Abstract
Oncogene-mediated signaling to the host environment induces a subset of cytokines and chemokines. The Drosophila Dac gene promotes migration of the morphogenetic furrow during eye development. Expression of the cell-fate determination factor Dachshund (DACH1) was lost in poor prognosis invasive breast cancer. Mouse embryo fibroblasts derived from Dach1−/− mice demonstrated endogenous Dach1 constitutively represses cellular migration. DACH1 inhibited cellular migration and invasion of oncogene (Ras, Myc, ErbB2, c-Raf)-transformed human breast epithelial cells. An unbiased proteomic analysis identified and immunoneutralizing antibody and reconstitution experiments demonstrated IL-8 is a critical target of DACH1 mediating breast cancer cellular migration and metastasis in vivo. DACH1 bound the endogenous IL-8 promoter in ChIP assays and repressed the IL-8 promoter through the AP-1 and NF-κB binding sites. Collectively, our data identify a pathway by which an endogenous cell-fate determination factor blocks oncogene-dependent tumor metastasis via a key heterotypic mediator.
Keywords: DACH1, metastasis
The molecular events governing the onset and progression of tumorigenesis involve the sequential inactivation of endogenous growth suppressors and the acquisition of oncogenic mutations (1). The functional characteristics acquired by oncogene-transformed cells include the release from normal growth constraints, changes in cellular metabolism, and the ability to migrate and invade the local host environment (2). Cellular migration is essential for normal mammalian development, for tissue repair and contributes to the metastatic phenotype of cancerous cells. Adhesion to the cellular substratum initiates the generation of protrusive activity (3, 4). Directional migration of a cell requires the formation of new adhesive structures at the leading edge and detachment of the posterior edge (5).
Oncogenes sustain host-cell interactions, promoting invasiveness, recruiting inflammatory mediators and developing angiogenic networks. Ras and Myc promote tumor angiogenesis through up-regulation of VEGF and the inhibition of thrombospondin 1 (TSP-1) (6). In addition, inflammatory mediators are induced by Ras activation. The inflammatory mediator, interleukin-8 (CXCL-8/IL-8), is a transcriptional target of Ras and plays an essential role in Ras-induced tumor growth and angiogenesis in vivo (7, 8). The molecular mechanisms normally restraining these oncogenic pathways in human breast cancer are poorly understood.
Reduced expression of the cell-fate determination factor DACH1 in human breast cancer is associated with poor prognosis (9). Analysis of >2,000 human breast cancer samples revealed that the loss of DACH1 expression correlated with death from breast cancer 40 months earlier. The Drosophila dachshund (dac) gene is the founding member of the DACH subfamily of nuclear proteins that conduct an essential role in promoting differentiation of the Drosophila eye and limb (10). The dac gene forms part of a retinal determination (RD) signaling pathway in Drosophila. The RD pathway requires eyeless (eya/Pax6), sine oculis (So, Six), eyes absent (eya/Eya), and dachshund (dac/Dach), which function together as genetic components of this network (11–13). In Drosophila, So functions as a DNA-binding factor and dac/eya are transcriptional cofactors. In mammalian cells, c-Jun functions as a DNA binding factor through which DACH1 regulates gene transcription and thereby inhibits cellular growth (14). The role of DACH1 in cellular migration or invasion remains unknown. In view of the reduction of DACH1 expression in metastatic human breast cancer, we examined the role of DACH1 in regulating human breast cancer cell migration and invasion.
