Abstract
Apolipoprotein D (ApoD) expression increases in several neurological disorders and in spinal cord injury. We provide a report of a physiological role for human ApoD (hApoD): Flies overexpressing hApoD are long-lived and protected against stress conditions associated with aging and neurodegeneration, including hyperoxia, dietary paraquat, and heat stress. We show that the fly ortholog, Glial Lazarillo, is strongly up-regulated in response to these extrinsic stresses and also can protect in vitro-cultured cells in situations modeling Alzheimer's disease (AD) and Parkinson's disease (PD). In adult flies, hApoD overexpression reduces age-associated lipid peroxide accumulation, suggesting a proximal mechanism of action. Similar data obtained in the mouse [Ganfornina, M.D., et al., (2008) Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell 10.1111/j.1474-9726.2008.00395.] as well as in plants (Charron et al., personal communication) suggest that ApoD and its orthologs play an evolutionarily conserved role in response to stress, possibly managing or preventing lipid peroxidation.
Keywords: aging, Alzheimer, β-amyloid, GLaz, oxidative stress
Apolipoprotein D (ApoD) is a small, soluble lipid carrier found in most human tissues, but especially in glia of the nervous system (1–4). The protein is highly conserved among mammals, and close homologs also can be found in plants and bacteria, implying an important basic function. ApoD is elevated in many pathological situations, including Alzheimer's disease (AD), Parkinson's disease (PD), stroke, schizophrenia, and bipolar disorder (5–11). In AD, it can be found in amyloid plaques within the brains of patients (12). It is up-regulated 500-fold at the site of the sciatic nerve crush injury in the rat (13, 14). In vitro evidence indicates that it can carry membrane lipids, such as arachidonic acid and sterols (15–18), and may be involved in the clearance and/or repair of damaged membranes, perhaps quenching harmful material released by neurons and glial cells in response to damage or recruiting lipids to expanding membranes. In the etiology of many of the disorders in which ApoD is elevated, oxidative stress is thought to play an important part (19, 20). For example, in AD, β-amyloid (Aβ42) is known to produce free radicals and hydrogen peroxide, which can in turn damage surrounding cells (21), causing an accumulation of lipid peroxides and protein carbonylation adducts. Recently, mitochondrial oxidative stress also has been implicated in the genesis of hyperphosphorylated τ, another canonical feature of AD (22).
In an unbiased screen to identify genes that protect Drosophila against hyperoxia (100% O2), we discovered that transgenic overexpression of a fly ortholog of ApoD, Glial Lazarillo (Glaz), could protect against a range of extrinsic stressors and extend the lifespan (23). The relatively high level of homology between human ApoD (hApoD) and GLaz led us to propose GLaz as a fly model of ApoD [see supporting information (SI) Fig. S1] (24, 25). We asked whether this effect of GLaz overexpression could provide insight into the role of ApoD up-regulation in neurological disorders because, to date, no correlation had been established between high ApoD levels and protection against degeneration. In the first part of this article, we demonstrate, in our transgenic system, that hApoD can protect Drosophila under stress conditions relevant to pathological processes and extend the lifespan. Overexpression of hApoD also reduces the age-related accumulation of lipid peroxides.
We show that, in adult flies, extrinsic stressors also induce GLaz expression, reinforcing the notion that GLaz, the fly ortholog of ApoD, also is part of a canonical stress response. Moreover, GLaz overexpression in Drosophila S2 cell cultures can protect against Aβ42-induced cytotoxicity and paraquat, suggesting that the elevation of ApoD in AD or PD may play a role in salvaging neurons under oxidative stress. This study looks at the targeted effects of overexpressed hApoD on stress resistance and longevity in a model organism.
Results
Generation of hApoD Transgenic Flies.
