Abstract
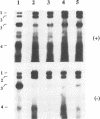
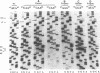
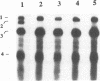
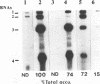
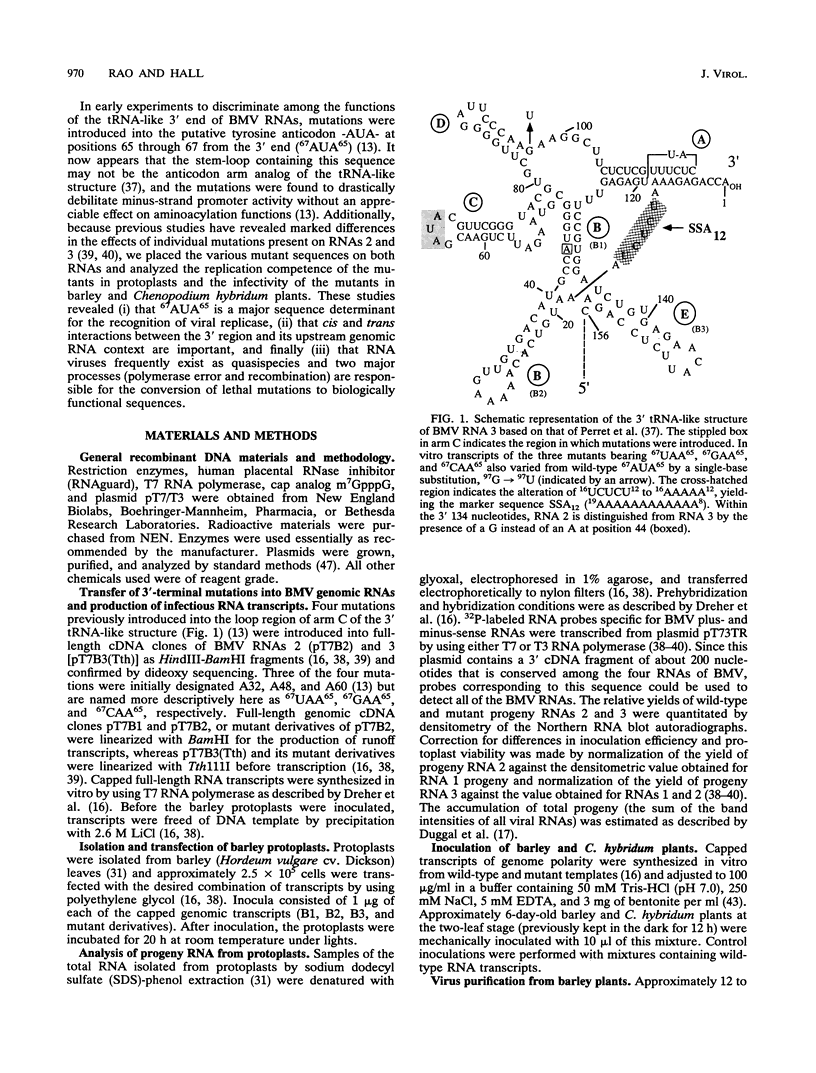
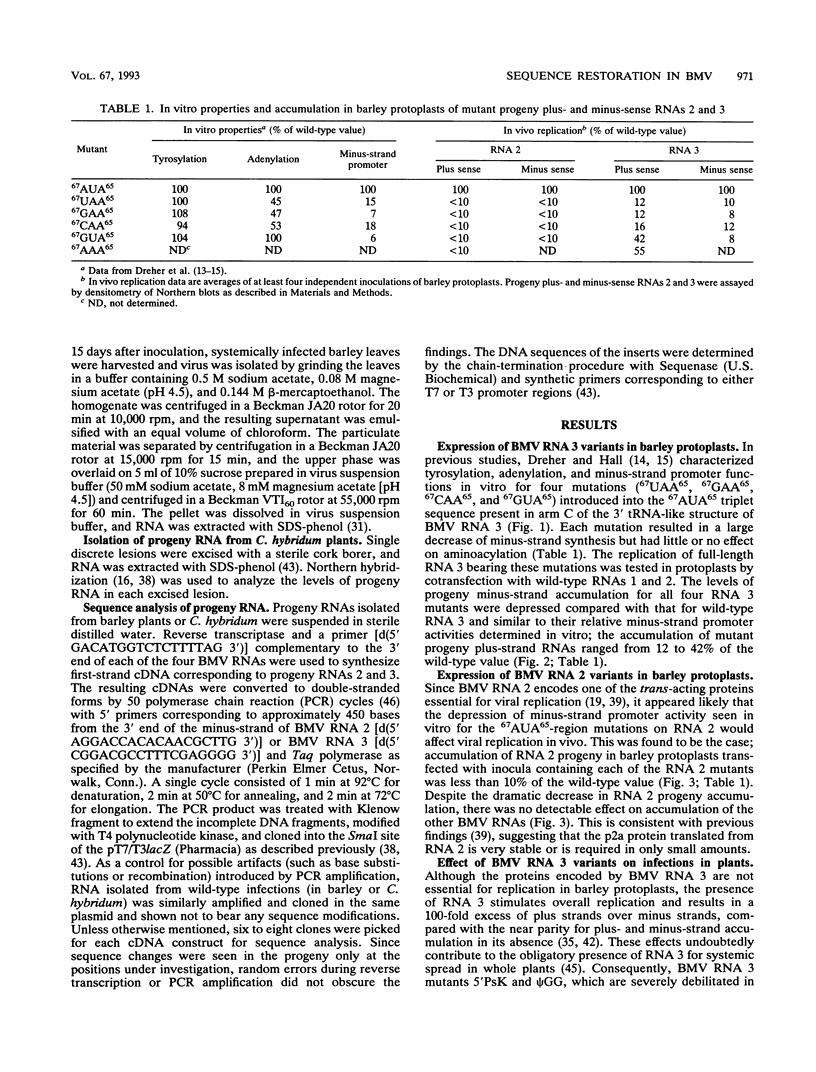
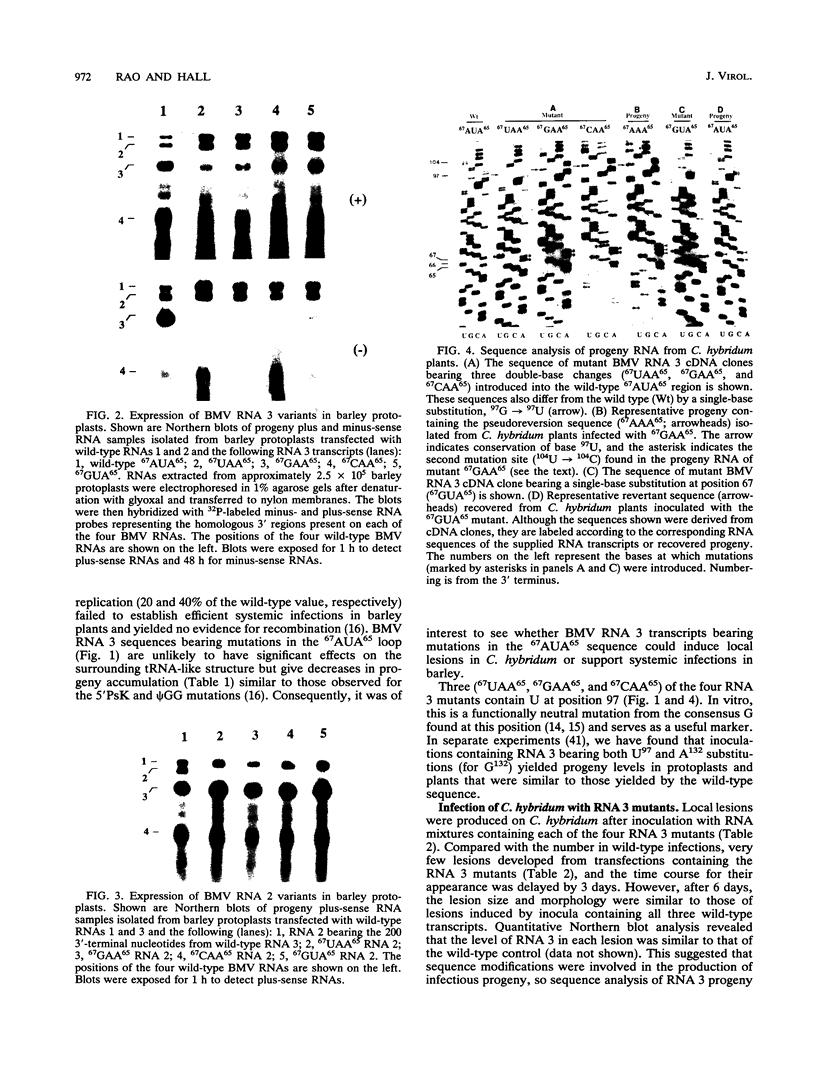
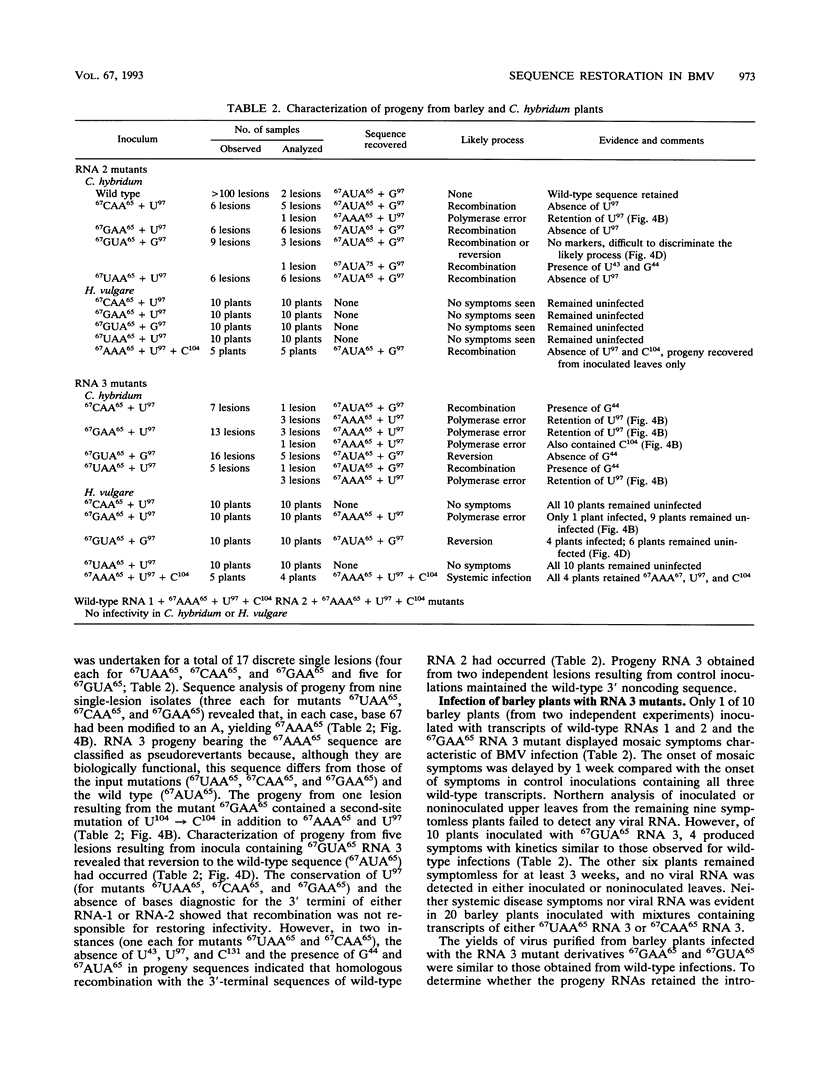
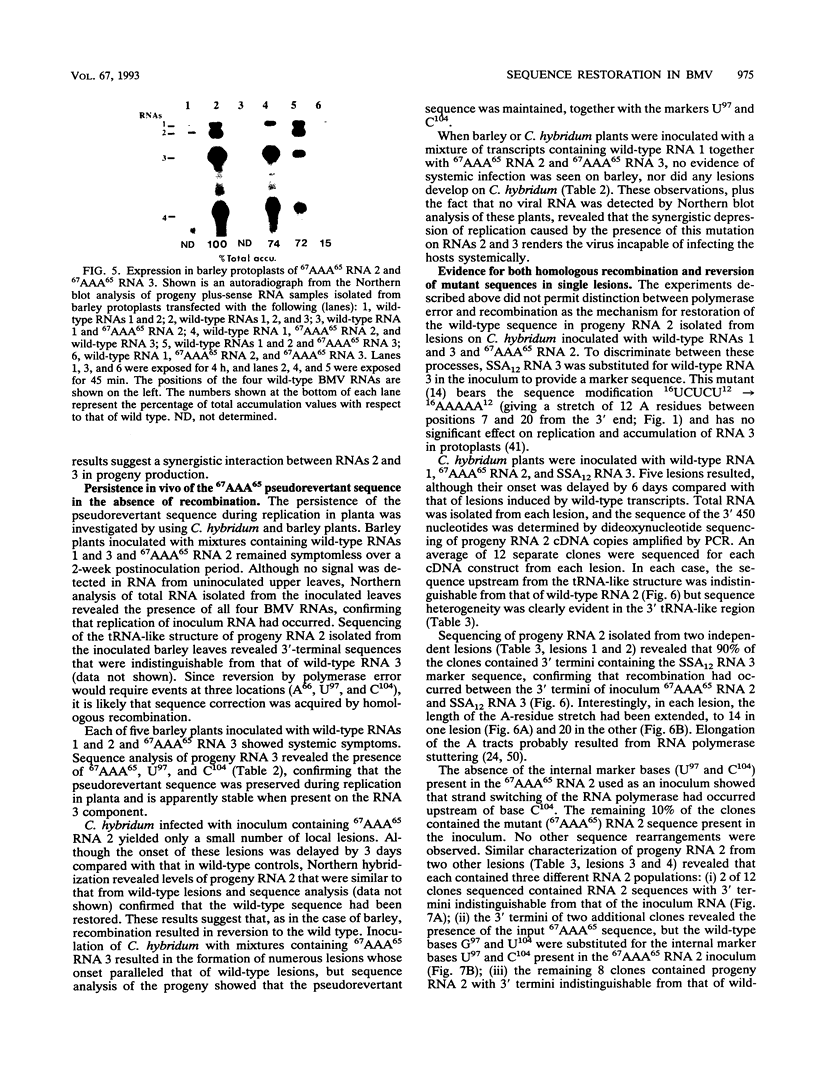
The tRNA-like structure present in the 3' noncoding region of each of the four virion RNAs of brome mosaic virus possesses a conserved A-67-U-A-65 (67AUA65) sequence. Four mutations in this region (67UAA65, 67GAA65, and 67CAA65, each with a double base change, and 67GUA65, containing a single point mutation), previously shown in vitro to be defective in minus-strand promoter function, were introduced into full-length genomic RNAs 2 and 3, and their replicative competence was analyzed in barley protoplasts. All four RNA 3 mutants were capable of replication, although progeny plus-sense RNA 3 accumulation was only 12 to 42% of that of the wild type. Replication of RNA 2 transcripts bearing these mutations was even more severely debilitated; the accumulation of each mutant progeny plus-strand RNA 2 was < 10% of that of the wild type. Analysis of mutant RNA 3 progeny recovered from local lesions induced in Chenopodium hybridum and systemic infections in barley (Hordeum vulgare) plants revealed that the mutant base at position 67 from the 3' end had in each case been modified to an A. These changes generated RNAs with functional pseudorevertant (67AAA65 for mutants 67UAA65, 67GAA65, and 67CAA65) or