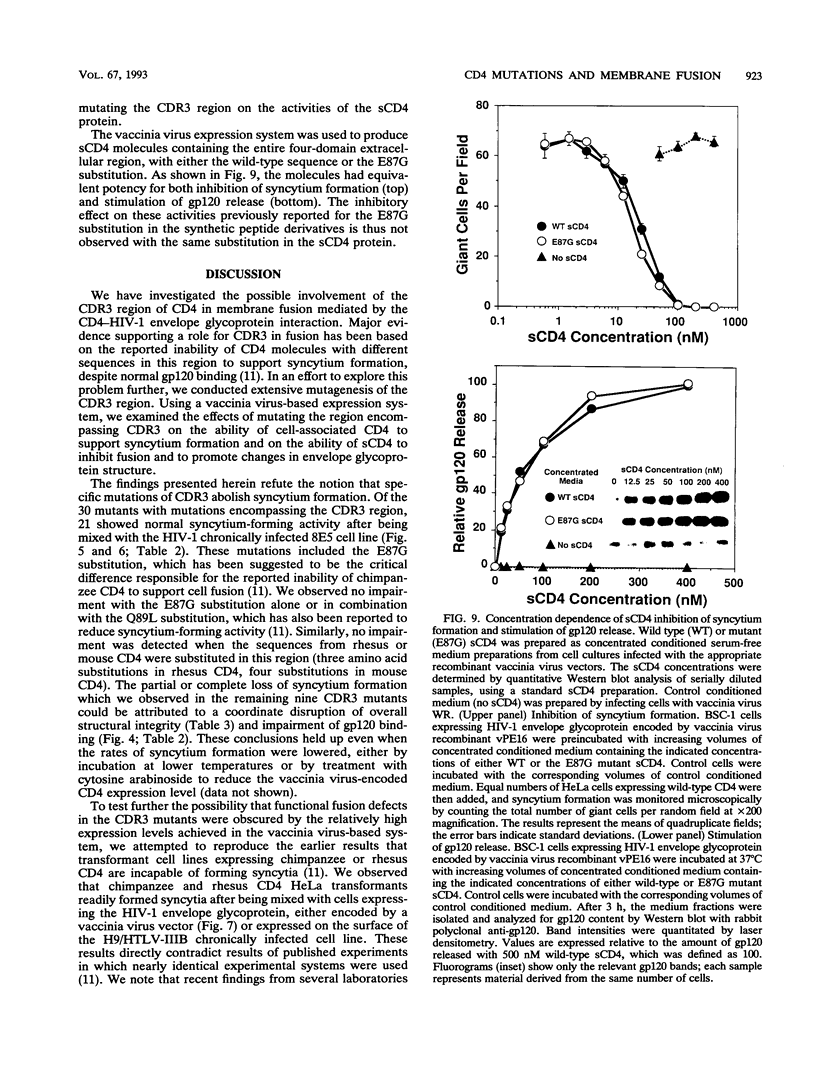
The third complementarity-determining region (CDR3) within domain 1 of the human CD4 molecule has been suggested to play a critical role in membrane fusion mediated by the interaction of CD4 with the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein. To analyze in detail the role of CDR3 and adjacent regions in the fusion process, we used cassette mutagenesis to construct a panel of 30 site-directed mutations between residues 79 and 96 of the full-length CD4 molecule. The mutant proteins were transiently expressed by using recombinant vaccinia virus vectors and were analyzed for cell surface expression, recombinant gp120-binding activity, and overall structural integrity as assessed by reactivity with a battery of anti-CD4 monoclonal antibodies. Cells expressing the CD4 mutants were assayed for their ability to form syncytia when mixed with cells expressing the HIV-1 envelope glycoprotein. Surprisingly in view of published data from others, most of the mutations had little effect on syncytium-forming activity. Normal fusion was observed in 21 mutants, including substitution of human residues 85 to 95 with the corresponding sequences from either chimpanzee, rhesus, or mouse CD4; a panel of Ser-Arg double insertions after each residue from 86 to 91; and a number of other charge, hydrophobic, and proline substitutions and insertions within this region. The nine mutants that showed impaired fusion all displayed defective gp120 binding and disruption of overall structural integrity. In further contrast with results of other workers, we observed that transformant human cell lines expressing native chimpanzee or rhesus CD4 efficiently formed syncytia when mixed with cells expressing the HIV-1 envelope glycoprotein. These data refute the conclusion that certain mutations in the CDR3 region of CD4 abolish cell fusion activity, and they suggest that a wide variety of sequences can be functionally tolerated in this region, including those from highly divergent mammalian species. Syncytium formation mediated by several of the CDR3 mutants was partially or completely resistant to inhibition by the CDR3-directed monoclonal antibody L71, suggesting that the corresponding epitope is not directly involved in the fusion process.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



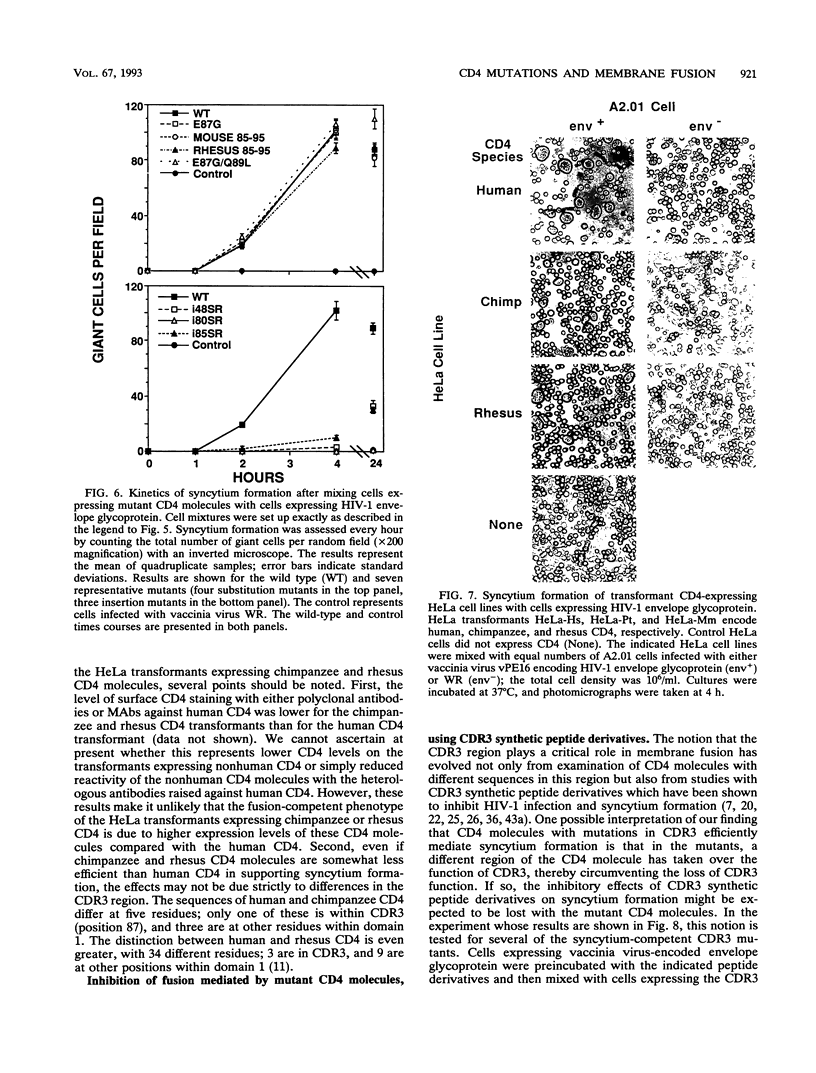



