Abstract
The membrane protein syntaxin participates in several protein–protein interactions that have been implicated in neurotransmitter release. To probe the physiological importance of these interactions, we microinjected into the squid giant presynaptic terminal botulinum toxin C1, which cleaves syntaxin, and the H3 domain of syntaxin, which mediates binding to other proteins. Both reagents inhibited synaptic transmission yet did not affect the number or distribution of synaptic vesicles at the presynaptic active zone. Recombinant H3 domain inhibited the interactions between syntaxin and SNAP-25 that underlie the formation of stable SNARE complexes in vitro. These data support the notion that syntaxin-mediated SNARE complexes are necessary for docked synaptic vesicles to fuse.
Keywords: exocytosis, botulinum toxin, vesicle docking, SNAREs, transmitter release
Synaptic transmission is based on the rapid exocytosis of neurotransmitters from synaptic vesicles (1). Exocytosis occurs at active zones, where vesicles are docked for Ca2+-triggered fusion with the presynaptic plasma membrane (2). The exocytotic event is coupled to an endocytotic cascade that recycles fused synaptic vesicles for additional rounds of regulated fusion (3). Several proteins of the synaptic vesicle, plasma membrane, and presynaptic cytosol have been implicated in neurotransmitter release (4, 5). Syntaxin-1A, a neuronal homologue of a family of proteins involved in membrane fusion (6–8), plays a key role in exocytosis. First, this plasma membrane protein is a substrate of botulinum C1 (Bot-C1), a toxin that inhibits transmitter release by the proteolytic activity of its disulfide-linked light chain (9). Second, Drosophila lacking syntaxin suffer embryonic lethality and a loss of synaptic transmission (10, 11).
In vitro, syntaxin 1A participates in a number of interactions that are believed to be essential for neurotransmitter release. Syntaxin-binding partners include Ca2+ channels, complexin, and synaptotagmin, a putative Ca2+ receptor for transmitter release, as well as mammalian homologues of the Unc13 and sec−1 protein families (4, 5, 12). Syntaxin also binds SNAP-25 (synaptosomal associated protein of 25 kDa), a peripheral plasma membrane protein, and synaptobrevin, a major integral membrane protein of synaptic vesicles (13, 14). Together these proteins form a stable ternary complex of 7S (15) that resists dissociation by SDS (14). This 7S complex has been proposed to mediate the docking and fusion of synaptic vesicles at the presynaptic plasma membrane, because it binds the fusion catalyzing proteins SNAP (soluble NSF attachment protein) and NSF (for N-ethylmaleimide-sensitive fusion protein) (15, 16). The resulting higher-order SNARE (SNAP receptor) complex of 20S dissociates upon hydrolysis of ATP by NSF, and this dissociation is thought to promote membrane fusion (15–17).
To investigate how syntaxin’s interactions with presynaptic proteins contribute to the trafficking events that underlie neurotransmitter release, we have perturbed syntaxin function in vivo by microinjecting Bot-C1 and recombinant fragments of syntaxin into the presynaptic terminal of the squid giant synapse. Our results indicate that syntaxin binding to SNAP-25, and subsequent formation of the SNARE complex, are essential for transmitter release. Further, we provide ultrastructural evidence that SNARE complex formation does not dock synaptic vesicles at the presynaptic plasma membrane but is required for downstream reactions that yield vesicle fusion.
MATERIALS AND METHODS
Cloning of a Squid Syntaxin cDNA.
A syntaxin probe, generated from squid optic lobe cDNA by PCR using degenerate primers corresponding to the codons of amino acids 36–42 and 255–261 of rat syntaxin 1A (6), was used to screen a squid λgt10 stellate ganglion cDNA library (1.5 × 106 pfu) under high-stringency conditions. After subcloning, the longest hybridization-positive clone was sequenced in both directions.
Recombinant Proteins.
The cDNA used in the production of recombinant proteins were generated by PCR using squid (sq) synaptobrevin (18), squid syntaxin (this study), and bovine syntaxin 1A (19) cDNA as templates. Fragments encoding sq-synaptobrevin (2–98), sq-syntaxin (2–265), TAX74 (195–268), TAX86 (180–265), and TAX50 (216–265) (numbers indicate native amino acid positions) were subcloned into pQE-9 (Qiagen) or pRSET A (Novagen) vectors to add an N-terminal His6-tag to the expressed proteins. A construct encoding the light chain of Bot-C1 with an N-terminal His6-tag was kindly provided by T. Binz and H. Niemann (University of Hannover, Germany). Recombinant proteins produced in the X-L1 Blue or BL21 (DE3) Escherichia coli strains were purified either as described (20) or by extracting bacteria with 5–8 M urea in 50 mM Tris/100 mM KCl, pH 8.0. Urea extracts were loaded onto Ni-agarose (Qiagen) or Zn-charged columns (Pharmacia) before washing and stepwise removal of urea to allow renaturation of bound protein. Proteins were eluted from the Zn2+ columns by 50 mM EGTA and from Ni2+ columns by 200 mM imidazole.