revertant (67GUA65-->67AUA65) sequences. In most instances, the presence of internal markers permitted discrimination between polymerase error and RNA recombination as the process by which sequence restoration occurred. The pseudorevertant sequence was found to be capable of persistence during subsequent propagation in plants when present on RNA 3 but not when present on RNA 2. These data document the fluidity of the RNA genome and reveal situations in which polymerase error or recombination can function preferentially to restore an optimal sequence. They also support the concept that RNA viruses frequently exist as quasispecies and have implications concerning evolutionary strategies for positive-strand RNA viruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Nucleotide sequence of the brome mosaic virus genome and its implications for viral replication. J Mol Biol. 1984 Feb 5;172(4):369–383. doi: 10.1016/s0022-2836(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. L., Dawson W. O. Deletion of repeated sequences from tobacco mosaic virus mutants with two coat protein genes. Virology. 1990 Aug;177(2):462–469. doi: 10.1016/0042-6822(90)90510-x. [DOI] [PubMed] [Google Scholar]
- Bozarth C. S., Weiland J. J., Dreher T. W. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology. 1992 Mar;187(1):124–130. doi: 10.1016/0042-6822(92)90301-5. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dzianott A. M. Generation and analysis of nonhomologous RNA-RNA recombinants in brome mosaic virus: sequence complementarities at crossover sites. J Virol. 1991 Aug;65(8):4153–4159. doi: 10.1128/jvi.65.8.4153-4159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O. Tobamovirus-plant interactions. Virology. 1992 Feb;186(2):359–367. doi: 10.1016/0042-6822(92)90001-6. [DOI] [PubMed] [Google Scholar]