Results
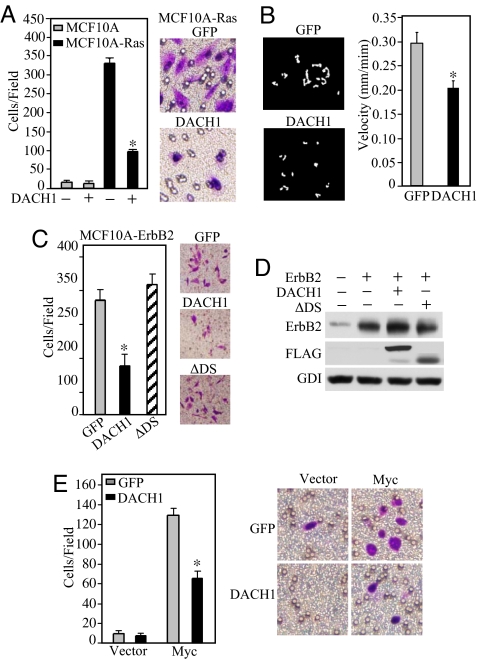
To determine whether DACH1 regulated the promigratory effects of distinct oncogenes, the immortal human breast MCF10A cells were used. MCF10A-Ras cells showed an ≈30-fold increase in migration compared to parental cells and a >75% reduction in transwell migration upon induction of DACH1 (Fig. 1A). DACH1 expression reduced the rate of cellular movement ≈30% (Fig. 1B). The crystal structure of DACH1 predicts a helix–turn–helix structure with a conserved domain (Dac and Ski/Sno domain 1). Deletion of the DS domain abrogated the effect of DACH1 to inhibit cellular transwell migration (Fig. 1C). MCF10A cells transduced with an activating ErbB2 expression vector induced migration 15-fold with a 60% reduction upon expression of DACH1 (Fig. 1C) without affecting ErbB2 expression (Fig. 1D). DACH1 induction reduced cellular migration of c-Myc-transformed MCF10A cells by 50% (Fig. 1E) without affecting c-Myc abundance [supporting information (SI) Fig. S1A].
Fig. 1.
DACH1 inhibits oncogene-induced migration of breast cancer cells. (A) MCF10A or MCF10A-Ras cells were transduced with retroviral expression vectors encoding GFP or DACH1-IRES-GFP and subjected to GFP-FACS sorting with subsequent analysis of cell migration by transwell assay. The data are shown as mean ± SEM of the number of cells migrated in n > 5 separate experiments. Crystal violet dye staining of MCF10A-Ras cells that migrated in the transwell assays is shown. (B) Videomicroscopy of individual cells were quantitated to determine the distance and direction of cellular migration of MCF10A-Ras cells transfected with either control (GFP) or DACH1 expression vector. (C) MCF10A-ErbB2 (NeuT) transformed cells were analyzed for migration. The mean data for the number of cells migrated per field is shown for MCF-10A oncogene-transformed cells transduced with retroviral vector encoding GFP or DACH1-IRES-GFP. (D) Western blot analysis of MCF10A-ErbB2 cells shown in C with antibodies as indicated. (E) MCF10A-c-Myc cells transduced with DACH1 or control vector analyzed for migration as shown in A. *, P < 0.01.
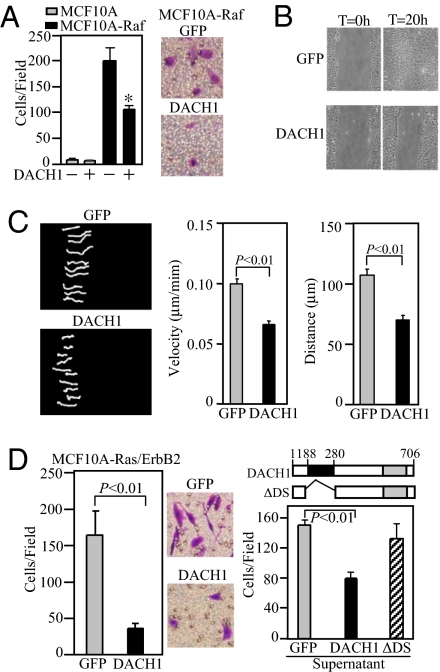
To determine whether DACH1 inhibited Ras-induced migration downstream of Ras, we examined the Ras-effector c-Raf. DACH1 expression reduced MCF10A-Raf transwell migration by ≈50% (Fig. 2A) and inhibited wound closure by >80% (Fig. 2B). DACH1 inhibited the persistence of migratory directionality reducing cellular velocity by 40% and the cellular distance migrated by 50% (Fig. 2C).