Overlay Assay.
Recombinant proteins (200 μg/ml) were biotinylated in 50 mM Mops, pH 7.8, by incubation (1 h) with biotinyl-aminocaproic acid-succinimidylester (Fluka) at a 100-fold molar excess. The reaction was quenched with 100 mM glycine and dialyzed against phosphate-buffered saline. Immobilon-P membranes blotted with squid optic lobe proteins were blocked in 10 mM Tris⋅HCl, pH 7.5/100 mM MgCl2/0.5% (wt/vol) Tween-20/1% (wt/vol) Triton X-100/1% (wt/vol) BSA/5% (vol/vol) fetal calf serum before incubation with biotinylated proteins (0.2–5 μg/ml). After incubation with peroxidase-coupled anti-biotin antibodies (1:2,000; Sigma) for 1 h at room temperature, the membranes were washed and bound biotinylated proteins were detected using enhanced chemiluminescence (Amersham). In some experiments the strips were washed in 1% (wt/vol) SDS for 15 min at room temperature before incubation with anti-biotin antibodies.
Biochemical Methods.
SNARE complex intermediates were generated in vitro and analyzed by immunoprecipitation and immunoblotting as described previously (20). To assess the inhibitory effect of H3 domain fragments, Triton X-100 extracts (200 μg protein) were preincubated for 30 min at 4°C with or without 40 μg of TAX86, equivalent to a 40-fold molar excess over immunologically estimated endogenous syntaxin. Protein concentrations were determined using the Dc protein assay (Bio-Rad) or Coomassie staining after SDS/PAGE.
Physiological Analysis of Neurotransmitter Release.
Electrophysiological measurements of synaptic transmission and microinjection into the presynaptic terminal of the squid giant synapse were performed as described previously (18, 21). Solutions containing recombinant proteins (1–2 mg/ml) or Bot-C1 holotoxin (2 μM prereduced with 2 mM DTT) were centrifuged before microinjection. Occasionally, microinjection solutions contained 2 mM 2-mercaptoethanol, 2 mM DTT, 200 mM imidazole, or 50 mM EGTA, reagents whose effects were monitored by control buffer injections. Data were acquired and analyzed with software from T. Goldthorpe (University of Toronto) or F. Schweizer (Duke University, Durham, NC).
Electron Microscopy.
Nerve terminals were processed for electron microscopy as detailed previously (18, 21). Sections were imaged in a JEOL electron microscope and photographed. For each treatment group, >200 active zone profiles from two terminals were analyzed with image-1 software (Universal Imaging, Philadelphia).
RESULTS
Molecular Characterization of Squid Syntaxin.
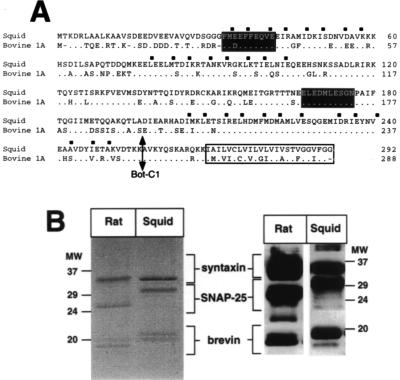
To investigate the in vivo functions of syntaxin, we first cloned cDNAs encoding its squid homologue. The deduced ORF (Fig. 1A) predicts a protein of 35 kDa with a C-terminal transmembrane region. Squid syntaxin shows greatest homology to syntaxin 1A, the major mammalian brain isoform (6, 8). Bovine syntaxin 1A and squid syntaxin are 71% identical (Fig. 1A), with regions of higher homology in the three putative coiled-coil helices H1–H3 (13, 22). A monoclonal antibody (mAb 6D2) that recognizes vertebrate syntaxins (22) revealed an immunoreactive band of 34 kDa in rat, squid, Drosophila, and crayfish neural membranes (Fig. 1B; data not shown).
Figure 1.