- Domingo E., Martínez-Salas E., Sobrino F., de la Torre J. C., Portela A., Ortín J., López-Galindez C., Pérez-Breña P., Villanueva N., Nájera R. The quasispecies (extremely heterogeneous) nature of viral RNA genome populations: biological relevance--a review. Gene. 1985;40(1):1–8. doi: 10.1016/0378-1119(85)90017-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Browning K. S., Clark J. M., Jr Sequence heterogeneity in satellite tobacco necrosis virus RNA. Virology. 1981 Apr 15;110(1):43–54. doi: 10.1016/0042-6822(81)90006-4. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Bujarski J. J., Hall T. C. Mutant viral RNAs synthesized in vitro show altered aminoacylation and replicase template activities. Nature. 1984 Sep 13;311(5982):171–175. doi: 10.1038/311171a0. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Hall T. C. Mutational analysis of the sequence and structural requirements in brome mosaic virus RNA for minus strand promoter activity. J Mol Biol. 1988 May 5;201(1):31–40. doi: 10.1016/0022-2836(88)90436-6. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Hall T. C. Mutational analysis of the tRNA mimicry of brome mosaic virus RNA. Sequence and structural requirements for aminoacylation and 3'-adenylation. J Mol Biol. 1988 May 5;201(1):41–55. doi: 10.1016/0022-2836(88)90437-8. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Rao A. L., Hall T. C. Replication in vivo of mutant brome mosaic virus RNAs defective in aminoacylation. J Mol Biol. 1989 Apr 5;206(3):425–438. doi: 10.1016/0022-2836(89)90491-9. [DOI] [PubMed] [Google Scholar]