Fig. 2.
DACH1 inhibits wound healing and persistence of migratory directionality. (A) Migration assays of MCF10A-Raf transformed cells. Cells were transduced with either retroviral vectors encoding GFP or DACH1. Data are mean ± SEM of five separate experiments for either transwell migration (A), wound closure assays (B) or phase contrast video microscopy (C). The velocity and distance migrated was determined for individual MCF10A-Raf cells transduced with either control vector or DACH1. Data mean ± SEM of n > 5 separate events. (D) Migration assays of MCF10A-Ras/ErbB2 cells transformed with either control (GFP) or DACH1. Data mean ± SEM of n > 5 separate experiments (Left) or transwell assays were conducted either in the presence of supernatant derived from cells expressing GFP, DACH1 or a mutant of the DACH1 DS domain (DACH1 ΔDS) (Right). Migration is reduced in media derived from DACH1 tranduced cells. Schematic representation of DACH1 and DACH1 ΔDS domain. *, P < 0.01.
Oncogene-transduced MCF10A cells (Ha-Ras/ErbB2) enhanced cellular migration compared to MCF10A cells, which was reduced 6-fold by DACH1 expression (Fig. 2D Left). The addition of conditioned media from DACH1-transduced MCF10A-Ras/ErbB2 cells reduced cellular migration >60% (Fig. 2D Right). In contrast, the supernatant derived from MCF10A-Ras/ErbB2 cells transduced with a DACH1 protein containing a mutation of the DACH1 DS domain, did not significantly inhibit cellular migration (Fig. 2D Right).
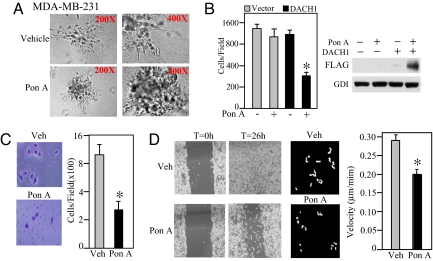
Collectively, these studies demonstrate DACH1 inhibits migration of MCF10A cells transformed with distinct oncogenes (Ha-Ras, c-Raf, c-Myc, and ErbB2). The highly metastatic MDA-MB-231 human breast cancer cell line was transduced with an expression vector encoding DACH1 under control of the ponasterone A-responsive enhancer (9, 14). MDA-MB-231 cells demonstrated spreading microprocesses at the distal end of the cellular colonies in matrigel (Fig. 3A Upper, 400× magnification). In contrast, the number of extending processes was essentially abrogated by the induction of DACH1 (Fig. 3A Lower).
Fig. 3.
DACH1 inhibits migration and invasion of highly metastatic MDA-MB-231 cells. (A) Phase contrast microscopy of MDA-MB-231 cells induced to express DACH1 (Lower) versus vehicle-treated cells (Upper). (Magnification: Left, 200×; Right, 400×.) (B–D) MDA-MB-231 cells stably expressing ponasterone A inducible DACH1 were assessed for either transwell migration (B), matrigel invasion (C), wound closure (D Left) or velocity-analyzed by videomicroscopy at a single-cell level (D Right), with mean data shown at 360 min. *, P < 0.01.
Invasion assays were conducted comparing cells induced to express DACH1 (Fig. 3B). DACH1 expression induced by Ponasterone A addition reduced the number of cells traversing the transwell chamber by >50% (Fig. 3B). In contrast, Ponasterone A alone had no direct effect on cellular migration (Fig. 3B). The induction of DACH1 expression reduced the number of cells invading the matrigel product by ≈65% (P < 0.001 Fig. 3C). Similarly, DACH1 expression reduced wound closure (Fig. 3D Left) and migration velocity (Fig. 3D Right) by ≈60%.