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthos J., Deen K. C., Chaikin M. A., Fornwald J. A., Sathe G., Sattentau Q. J., Clapham P. R., Weiss R. A., McDougal J. S., Pietropaolo C. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989 May 5;57(3):469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Presta L. G., Marsters S. A., Camerato T. R., Rosenthal K. A., Fendly B. M., Capon D. J. Mapping the CD4 binding site for human immunodeficiency virus by alanine-scanning mutagenesis. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7150–7154. doi: 10.1073/pnas.87.18.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashorn P. A., Berger E. A., Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990 May;64(5):2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinić D., Robey F. A. The V3 region of the envelope glycoprotein of human immunodeficiency virus type 1 binds sulfated polysaccharides and CD4-derived synthetic peptides. J Biol Chem. 1992 Apr 5;267(10):6664–6671. [PubMed] [Google Scholar]
- Berger E. A., Fuerst T. R., Moss B. A soluble recombinant polypeptide comprising the amino-terminal half of the extracellular region of the CD4 molecule contains an active binding site for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2357–2361. doi: 10.1073/pnas.85.7.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Lifson J. D., Eiden L. E. Stimulation of glycoprotein gp120 dissociation from the envelope glycoprotein complex of human immunodeficiency virus type 1 by soluble CD4 and CD4 peptide derivatives: implications for the role of the complementarity-determining region 3-like region in membrane fusion. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8082–8086. doi: 10.1073/pnas.88.18.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Ellens H., Hart T. K., Kirsh R. L. Soluble CD4 and dextran sulfate mediate release of gp120 from HIV-1: implications for clinical trials. J Acquir Immune Defic Syndr. 1991;4(9):923–924. [PubMed] [Google Scholar]
- Callahan L. N., Phelan M., Mallinson M., Norcross M. A. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J Virol. 1991 Mar;65(3):1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini D., Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the virus binding site. Cell. 1990 Mar 9;60(5):747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Ward R. H. The CD4-gp120 interaction and AIDS pathogenesis. Annu Rev Immunol. 1991;9:649–678. doi: 10.1146/annurev.iy.09.040191.003245. [DOI] [PubMed] [Google Scholar]
- Chao B. H., Costopoulos D. S., Curiel T., Bertonis J. M., Chisholm P., Williams C., Schooley R. T., Rosa J. J., Fisher R. A., Maraganore J. M. A 113-amino acid fragment of CD4 produced in Escherichia coli blocks human immunodeficiency virus-induced cell fusion. J Biol Chem. 1989 Apr 5;264(10):5812–5817. [PubMed] [Google Scholar]