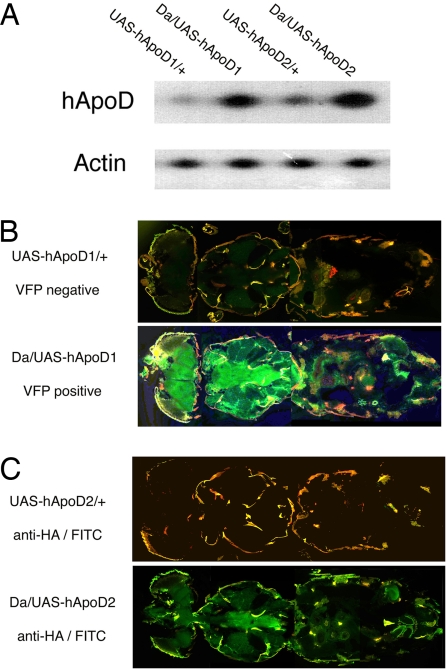
We generated two independent lines carrying hApoD cDNA under the control of GAL4-binding sites (UAS) by using a gateway-modified pUASt expression vector. In the line UAS-hApoD1, hApoD cDNA is fused with Venus Fluorescent Protein (VFP) cDNA at its C terminus, whereas in UAS-hApoD2, hApoD cDNA is fused with a C-terminal HA (influenza hemagglutinin) tag. Both insertions map to the third chromosome. In both lines, there is a GAL4-dependent increase in the amount of hApoD mRNA (Fig. 1A) when driven by the ubiquitous driver Daughterless-GAL4 (Da). This mRNA was not detected in control flies (w1118) (data not shown), but there was some weak transcription in heterozygous flies lacking the driver. Fig. 1B displays the green fluorescence (at 485 nm) induced by Da on UAS-hApoD1 (Fig. 1B Lower), confirming that the C-terminus fusion allows proper folding of VFP. Da/+ flies are not fluorescent (data not shown), whereas UAS-hApoD1/+ flies have a low background level of fluorescence, consistent with the leaky transcription detected by RT-PCR. Fig. 1C shows Da/UAS-hApoD2 overexpressing the hApoD-HA fusion. Staining with an anti-HA primary antibody, followed by a FITC-conjugated secondary antibody (Fig. 1C Lower), reveals expression of the HA tag in a GAL4-dependent fashion. Although we know from the RT-PCR result that some hApoD-HA is transcribed, no fluorescent signal was observed in UAS-hApoD2/+ flies (Fig. 1C Upper) probably because of the lower detection sensitivity of this protocol.
Fig. 1.
Generation of two hApoD transgenic lines by using the UAS/GAL4 system. (A) RT-PCR shows hApoD driven by Da in each strain compared with control strains. (B) Horizontal cryosection through Da/UAS-hApoD1 shows strong, ubiquitous green VFP signal (the red color is due to cuticle autofluorescence). Normal signal is seen in UAS-hApoD1/+ control flies. (C) Horizontal cryosection through Da/UAS-hApoD2, which carries the HA-tag. Green fluorescence (FITC) reveals the presence of hApoD, stained by HA antibody, in most tissues (less intense than the VFP marker).
hApoD Overexpression Extends Normal Lifespan in Drosophila.
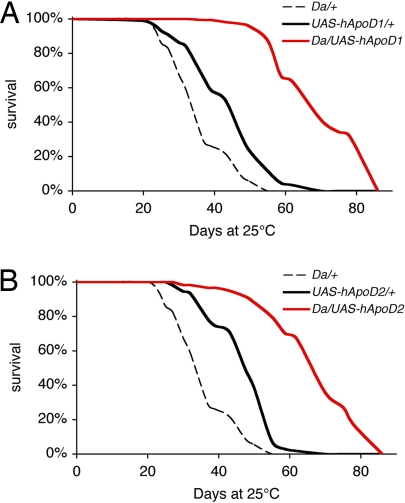
In humans, hApoD is known to be up-regulated with age possibly in response to accumulated damage (7). We used the ubiquitous Da to strongly drive expression of UAS-hApoD transgenes in the whole organism. By using the fact that GAL4 expression is reduced at lower temperatures (26), expression was minimized during early development by raising the larvae and pupae at 18°C on standard food (27). Crosses of w1118 to either transgene or driver were used as genetic controls and also raised at 18°C on standard food. For all genotypes, we raised adult flies on standard food at 25°C and followed their survival. Flies overexpressing hApoD (Da/UAS-hApoD1) had 40% longer median lifespans (Fig. 2A) than the longest-lived control (UAS-hApoD1/w1118). For UAS-hApoD2, the median lifespan was increased by 41% (Fig. 2B). In each case, maximum lifespan also was improved.