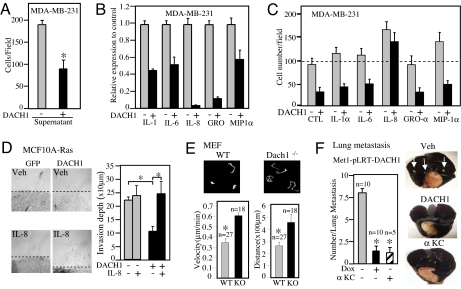
As with the oncogene-transformed MCF10A cells (Fig. 2E), the supernatant of DACH1 transduced MDA-MB-231 cells was sufficient to block migration of MDA-MB-231 cells (Fig. 4A). An unbiased proteomic approach to identify candidate proteins regulated by DACH1 expression, using antibody array analysis, demonstrated DACH1 expression correlated with reduced secretion of IL-8 and the related chemokines IL-1, IL-6, GRO, and MIP1 (α, β, γ) (Fig. 4B). IL-8, but not the related chemokines GRO, MCP1, or MIP1α, was capable of rescuing the DACH1-mediated migratory defect (Fig. 4C).
Fig. 4.
Proteomic analysis of DACH1-regulated chemokines. (A) Transwell migration assay of MDA-MB-231 cells treated with conditioned media derived from either vehicle control or ponasterone A-treated cells. (B) Cytokine array analysis of supernatant derived from MDA-MB-231 cells treated with either vehicle or ponasterone A to induce DACH1 identified a subset of cytokines differentially regulated by DACH1. Relative abundance is shown, comparing DACH1 expressing cells (PonA) with control. (C) Transwell migration assays of MDA-MB-231 cells treated with cytokines as indicated, including interleukin-8 or control IgG at 5 ng/ml. Migration of cells is shown as cell number per field with data as mean ± SEM for n > 5 separate experiments. (D) 3D collagen invasion assay of MCF10A-Ras cells expressing control (GFP) or DACH1 in the presence of IL-8 or vehicle. The mean depth of migration is shown quantified as mean ± SEM. (E) Cellular migration of MEFs derived from Dach1 WT or knockout mice, using time lapse video microscopy. Dach1−/− (KO) MEFs display enhanced cellular migratory velocity and distance (P < 0.01). (F) The number of lung metastasis identified in nude mice injected with Met-1 cells transduced with pLRT-DACH1, treated with vehicle or Doxycycline DACH1 or immunoneutralizing antibody to CRCL1/KC. *, P < 0.01.
The ability of MCF10A-Ras cells to invade a collagen matrix was assessed (15), because this process may more closely recapitulate the in vivo invasive phenotype. The refractile cells identified distal to the cellular margin after 5 days were used as a measure of cellular invasion of collagen. DACH1 expression reduced cellular invasion of a collagen matrix by ≈50% (Fig. 4D). The addition of IL-8 rescued DACH1-mediated repression of cellular migration. Mouse embryo fibroblasts (MEFs) derived from Dach1 knockout mice demonstrated a doubling of migratory velocity and distance traveled by time-lapse video microscopy (Fig. 4E). Thus, endogenous Dach1 inhibits cellular migration.
Next, we examined the role of DACH1 and IL-8 in regulating breast tumor metastasis in vivo, using the highly metastatic breast cancer cell line. Induced expression of DACH1 by doxycycline in Met-1 cells inhibited migration in transwell assay (Fig. S2 A and B). All mice injected with Met-1 cells developed widespread lung metastasis, as evidenced by India ink staining and histopathology (Fig. 4F). Because DACH1 inhibited IL-8 production in MDA-MB-231 cells and inhibited production of the murine homologue of IL-8, CRCL1/KC (16), in Met-1 cells (Fig. S2C), we determined the role of KC (Cytokine-induced neutrophil chemoattractant) in Met-1 cell metastasis in vivo, using an immunoneutralizing antibody to KC after introduction of Met-1 cells into nude mice. The administration of αKC antibody blocked breast tumor metastasis (Fig. 4F).