- Duggal R., Rao A. L., Hall T. C. Unique nucleotide differences in the conserved 3' termini of brome mosaic virus RNAs are maintained through their optimization of genome replication. Virology. 1992 Mar;187(1):261–270. doi: 10.1016/0042-6822(92)90314-f. [DOI] [PubMed] [Google Scholar]
- French R., Ahlquist P. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J Virol. 1987 May;61(5):1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- García-Arenal F., Palukaitis P., Zaitlin M. Strains and mutants of tobacco mosaic virus are both found in virus derived from single-lesion-passaged inoculum. Virology. 1984 Jan 15;132(1):131–137. doi: 10.1016/0042-6822(84)90097-7. [DOI] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Rey M. E., Dodds J. A. Analysis of genetic heterogeneity within the type strain of satellite tobacco mosaic virus reveals variants and a strong bias for G to A substitution mutations. Virology. 1992 Jul;189(1):233–244. doi: 10.1016/0042-6822(92)90699-p. [DOI] [PubMed] [Google Scholar]
- Lai M. M. RNA recombination in animal and plant viruses. Microbiol Rev. 1992 Mar;56(1):61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane L. C. The bromoviruses. Adv Virus Res. 1974;19:151–220. doi: 10.1016/s0065-3527(08)60660-0. [DOI] [PubMed] [Google Scholar]