Fig. 2.
Overexpression of hApoD extends normal lifespan of Drosophila. Survival was recorded on standard food in normoxia at 25°C. (A) Da/UAS-hApoD1 flies had a 40% longer median lifespan than UAS-hApoD1/+ controls (P < 0.001). Maximum lifespan also was improved by 20%. (B) Da/UAS-hApoD2 flies had a 41% longer median lifespan than UAS-hApoD2/+ controls (P < 0.001). Maximum lifespan also was improved by 19%.
Overexpression of hApoD Protects Drosophila Against Various Extrinsic Stresses.
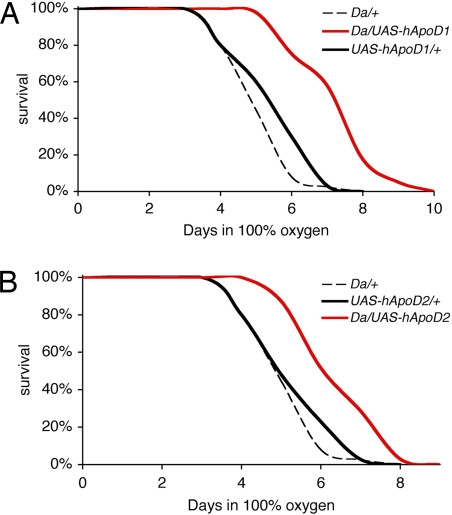
We next exposed the hApoD transgenic flies and their genetic controls to 100% O2 for their entire adult lives in the paradigm originally used to isolate GLaz as a stress-resistance gene. As previously shown (28), such an exposure, for normal flies, results in mitochondrial damage consistent with elevated oxidative stress, as well as apoptotic muscle degeneration and death within a week. This treatment can be considered to be a model of accelerated pathological aging. Flies overexpressing UAS-hApoD1 (Da/UAS-hApoD1) throughout adult life lived on average 36% longer than the longest-lived control (UAS-hApoD1/+) under hyperoxia (Fig. 3A). Da/UAS-hApoD2 flies lived 25% longer than UAS-hApoD2/+ flies (Fig. 3B) under the same conditions. Maximum lifespans also were improved significantly.
Fig. 3.
Overexpression of hApoD protects against hyperoxia in Drosophila. Survival was recorded on standard food in 100% O2 at 25°C. (A) Da/UAS-hApoD1 flies had a 25% longer median lifespan than UAS-hApoD1/+ controls (P < 0.001). Maximum survival also was improved by 20%. (B) Da/UAS-hApoD2 flies had a 20% longer median survival than UAS-hApoD2/+ controls (P < 0.001). Maximum survival also was improved by 15%.
Next, we assessed whether hApoD overexpression increases resistance to dietary paraquat, which is thought to target complex I of the mitochondrial respiratory chain, thereby producing deleterious reactive oxygen species. Paraquat has been used to model PD (29). When hApoD was overexpressed in flies exposed to 20 mM paraquat (see Materials and Methods), there was a 40% longer median survival on paraquat in Da/UAS-hApoD1 than in their control counterparts (Fig. S2A). Similarly, Da/UAS-hApoD2 were 38% longer-lived, on average, than either of their genetic controls (Fig. S2B), although the maximum lifespans in the population were not affected.
Heat stress was tested by exposing the flies in standard food vials to 37°C. Da/UAS-hApoD1 flies had a median lifespan 35% longer than UAS-hApoD1/+ controls (Fig. S3A), and Da/UAS-hApoD2 lived on average 40% longer than UAS-hApoD2/+ controls at 37°C (Fig. S3). In this case, maximum lifespan also increased. All of the control fly genotypes were dead within 30 h, whereas hApoD overexpressors survived to the 38-h mark.
hApoD Overexpression Reduces Accumulation of Lipid Peroxides in Old Flies.