- Earl P. L., Hügin A. W., Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990 May;64(5):2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden L. E., Lifson J. D. HIV interactions with CD4: a continuum of conformations and consequences. Immunol Today. 1992 Jun;13(6):201–206. doi: 10.1016/0167-5699(92)90154-Y. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Ikuta K., Fujii N., Ezawa K., Kato S. Inhibition of HIV-1 replication and syncytium formation by synthetic CD4 peptides. Arch Virol. 1989;105(1-2):129–135. doi: 10.1007/BF01311123. [DOI] [PubMed] [Google Scholar]
- Hillman K., Shapira-Nahor O., Gruber M. F., Hooley J., Manischewitz J., Seeman R., Vujcic L., Geyer S. J., Golding H. Chemically induced CD4 mutants of a human T cell line. Evidence for dissociation between binding of HIV I envelope and susceptibility to HIV I infection and syncytia formation. J Immunol. 1990 Mar 15;144(6):2131–2139. [PubMed] [Google Scholar]
- Kalyanaraman V. S., Rausch D. M., Osborne J., Padgett M., Hwang K. M., Lifson J. D., Eiden L. E. Evidence by peptide mapping that the region CD4(81-92) is involved in gp120/CD4 interaction leading to HIV infection and HIV-induced syncytium formation. J Immunol. 1990 Dec 15;145(12):4072–4078. [PubMed] [Google Scholar]
- Kirsh R., Hart T. K., Ellens H., Miller J., Petteway S. A., Jr, Lambert D. M., Leary J., Bugelski P. J. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1209–1212. doi: 10.1089/aid.1990.6.1209. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Hwang K. M., Nara P. L., Fraser B., Padgett M., Dunlop N. M., Eiden L. E. Synthetic CD4 peptide derivatives that inhibit HIV infection and cytopathicity. Science. 1988 Aug 5;241(4866):712–716. doi: 10.1126/science.2969619. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Rausch D. M., Kalyanaraman V. S., Hwang K. M., Eiden L. E. Synthetic peptides allow discrimination of structural features of CD4(81-92) important for HIV-1 infection versus HIV-1-induced syncytium formation. AIDS Res Hum Retroviruses. 1991 Jun;7(6):521–527. doi: 10.1089/aid.1991.7.521. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., McDougal J. S., Clapham P. R., Dalgleish A. G., Jamal S., Weiss R. A., Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988 Sep 9;54(6):865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Marsh M., Weiss R. A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Klatzmann D. R., Maddon P. J. CD4-gp120 interactions. Curr Opin Immunol. 