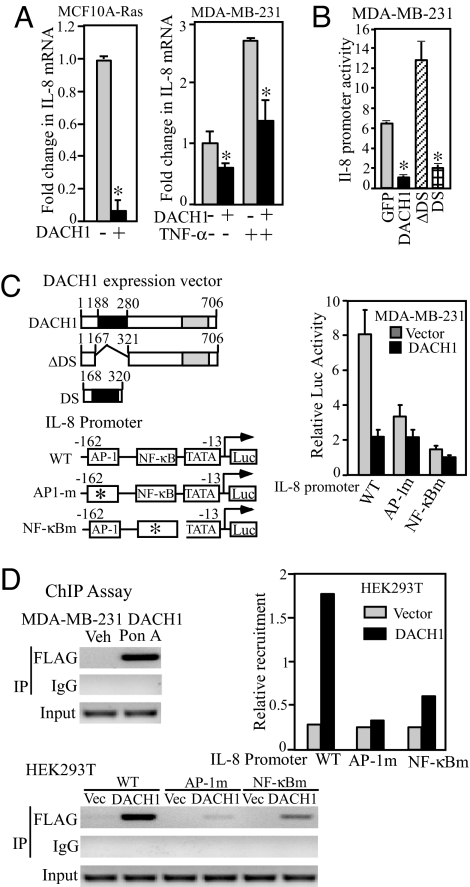
Altered expression of the IL-8 gene is seen in many cancers and increased expression correlates strongly with metastatic potential of breast carcinomas cells (17) Ras-induced IL-8 expression is essential for tumor growth and angiogenesis (7). The IL-8 gene has been strongly implicated in promoting breast tumor cellular migration. IL-8 mRNA levels were reduced ≈90% by DACH1 in MCF10A-Ras and MDA-MB-231 cells (Fig. 5A). TNF-α-dependent IL-8 expression (18) was inhibited by DACH1 expression (Fig. 5A). The IL-8 promoter was repressed by DACH1 in a dose-dependent manner, requiring the DS domain (Fig. 5B).
Fig. 5.
The IL-8 promoter is repressed by DACH1 requiring the DS domain. (A) IL-8 mRNA abundance was determined by QT-PCR in MCF10A-Ras or MDA-MB-231 cells expressing DACH1. In MDA-MB-231 cells, TNF-α was used to induce IL-8 production. (B and C) Schematic representation of DACH1 expression vectors and mutants, IL-8 promoter and point mutant luciferase reporter gene contractions. The effect of DACH1 on IL-8 promoter activity is shown normalized to Renilla Luciferase activity as an internal control. Data are mean ± SEM of n > 5 separate experiments. (D) ChIP analysis of DACH1 recruitment to either the endogenous IL-8 promoter in MDA-MB-231 cells (above) or to transfected IL-8 promoter constructions encoding either the WT, AP-1, or NFκB point mutants of the IL-8 promoter in HEK293 cells. Each experiment was conducted on at least two separate occasions with the FLAG antibody and was also conducted by using an antibody directed to DACH1 with similar results. Quantitative data of relative abundance of DACH1 recruitment to the IL-8 promoter is shown as mean data. *, P < 0.01.