Molecular characterization of squid syntaxin. (A) Alignment of the deduced amino acid sequences of squid syntaxin and bovine syntaxin 1A. The hydrophobic residues defining predicted coiled-coil domains H1-H3 (7) are indicated by squares. Black shading shows the predicted toxin binding sites (24), and an arrow marks the Bot-C1 cleavage site (23). The putative transmembrane domain is boxed. (B) Syntaxin-associated proteins in detergent extracts of rat brain or squid optic lobe. SNARE complexes were immunoprecipitated with mAb 6D2 prior to SDS/PAGE and Coomassie blue staining or Western blotting with antibodies specific for synaptobrevin (18), syntaxin (22), and SNAP-25 (Alamone labs, Jerusalem). The band marked with an asterisk has been identified as myelin basic protein (16).
Detergent-solubilized squid syntaxin associated into high-molecular-mass oligomers that resisted dissociation by SDS if protein samples were not boiled prior to SDS/PAGE (not shown). In mammalian brain extracts, these oligomers correspond to ternary complexes of syntaxin, SNAP-25, and synaptobrevin (14). Immunoprecipitation of a Triton X-100 extract of squid optic lobe membranes, under conditions that isolate the 7S complex from rat brain extracts, showed that squid syntaxin interacts with two major polypeptide species, a doublet band at 20 kDa and a protein of 30 kDa (Fig. 1B). Immunoblotting identified these proteins as squid synaptobrevin and SNAP-25, respectively (Fig. 1B), showing that squid syntaxin also forms 7S SNARE complexes.
Bot-C1 Inhibits Neurotransmission at the Giant Synapse.
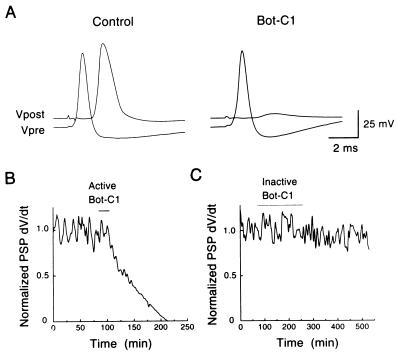
Squid syntaxin contains both the cleavage site (23) and the distal sequences (24) that allow proteolytic cleavage by Bot-C1 in vitro (Fig. 1A; V.O., unpublished data). In contrast, squid SNAP-25 appears insensitive to clostridial toxin cleavage (M. E. Burns, C.H., T.D., V.O., T.S., H.B., and G.J.A., unpublished data). Thus, unlike in mammalian systems, Bot-C1 may act in squid by selectively cleaving syntaxin (9). Microinjection of reduced Bot-C1 holotoxin or recombinant Bot-C1 light chain into the presynaptic terminal of the squid giant synapse produced an irreversible block of synaptic transmission evoked by presynaptic action potentials (Fig. 2; Table 1). This inhibition of release developed slowly and was not due to changes in the electrical characteristics of the terminal, because toxin injection did not alter presynaptic resting or action potentials (not shown). In two experiments, injection of different toxin preparations had no effect (Fig. 2; Table 1). We attribute this variability to chemical instability of Bot-C1, and we used the inactive toxin preparations as controls in morphological experiments (see below).
Figure 2.
Bot-C1 inhibits neurotransmitter release. (A) Injection of recombinant Bot-C1 light chain (20 μM in pipette) into the squid giant synapse caused a slow, irreversible block of neurotransmitter release, as indicated by an almost complete loss of the postsynaptic potential. (B) Time course of Bot-C1 inhibition of transmitter release shown by the progressive reduction in the initial slope (dv/dt) of the postsynaptic potential. The period of Bot-C1 injection is indicated by a bar. (C) As in B, but an inactive Bot-C1 preparation was used.
Table 1.
Effects of Bot-Cl and syntaxin fragments on neurotransmitter release
| Reagent | Inhibition, % ± SEM |
|---|---|
| Bot-Cl holotoxin | 65 ± 21 (2) |
| Bot-Cl light chain | 87 ± 5 (5) |
| Bot-Cl inactive preps. | 10 ± 4 (2) |
| Sq-syntaxin | 13 ± 6 (5) |
| TAX86 | 61 ± 2 (10) |
| TAX74 | 44 ± 7 (4) |
| TAX50 | 13 ± 2 (5) |
Reagents were microinjected into the squid giant synapse, and inhibition of neurotransmitter release was calculated as percentage change in postsynaptic potential slope in comparison to the preinjection control. The number of experiments is given in parentheses.
Identification of Syntaxin Fragments that Bind to Squid SNAP 25.