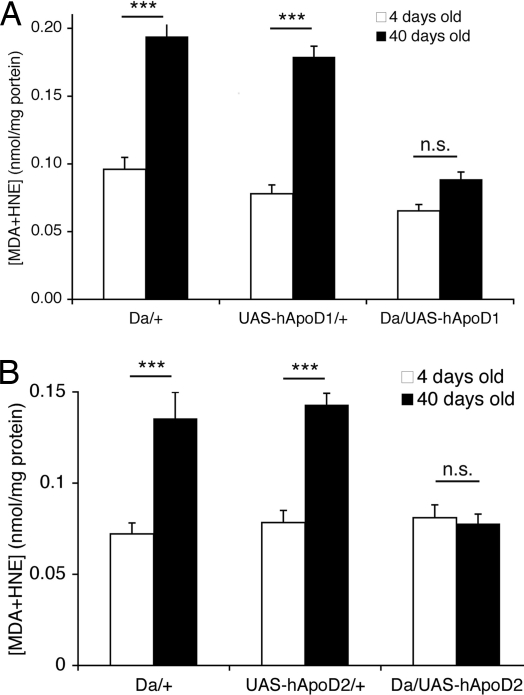
Lipid peroxides are formed when free radicals such as superoxide produced by mitochondria react with membrane and storage lipids (30). These lipid peroxides alter the normal properties of the membrane and impair cellular function. Their accumulation is a measure of oxidative stress (31), and we observed that lipid peroxides normally accumulate in wild-type Canton-S (CS) flies over the course of their life (data not shown). Here we report that hApoD appears to function, at least in part, by constraining lipid peroxides. Whereas control flies display a significantly increased lipid peroxide burden between days 4 and 40 of adult life, flies overexpressing hApoD1 or hApoD2 clearly accumulate less, maintaining the levels of younger flies (Fig. 4). These data do not address whether the reduction is at the level of generation of lipid peroxides or on their clearance.
Fig. 4.
hApoD overexpression reduces lipid peroxide accumulation in old Drosophila. Adult flies were maintained on standard food at 25°C. (A) Da/UAS-hApoD1 flies did not display any significant increase in lipid peroxide burden at 40 days of age, whereas control flies saw this burden increase 2-fold during the same period (***, P < 0.005, t test). (B) Similar results were found with the Da/UAS-hApoD2 flies compared with controls (***, P < 0.005, t test).
Up-Regulation of the Fly Ortholog of hApoD, GLaz, by Extrinsic Stress.
Given the up-regulation of hApoD in various diseases involving chronic stress, as in the degeneration seen in stroke, AD, or PD, we wondered whether individual stresses also would regulate expression of the fly ortholog in our model in situations of acute exposure and within a short timeframe.
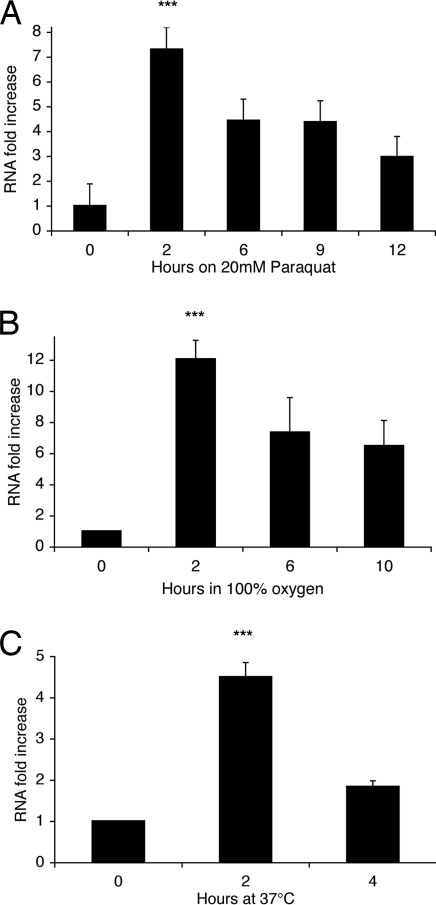
Flies lacking GLaz are particularly sensitive to paraquat treatment (32), whereas flies overexpressing GLaz have enhanced resistance (J.M., unpublished data). Paraquat feeding can be viewed as a model of environmentally induced neurodegeneration. We compared, by quantitative real-time PCR (qRT-PCR), the levels of GLaz mRNA in flies fed paraquat plus sucrose to flies fed sucrose only over the course of 12 h (Fig. 5A). Even at the earliest time point of 2 h, GLaz was dramatically up-regulated, with a 7-fold induction over its normal level. This transcriptional induction decreased gradually, but was still up-regulated 3-fold at 12 h.