To determine the molecular mechanisms by which DACH1 repressed the IL-8 promoter, a series of point mutants within key transcriptional regulatory elements were examined (Fig. 5C). DACH1 repressed the IL-8 promoter by 80%. Mutation of the IL-8 promoter AP-1 site reduced IL-8 promoter activity 75% resulting in a promoter fragment that was significantly reduced in DACH1-mediated repression. Similarly, mutation of the NFκB binding site substantially reduced DACH1 repression (Fig. 5C). DACH1 was identified by ChIP analysis in the MBA-MB-231 cell, using the FLAG antibody in the context of the endogenous IL-8 promoter (Fig. 5D). ChIP analysis, using the FLAG antibody to immunoprecipate DACH1, demonstrated reduced recruitment upon mutation of either the AP-1 or NFκB sites (Fig. 5D).
Discussion
The molecular mechanisms attenuating heterotypic signaling that govern oncogene-induced tumor progression and invasion are poorly understood. The current studies demonstrate that the cell-fate determination factor DACH1, the expression of which is lost in poor prognosis invasive breast cancer (9), inhibits cellular migration and metastasis. Our result of >2,000 breast cancer samples correlated with death from breast cancer 40 months earlier in patients with reduced tumor DACH1 expression (9). The expression of DACH1 was dramatically reduced in invasive breast cancer compared with normal breast tissue and DACH1 expression was reduced in invasive cancer compared with noninvasive (9). Using mouse embryo fibroblasts derived from Dach1−/− mice, we demonstrate herein that endogenous Dach1 serves to constitutively inhibits cellular migration and cellular migratory velocity.
Ras-induced IL-8 production plays a key role in tumor progression (7). Herein, DACH1 inhibited IL-8 expression and secretion. Restoration of IL-8 rescued the cellular migratory defect induced by DACH1 and, either DACH1 or immunoneutralizing antibody to the murine homologue of IL-8 (CRCL1/KC) was sufficient to block breast cancer metastasis to the lungs in vivo. Collectively, these studies indicate that DACH1 repression of IL-8 production is a key mechanism contributing to the anti-migratory function of DACH1. DACH1 inhibited breast cancer cellular migration involving a secreted factor that was identified as IL-8 through an unbiased proteomic analysis. DACH1 directly repressed the IL-8 promoter, defining a mechanism by which breast tumor chemokine production and breast cancer progression may be attenuated.
IL-8 production was repressed by physiological levels of DACH1. The abundance of DACH1 varies during the cell cycle 2- to 3-fold, and the relative abundance of total cellular DACH1 induced by ponasterone A was a relatively modest 2- to 3-fold increase (9). The inhibition of IL-8 production was observed in cells stably expressing DACH1 and in cells induced to acutely express DACH1, suggesting that the reduction in IL-8 is not the consequence of a compensatory delayed secondary event consequence upon sustained DACH1 expression. DACH1 inhibited IL-8 production from Ras or c-Myc transformed MCF-10A cells and repressed IL-8 promoter activity (Fig. S1 B and C). Interleukin-8 (IL-8) is a CXC chemokine, initially purified as a small basic protein that functions as a neutrophil chemoattractant (19). Highly metastatic breast carcinoma cell lines produce large amounts of IL-8 compared to low metastatic breast cancer cell lines (20). The current studies demonstrate that the murine homologue of IL-8 (CRCL1/KC) also governs cellular metastasis and that DACH1 is a key regulator of CRCL1/KC production.
Clinical studies have documented up-regulation of CXCL-8 in human breast cancer (17) and non-small cell lung cancer (17), melanoma (21), and colon cancer. The expression of CXCL-8 is linked to poor prognosis and metastatic phenotype in several tumor types, including human colon, pancreatic, and prostate cancer. DACH1 expression inhibited IL-8 secretion, mRNA abundance, and IL-8 promoter activity. DACH1 repression of the IL-8 promoter required the AP-1 and NF-κB binding site and DACH1 was identified at the IL-8 promoter in the context of local chromatin in ChIP assays. Studies have shown DACH1 is recruited to either Six binding sites or AP-1 binding sites and that DACH1 is recruited in the context of local chromatin to conduct transcriptional regulatory functions (9, 11). DACH1 is not thought to bind DNA directly in a DNA sequence specific manner. In contrast, DACH1 is known to inhibit c-Jun transactivation through recruitment of HDAC1 and HDAC3 to the γ domain of c-Jun (14) and herein did not affect c-Jun or p50 occupancy at the IL-8 promoter in ChIP assays (Fig. S1D).