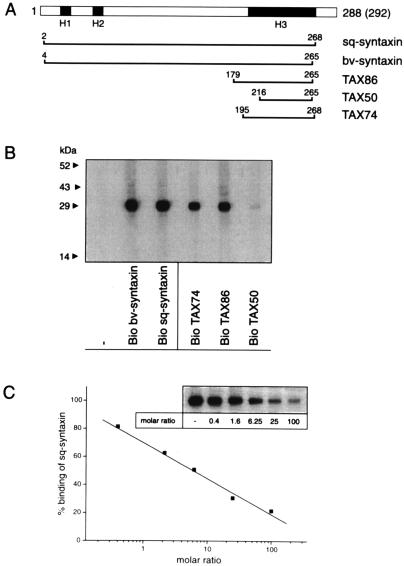
To examine the binding of syntaxin to squid synaptic proteins, we made recombinant proteins representing the cytoplasmic domains of squid (sq) and bovine (bv) syntaxin (Fig. 3A). After biotinylation, both proteins bound exclusively to SNAP-25 in immobilized samples of squid synaptosomal proteins (Fig. 3B). Consistent with previous reports (25, 26), the C-terminal H3 domains of squid (TAX74) and bovine (TAX86) syntaxin also bound SNAP-25 (Fig. 3 A and B). The initial segment of the H3 domain was essential for SNAP-25 binding (25), because TAX50, an N-terminally truncated fragment of TAX86, did not bind SNAP-25 (Fig. 3B). TAX86, but not TAX50, competed with sq-syntaxin for binding to SNAP-25 in a dose-dependent fashion, with approximately a 6-fold molar excess producing 50% inhibition (Fig. 3C). These results demonstrate that this H3 domain fragment is a competitive inhibitor of the interaction between syntaxin and SNAP-25.
Figure 3.
Analysis of syntaxin interactions in vitro. (A) Representation of the syntaxin fragments used. The three predicted coil-coiled domains (H1 to H3) are indicated in black. For amino acid numbering, see Fig. 1. (B) Syntaxin fragments containing the entire H3 domain bind to immobilized SNAP-25. (C) TAX86 competes with squid syntaxin for binding to SNAP-25. Immobilized squid optic lobe proteins were incubated with biotinylated sq-syntaxin (200 ng/ml) in the presence of increasing amounts of TAX86. The amount of bound biotinylated syntaxin, estimated by densitometry, is given as percentage bound in the absence of TAX86.
H3 Domain Fragments Inhibit Neurotransmitter Release.
Injection of TAX86 into the squid giant presynaptic terminal blocked neurotransmitter release, as revealed by a decline in the rate of rise of the evoked postsynaptic potentials (PSPs; Fig. 4A; Table 1). Like Bot-C1, TAX86 inhibited release without changing presynaptic resting or action potentials. The inhibition produced by TAX86 developed slowly and partially recovered after terminating the injection (Fig. 4 A and B). This reversibility indicates that microinjection did not lead to mechanical damage of the terminal. A concentration of about 10 μM was required to inhibit release by about 50% (n = 5), as estimated by measuring the quantity of fluorescent dextran coinjected with TAX86 (27). The prolonged time course of TAX86 inhibition is likely to be due to the time required to achieve this concentration in the terminal. Injections of TAX74 also reversibly inhibited transmitter release (Table 1). A decreased potency of TAX74 probably reflects additional sequence in TAX86 that help stabilize H3 domain structure (28). In contrast to the above, microinjecting buffer alone (not shown) or the truncated H3 domain TAX50 to a concentration at which TAX86 produced maximal effects (Fig. 4C) caused little inhibition (Table 1). Thus, the N-terminal portion of the H3 domain seems to be required for both inhibition of release and SNAP-25 binding in vitro. Injection of sq-syntaxin had no obvious effect on transmitter release (Table 1), although this protein bound immobilized SNAP-25 in vitro. This may relate to the presence of the N-terminal H1 and H2 coil-coiled domains, which can prevent SNARE interactions in soluble syntaxin (29).
Figure 4.
The H3 domain of syntaxin inhibits neurotransmitter release. (A) TAX86 injection into the giant terminal produced a reversible inhibition of neurotransmitter release. Prior to TAX86 injection (control), presynaptic stimulation caused a suprathreshold postsynaptic potential. Injection of TAX86 inhibited transmitter release, resulting in a subthreshold postsynaptic potentials. After terminating the injection, transmitter release recovered partially to suprathreshold levels. (B) Time course of TAX86 inhibition. Successive injections of TAX86 caused a strong inhibition of transmitter release that slowly reversed after terminating the injection. (C) Injection of TAX50 had little effect on release.