- Luo G. X., Chao M., Hsieh S. Y., Sureau C., Nishikura K., Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990 Mar;64(3):1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L. E., Dreher T. W., Hall T. C. Mutational analysis of the core and modulator sequences of the BMV RNA3 subgenomic promoter. Nucleic Acids Res. 1988 Feb 11;16(3):981–995. doi: 10.1093/nar/16.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L. E., Huntley C. C., Pogue G. P., Connell J. P., Hall T. C. Regulation of (+):(-)-strand asymmetry in replication of brome mosaic virus RNA. Virology. 1991 May;182(1):76–83. doi: 10.1016/0042-6822(91)90650-z. [DOI] [PubMed] [Google Scholar]
- Perret V., Florentz C., Dreher T., Giege R. Structural analogies between the 3' tRNA-like structure of brome mosaic virus RNA and yeast tRNATyr revealed by protection studies with yeast tyrosyl-tRNA synthetase. Eur J Biochem. 1989 Nov 6;185(2):331–339. doi: 10.1111/j.1432-1033.1989.tb15120.x. [DOI] [PubMed] [Google Scholar]
- Rao A. L., Dreher T. W., Marsh L. E., Hall T. C. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5335–5339. doi: 10.1073/pnas.86.14.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Interference in trans with brome mosaic virus replication by RNA-2 bearing aminoacylation-deficient mutants. Virology. 1991 Jan;180(1):16–22. doi: 10.1016/0042-6822(91)90004-u. [DOI] [PubMed] [Google Scholar]
- Rao A. L., Hall T. C. Requirement for a viral trans-acting factor encoded by brome mosaic virus RNA-2 provides strong selection in vivo for functional recombinants. J Virol. 1990 May;64(5):2437–2441. doi: 10.1128/jvi.64.5.