1991 Aug;3(4):552–558. doi: 10.1016/0952-7915(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Mizukami T., Fuerst T. R., Berger E. A., Moss B. Binding region for human immunodeficiency virus (HIV) and epitopes for HIV-blocking monoclonal antibodies of the CD4 molecule defined by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9273–9277. doi: 10.1073/pnas.85.23.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Norton W. A., Sattentau Q. J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991 Mar;65(3):1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Nara P. L. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5 (Suppl 2):S21–S33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Hwang K. M., Rausch D. M., Lifson J. D., Eiden L. E. CD4 antigen-based antireceptor peptides inhibit infectivity of human immunodeficiency virus in vitro at multiple stages of the viral life cycle. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7139–7143. doi: 10.1073/pnas.86.18.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repke H., Gabuzda D., Palù G., Emmrich F., Sodroski J. Effects of CD4 synthetic peptides on HIV type I envelope glycoprotein function. J Immunol. 1992 Sep 1;149(5):1809–1816. [PubMed] [Google Scholar]
- Ryu S. E., Kwong P. D., Truneh A., Porter T. G., Arthos J., Rosenberg M., Dai X. P., Xuong N. H., Axel R., Sweet R. W. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 1990 Nov 29;348(6300):419–426. doi: 10.1038/348419a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Urdea M. S. Use of unpurified synthetic deoxynucleotide primers for rapid dideoxynucleotide chain termination sequencing. DNA. 1984 Aug;3(4):339–343. doi: 10.1089/dna.1.1984.3.339. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols D., Pauwels R., Desmyter J., De Clercq E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology. 1990 Apr;175(2):556–561. doi: 10.1016/0042-6822(90)90440-3. [DOI] [PubMed] [Google Scholar]
- Shapira-Nahor O., Golding H., Vujcic L. K., Resto-Ruiz S., Fields R. L., Robey F. A. CD4-derived synthetic peptide blocks the binding of HIV-1 GP120 to CD4-bearing cells and prevents HIV-1 infection. Cell Immunol. 1990 Jun;128(1):101–117. doi: 10.1016/0008-8749(90)90010-o. [DOI] [PubMed] [Google Scholar]
- Sinangil F., Loyter A., Volsky D. J. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 1988 Oct 24;239(1):88–92. doi: 10.1016/0014-5793(88)80551-9. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Truneh A., Buck D., Cassatt D. R., Juszczak R., Kassis S., Ryu S. E., Healey D., Sweet R., Sattentau Q. A region in domain 1 of CD4 distinct from the primary gp120 binding site is involved in HIV infection and virus-mediated fusion. J Biol Chem. 1991 Mar 25;266(9):5942–5948. [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]