DACH1 inhibited ERK but not Akt activity (Fig. S1E), suggesting a potential role for ERK in the intracellular signaling pathway that regulates IL-8 and is inhibited by DACH1. Hypoxia is known to induce IL-8, although the mechanism is not directly mediated by hypoxia inducible factor 1 (HIF1). The induction of IL-8 involves NF-κB (8). Oncogenic Ras and Rac are known to induce NF-κB (22) and IL-8 abundance (7). Whether DACH1 repression involves NF-κB signals remains to be determined.
DACH1 expression is reduced in human breast cancer and DACH1 inhibited breast tumor cellular proliferation and DNA synthesis (9). The DACH1 gene product associates with Six, and Dac/Six together contribute to the RD pathway in development. Misregulated expression of Six proteins also occurs in human cancer (23). HSIX1, the human homologue of the Drosophila Six gene, is overexpressed in breast cancer and forced Six1 expression attenuates a G2 cell cycle check point (23). Six1 has been implicated in regulating metastasis, enhancing migration of poorly metastatic rhabdomyosarcoma tumors (24). The possibility that the migration and invasion pathway governed by DACH1 described herein functions through the genetic targets of the cell fate determination pathway that include Eya/Six remains to be determined.
Methods
Detailed materials and methods are in SI Methods. In brief, the expression plasmids for DACH1, DACH1 DS-domain alone (DS), or DACH1 DS-domain deleted (ΔDS) (which include an N-terminal FLAG peptide), the ponasterone-regulated expression system, the full-length and point mutants of the IL-8 promoter luciferase reporter construction, were described in refs. 25–28. MCF10A-ΔRaf-ER cells (29), MCF10A-Ras, Ras/ErbB2, ErbB2 (NeuT), c-Myc-transformed cells (9), and mouse embryo fibroblasts (MEF) prepared from Dach1−/− mice (30) were cultured (25, 31). Western blot analysis was performed by using antibodies to cyclin D1, ErbB2, phospho-ERK, phosphor-AKT, Myc, FLAG, and the loading control guanine dissociation inhibitor (GDI) (27, 32). 3D culture of cells (33), transwell migration assays (34), and 3D invasive activity (15) were assessed.
Migration and in Vivo Metastasis Assays.
In vivo metastasis were assessed in nude mice from the National Cancer Institute, after tail vein injection, using 1 × 104 cells (9). Doxycycline was used to induce the DACH1 transgene expression via drinking water at a final concentration of 1 μg/ml. Staining for lung metastasis of Met-1 cells, using india ink was conducted (35). Anti-mouse CRCL1/KC antibody (R&D Systems) (36) was given by i.p. injection (100 μg) as described for IL-8 antibody (8). In wound healing assays, cells were grown to confluence on 12-well plates (37). Time-lapse video images were collected and stored as images, using Metamorph software, Version 3.5 (34).
Cytokine Array Analysis.
Human cytokine arrays spotted on nitrocellulose membranes were obtained from Raybiotech (33). Conditioned medium from MDA-MB-231 cells expressing ponasterone-regulated DACH1 was prepared by culturing cells in serum free DMEM for 24–48 h.
Real-Time PCR for Assessment of Expression of IL-8 Transcripts and ChIP Assays.
Equal amounts of purified RNA samples were reverse-transcribed by using an Iscript reverse transcriptase kit (Bio-Rad) to form cDNA, which was subjected to SYBR Green based real-time PCR relative quantification method for amplification of IL-8 transcripts (15). Endogenous IL-8 promoter ChIP assays used antibodies directed to either the FLAG epitope of DACH1 or endogenous DACH1 and primers to the human IL-8 gene promoter.