H3 Domain Fragments Inhibit SNARE Complex Formation.
To characterize the mechanism by which TAX74 and TAX86 inhibit neurotransmitter release, we analyzed how these fragments affect SNARE complex formation. Dimeric and trimeric SNARE complexes were generated by incubating immobilized squid synaptic proteins with biotinylated sq-synaptobrevin. Although synaptobrevin alone bound poorly to SNAP-25, binding was markedly increased upon addition of sq-syntaxin (Fig. 5A). Both TAX86 and TAX74 also promoted the synaptobrevin–SNAP-25 interaction, whereas TAX50 had no effect (Fig. 5A). These results indicate that the H3 domain is sufficient for SNARE complex formation. However, in contrast to the ternary complexes assembled with sq-syntaxin, those containing TAX74 or TAX86 were largely disrupted upon washing in SDS (Fig. 5A). Thus, complexes formed by the H3 domain alone are less stable than those formed from intact syntaxin.
Figure 5.
Interaction of the H3 domain with SNARE complexes. (A) TAX74 and TAX86, but not TAX50 (0.5 μg/ml each), promoted synaptobrevin (1 μg/ml) binding to SNAP-25 in the overlay assay (− SDS wash, Left). However, only sq-syntaxin generated trimeric complexes that fully resisted dissociation by SDS (+ SDS wash, Right). (B) Formation of SDS-resistant SNARE oligomers is inhibited by TAX86 upon prior SNARE disassembly. Extracts were incubated in the absence (−) or presence (+) of TAX86 before generating SNARE complexes. Immunoprecipitation with syntaxin antibodies followed by SDS/PAGE of unboiled samples and immunoblotting with mAb 6D2 revealed monomeric syntaxin and SNARE-containing oligomers (20). TAX86 did not affect preformed 7S and 20S complexes but inhibited complex formation after one (D20S, DR20S) or two (DRD20S) rounds of disassembly.
In vivo, the effects of H3 domain injections are seen in the presence of endogenous SNARE complexes. Therefore, we treated SNARE complexes, formed as shown in Fig. 5A, with excess TAX86 and observed no displacement of biotinylated syntaxin (data not shown). Similarly, the SDS-resistant SNARE oligomers (7S) present in rat brain detergent extracts (14, 20) were not dissociated by a large molar excess of TAX86 even after forming 20S complexes by supplementing incubations with SNAP and NSF (Fig. 5B). However, after ATP induced dissociation of the 20S complexes (16), TAX86 inhibited the spontaneous reformation of ternary complexes that occurs upon ATP depletion (D20S) (20) or chelating Mg-ATP with EDTA (DR20S) (16). This was evident from a decrease in SDS-resistant oligomers concomitant with an increase in syntaxin monomers (Fig. 5C). After two rounds of disassembly (DRD20S), inhibition of SNARE complex formation by TAX86 was even more pronounced. These results indicate that TAX86 competes with free, but not bound, syntaxin in SNARE complex formation.
Nerve Terminal Morphology after TAX86 and Bot-C1 Injection.
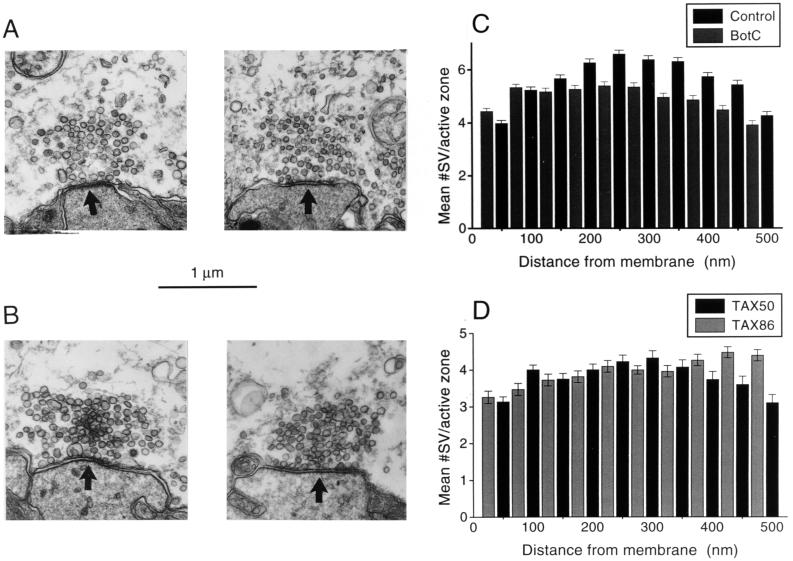
To identify the steps of the synaptic vesicle life cycle (3) at which syntaxin functions, we compared the ultrastructure of nerve terminals injected with Bot-C1 and TAX86 with that of control terminals injected with inactive toxin or TAX50, respectively. Electron microscopy of nerve terminals injected with Bot-C1 (Fig. 6A) or TAX86 (Fig. 6B) revealed no significant changes in the general morphology of their presynaptic active zones. Furthermore, the number and spatial distribution of synaptic vesicles within the active zones of terminals injected with either active Bot-C1 or TAX86 were not significantly different from those of controls (Fig. 6 C and D). This indicates that neither Bot-C1 nor TAX86 block release by preventing endocytosis. Notably, the number of docked vesicles (i.e., those whose centers were within 50 nm of the presynaptic plasma membrane) also was unaltered. Therefore, we conclude that Bot-C1 and TAX86 inhibit vesicle fusion by acting at a step that follows docking.