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. L., Huntley C. C., Marsh L. E., Hall T. C. Analysis of RNA stability and (-) strand content in viral infections using biotinylated RNA probes. J Virol Methods. 1990 Dec;30(3):239–250. doi: 10.1016/0166-0934(90)90066-o. [DOI] [PubMed] [Google Scholar]
- Rao A. L., Sullivan B. P., Hall T. C. Use of Chenopodium hybridum facilitates isolation of brome mosaic virus RNA recombinants. J Gen Virol. 1990 Jun;71(Pt 6):1403–1407. doi: 10.1099/0022-1317-71-6-1403. [DOI] [PubMed] [Google Scholar]
- Rietveld K., Pleij C. W., Bosch L. Three-dimensional models of the tRNA-like 3' termini of some plant viral RNAs. EMBO J. 1983;2(7):1079–1085. doi: 10.1002/j.1460-2075.1983.tb01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher R., Ahlquist P. Effects of deletions in the N-terminal basic arm of brome mosaic virus coat protein on RNA packaging and systemic infection. J Virol. 1989 Nov;63(11):4545–4552. doi: 10.1128/jvi.63.11.4545-4552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat D. E., Palukaitis P. A single nucleotide change within a plant virus satellite RNA alters the host specificity of disease induction. Plant J. 1992 Jan;2(1):43–49. [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Tyulkina L. G., Karpova O. V., Rodionova N. P., Atabekov J. G. Site-specific cleavage and religation of viral RNAs. I. Infectivity of barley stripe mosaic virus RNA religated from functionally active segments and restoration of the internal poly(A) tract in progeny. Virology. 1987 Aug;159(2):312–320. doi: 10.1016/0042-6822(87)90469-7. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]