Supplementary Material
Acknowledgments.
We thank Vassilis Altmazoglou and Trias Thiraiou for the mapping of the Affymetric gene ID to the Ensembl gene ID and the initial calculation on the hexamer distribution. This work was supported in part by National Institutes of Health Grants R01CA70896, R01CA75503, R01CA86072, R01CA107382 (R.G.P.) and by the Margaret Q. Landenberger Research Foundation (K.W.). The Kimmel Cancer Center was supported by the National Institutes of Health Cancer Center Core Grant P30CA56036 (to R.G.P.). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from the Pennsylvania Department of Health (to R.G.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802085105/DCSupplemental.
References
- 1.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 3.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 4.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 6.Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 7.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Mizukami Y, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 9.Wu K, et al. DACH1 is a cell fate determination factor that inhibits Cyclin D1 and breast tumor growth. Mol Cell Biol. 2006;26:7116–7129. doi: 10.1128/MCB.00268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardon G, Solomon NM, Rubin GM. Dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development (Cambridge, UK) 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- 11.Li X, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 12.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 13.Halder G, et al. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development (Cambridge, UK) 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- 14.Wu K, et al. The cell fate determination factor DACH1 inhibits c-Jun induced contact-independent growth. Mol Biol Cell. 2007:755–767. doi: 10.1091/mbc.E06-09-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katiyar S, Jiao X, Wagner E, Lisanti MP, Pestell RG. Somatic excision demonstrates c-Jun induces cellular migration and invasion through induction of stem cell factor. Mol Cell Biol. 2007;27:1356–1369. doi: 10.1128/MCB.01061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wislez M, et al. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- 17.Wang XL, et al. Localization of a tumour-suppressor gene associated with human oral cancer on 7q31.1. Int J Cancer. 1998;75:671–674. doi: 10.1002/(sici)1097-0215(19980302)75:5<671::aid-ijc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-α-induced NF-κB/RelA Ser276 phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cellular Signalling. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 20.De Larco JE, Wuertz BR, Yee D, Rickert BL, Furcht LT. Atypical methylation of the interleukin-8 gene correlates strongly with the metastatic potential of breast carcinoma cells. Proc Natl Acad Sci USA. 2003;100:13988–13993. doi: 10.1073/pnas.2335921100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetze S, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension. 2002;40:748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 22.Joyce D, et al. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- 23.Coletta RD, et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA. 2004;101:6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, et al. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–181. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 25.Albanese C, et al. Sustained mammary gland-directed, ponasterone A-inducible expression in transgenic mice. FASEB J. 2000;14:877–884. doi: 10.1096/fasebj.14.7.877. [DOI] [PubMed] [Google Scholar]
- 26.Albanese C, et al. IKKalpha regulates mitogenic signaling through transcriptional induction of Cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K, et al. DACH1 inhibits TGF-beta signaling through binding Smad4. J Biol Chem. 2003;278:51673–51684. doi: 10.1074/jbc.M310021200. [DOI] [PubMed] [Google Scholar]
- 28.Casola A, et al. Interleukin-8 gene regulation in intestinal epithelial cells infected with rotavirus: Role of viral-induced IkappaB kinase activation. Virology. 2002;298:8–19. doi: 10.1006/viro.2002.1475. [DOI] [PubMed] [Google Scholar]
- 29.Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis RJ, Pesah YI, Harding M, Paylor R, Mardon G. Mouse Dach2 mutants do not exhibit gross defects in eye development or brain function. Genesis. 2006;44:84–92. doi: 10.1002/gene.20188. [DOI] [PubMed] [Google Scholar]
- 31.Albanese C, et al. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;274:34186–34195. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- 32.Lee RJ, et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ju X, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci USA. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams TM, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 36.Witowski J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 37.Neumeister P, et al. Cyclin D1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.