Figure 6.
Ultrastructural effects of injecting Bot-C1 and TAX86. Presynaptic terminals inhibited by either Bot-C1 or TAX86 were analyzed by electron microscopy and compared with terminals injected with inactive Bot-C1 or TAX50, respectively. (A and B) Representative active-zone profiles from Bot-C1 (A) and TAX86 (B) treatments (Left) are displayed with controls (Right; inactive Bot-C1 and TAX50, respectively). Arrowheads located in the postsynaptic spines point toward active zones. (C and D) Quantitative analysis of active zone profiles assessed by counting synaptic vesicles at 50-nm intervals from the presynaptic membrane. (C) Data from Bot-C1-injected (gray bars, n = 398) and control-injected (black bars, n = 510) terminals. (D) Data from TAX86- (gray bars, n = 379) and TAX50- (black bars, n = 238) injected terminals. n, number of active zone profiles analyzed.
DISCUSSION
We have examined the widely held hypothesis that the tight interaction of syntaxin with SNAP-25 and synaptobrevin, seen in vitro, is important for neurotransmitter release in vivo. Using Bot-C1, a syntaxin-selective protease, and TAX86, a syntaxin fragment that prevents binding of SNAP-25 and formation of stable SNARE complexes, we found that interaction between these proteins is indeed essential for neurotransmitter release. Our data also indicate that syntaxin participates in a reaction that follows synaptic vesicle docking but lies upstream of the reactions responsible for Ca2+-triggered fusion.
The SNAP-25 Binding Domain of Syntaxin Inhibits Release by Preventing SNARE Complex Assembly.
The H3 domain of syntaxin has been implicated in several in vitro interactions, including binding of syntaxin to SNAP-25 and synaptobrevin (4, 5, 12, 25, 30), synaptotagmin (6, 31, 32), Ca2+ channels (33, 34), α-SNAP, and the mammalian sec1 protein (25, 26). However, in our overlay assays SNAP-25 always appeared as the prominent binding partner of the H3 domain, suggesting that inhibition of neurotransmitter release by this domain is due to selective disruption of the interaction between syntaxin and SNAP-25. Different observations support this idea. First, syntaxin’s binding site for synaptotagmin (32) was contained not only within TAX74 and TAX86 but also within TAX50, a fragment that did not inhibit release. Second, it appears unlikely that the interaction between syntaxin and presynaptic Ca2+ channels was affected, because the latter depends on the presence of a transmembrane domain (33) that was absent in the fragments used here. In addition, disruption of this interaction affects the kinetics of release (35), which was not observed following injection of TAX86 or TAX74. Third, under the conditions of our in vitro experiments TAX86 was unable to prevent α-SNAP from binding to endogenous syntaxin (data not shown). Similarly, the H3 domain of syntaxin is incapable of binding sec−1 in vitro (25), suggesting that this interaction is not disrupted by TAX74 and TAX86. Thus, the available data are consistent with the conclusion that the H3 domain inhibits release by interfering with syntaxin binding to SNAP-25.
The mechanism of H3 domain inhibition can be deduced from our in vitro observations. Both TAX86 and TAX74 bound SNAP-25 in a way that facilitated subsequent binding of synaptobrevin. Moreover, TAX86 inhibited the binding of full-length syntaxin to SNAP-25 but had no effect on preformed binary or ternary SNARE complexes. Similarly, SNARE cleavage by clostridial neurotoxins requires SNARE complex disassembly (16, 36). Notably, the SDS-resistance characteristic of the ternary SNARE complex (14) was reduced when TAX86 was bound to synaptobrevin and SNAP-25. Thus, TAX74 and TAX86 may act by preventing the formation of a functional “low-energy” SNARE complex (20). Our observation that reassembly of dissociated SNAREs was efficiently inhibited by TAX86 is consistent with this interpretation. Complexes containing synaptobrevin, SNAP-25, and a TAX86-like H3 domain construct have been reported to associate with SNAP and NSF but not to disassemble (25, 26). If such complexes formed in vivo, TAX86 should produce an irreversible inhibition of release, which was not observed (Fig. 4B). Also, in vitro competition studies using detergent extracts gave no evidence that TAX86 produces permanently assembled SNARE complexes when endogenous syntaxin is present (V.O., unpublished data). Therefore, we propose that H3 domain fragments inhibit release by forming nonfunctional SNARE complexes and that disassembly of ternary SNARE complexes during the synaptic vesicle cycle is required for inhibition by these fragments.
SNARE Complexes Are Needed for Docked Synaptic Vesicle to Fuse.
Our work showing that Bot-C1 inhibits neurotransmitter release at the squid giant synapse extends previous investigations with this agent (9, 37) by examining the structural consequences of toxin poisoning. We found that both Bot-C1 and TAX86 inhibited transmitter release without altering the distribution of synaptic vesicles at active zones. Although this is consistent with earlier experiments that revealed little change in the number of docked synaptic vesicles in Drosophila nerve terminals lacking syntaxin (11), it contrasts with findings that other treatments that prevent exocytosis cause an accumulation of docked vesicles (18, 21, 27). To explain this discrepancy, we assume that Bot-C1, TAX86, and genetic knock-out of syntaxin act on the pool of recently docked vesicles. In contrast, reagents that accumulate docked vesicles inhibit later reactions and thus additionally trap vesicle intermediates that are only transient under normal conditions (M. Burns, T. Sasaki, Y. Takai, and G. J. Augustine, unpublished data). The assumption of sequential pools of docked synaptic vesicles is supported by observations that docked endocrine secretory vesicles undergo multiple, kinetically distinct reactions before becoming capable of Ca2+-triggered fusion (38, 39). Although we suggest that Bot-C1 and TAX86 inhibit an early reaction, this does not preclude a role for the SNAREs in subsequent reactions that lie closer to fusion. Indeed, the accumulation of docked vesicles in tetanus toxin-treated terminals is consistent with such an idea (18).
In conclusion, SNAREs are not directly responsible for generating the vesicles pool that are docked at the plasma membrane. Our notion is that other processes initiate vesicle docking, and the subsequent formation of SNARE complexes then primes docked vesicles to make them competent for fusion. This interpretation can explain our results and is consistent with other experiments that disrupt SNARE protein function in neurons (11, 18, 27). Further, this interpretation is compatible with proposals that SNARE complexes may participate in the maturation and stabilization of the docked state (40). Recent studies on constitutive membrane fusion in yeast, however, suggest that SNAREs are needed for vesicle docking in vitro (41, 42). Although it is difficult to compare results obtained in yeast and neurons because of the different experimental paradigms used, this discrepancy may relate to identification of different stages of docking or an in vitro loss of components (or subcellular compartmentalization) required for physiological docking. Alternatively, neurons may have evolved a SNARE-independent docking mechanism as a specialization for rapid and regulated membrane fusion.
Acknowledgments
We thank M. Takahashi for syntaxin antibodies, H. Niemann for the Bot-C1 plasmid, J. Rothman for α-SNAP and NSF expression constructs, and J. Battey for the cDNA library. This work was supported by Deutsche Forschungsgemeinschaft and Human Frontier Science Program Organizationgrants, Fonds der Chemischen Industrie, National Institutes of Health Grant NS 21624, and a National Institutes of Health Postdoctoral Fellowship to J.H.
ABBREVIATIONS
- Bot-C1
botulinum C1 toxin
- NSF
N-ethylmaleimide-sensitive fusion protein
- SNAP
soluble NSF attachment protein
- SNARE
SNAP receptor
- SNAP-25
synaptosomal-associated protein of 25 kDa
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. Y14575).
References
- 1.Katz B. The Release of Neurotransmitter Substances. Liverpool: Liverpool Univ. Press; 1969. [Google Scholar]
- 2.Heuser J E, Reese T S, Dennis M J, Jan Y, Jan L, Evans L. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heuser J E, Reese T S. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calakos N, Scheller H. Physiol Rev. 1996;76:1–29. doi: 10.1152/physrev.1996.76.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Augustine G J, Burns M E, DeBello W M, Pettit D L, Schweizer F E. Annu Rev Pharmacol Toxicol. 1996;36:659–701. doi: 10.1146/annurev.pa.36.040196.003303. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 7.Inoue A, Obata K, Akagawa K. J Biol Chem. 1992;267:10613–10619. [PubMed] [Google Scholar]
- 8.Bennett M K, Garcia-Arraras J E, Elferink L A, Peterson K, Fleming A M, Hazuka C D, Scheller R. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 9.Schiavo G, Montecucco C. In: Bacterial Toxins. Aktories K, editor. Weinheim, Germany: Chapman & Hall; 1997. pp. 169–192. [Google Scholar]
- 10.Schulze K L, Broadie K, Perin M S, Bellen H J. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 11.Broadie K, Prokop A, Bellen H J, O’Kane C J, Schulze K L, Sweeney S T. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 12.Hanson P I, Jahn R. Curr Op Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 13.Pevsner J, Hsu S-C, Braun J E A, Calakos N, Ting A E, Bennett M K, Scheller R H. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 16.Söllner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 17.Hanson P I, Otto H, Barton N, Jahn R. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- 18.Hunt J H, Bommert K, Charlton M P, Kistner A, Habermann E, Augustine G J, Betz H. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 19.Hodel A, Schäfer T, Gerosa D, Burger M M. J Biol Chem. 1994;269:8623–8626. [PubMed] [Google Scholar]
- 20.Pellegrini L L, O’Connor V, Lottspeich F, Betz H. EMBO J. 1995;14:4705–4713. doi: 10.1002/j.1460-2075.1995.tb00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bommert K, Charlton M P, DeBello W M, Chin G J, Betz H, Augustine G J. Nature (London) 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida A, Oho C, Omori A, Kuwahara R, Ito T, Takahashi M. J Biol Chem. 1992;267:24925–24928. [PubMed] [Google Scholar]
- 23.Schiavo G, Shone C C, Bennett M K, Scheller R H, Montecucco C. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- 24.Rossetto O, Schiavo G, Montecucco C, Poulain B, Deloye F, Lozzi L, Shone C C. Nature (London) 1994;372:415–416. doi: 10.1038/372415a0. [DOI] [PubMed] [Google Scholar]
- 25.Kee Y, Lin R C, Hsu S-C, Scheller R H. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. EMBO J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBello W M, O’Connor V, Dresbach T, Whiteheart S W, Wang S S-H, Schweizer F E, Betz H, Rothman J E, Augustine G J. Nature (London) 1995;373:626–630. doi: 10.1038/373626a0. [DOI] [PubMed] [Google Scholar]
- 28.Zhong P, Chen Y A, Tam D, Chung D, Scheller R H, Miljanich G P. Biochemistry. 1997;36:4317–4326. doi: 10.1021/bi9625408. [DOI] [PubMed] [Google Scholar]
- 29.Calakos N, Bennett M K, Peterson K E, Scheller R H. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- 30.Chapman E R, An S, Barton N, Jahn R. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- 31.Li C, Ullrich B, Zhang J Z, Anderson R G W, Brose N, Südhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 32.Kee Y, Scheller R H. J Neurosci. 1996;16:1975–1981. doi: 10.1523/JNEUROSCI.16-06-01975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezprozvanny I, Scheller R H, Tsien R W. Nature (London) 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 34.Wiser O, Bennett M K, Atlas D. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 35.Mochida S, Sheng Z-H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrini L L, O’Connor V, Betz H. FEBS Lett. 1994;353:319–323. doi: 10.1016/0014-5793(94)01070-6. [DOI] [PubMed] [Google Scholar]
- 37.Mochida S, Saisu H, Kobayashi H, Abe T. Neuroscience. 1995;65:905–915. doi: 10.1016/0306-4522(94)00508-3. [DOI] [PubMed] [Google Scholar]
- 38.Parsons T D, Coorssen J R, Horstmann H, Almers W. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee A, Barry V A, DasGupta B R, Martin T F J. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 40.Sögaard M, Tani K, Ye R R, Geromanos S, Tempst P, Kirchhausen T, Rothman J E, Söllner T. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 41.Mayer A, Wickner W, Haas A. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 42.Nichols B J, Ungermann C, Pelham H R B, Wickner W, Haas A. Nature (London) 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]