Fig. 5.
Induction of GLaz mRNA in wild-type flies (CS) by extrinsic stress. Data represent fold increase in GLaz mRNA levels compared with flies in control conditions, assessed by qRT-PCR (t = 0, normoxia, standard food). (A) On 5% sucrose/20 mM paraquat, demonstrating a dramatic up-regulation of GLaz mRNA. Induction peaked at 7-fold after 2 h and decreased back to 3-fold after 12 h (control: t = 0, no paraquat). (B) In 100% O2 on standard fly food. GLaz mRNA induction peaked at 12-fold after 2 h and stabilized at 6-fold after 10 h. (C) At 37°C on standard fly food. GLaz mRNA was induced 4.5-fold at 2 h and remained up-regulated 2-fold after 4 h. During the time frame of each experiment, no death occurred. All values were normalized by using housekeeping genes rp49 and TBP as references (see Materials and Methods). Values are averages of three independent experiments ± SEM. Student's t test performed on the Ct values yielded P values (***, P < 0.005).
We subsequently performed qRT-PCR on GLaz mRNA on young wild-type flies (CS) exposed to 100% O2, compared with flies remaining under normoxic conditions. The transcript abundance of GLaz (Fig. 5B) is strongly regulated by hyperoxia exposure. Remarkably, the GLaz transcript signal rapidly increased 12-fold in the first 2 h and continued to be elevated 5-fold after 10 h.
Given that the heat-shock response lies downstream of most oxidative stress responses, we also looked at the induction of GLaz after exposure to high temperature. Flies in standard food vials were placed in a 37°C incubator. Fig. 5C shows that GLaz is once more a strong responder, with a 4.5-fold up-regulation at 2 h, followed by a decline.
Drosophila Cells Are Protected from Aβ42 and Paraquat Toxicity by GLaz Overexpression.
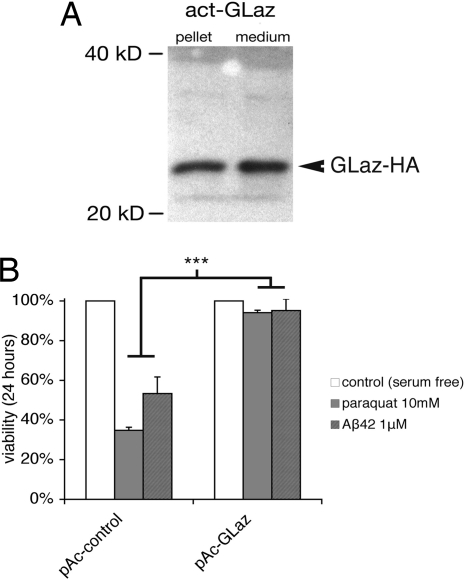
As a paradigm in which the beneficial effects of Glaz could be monitored more closely, at the cellular level, the effects of transient overexpression of GLaz in Drosophila S2 cell cultures were examined. We generated constructs allowing the expression of HA-tagged GLaz under an actin promoter. Transfected cells expressed the HA-tagged protein (Fig. 6A), secreting it in the culture medium. As reported by a cotransfected Blue Fluorescent Protein (BFP), the transfection efficiency was 30%. These cells were subsequently placed in serum-free medium to avoid interference by bovine lipoproteins and exposed to 10 mM paraquat for 24 h. After this oxidative insult, only 35% of cells transfected with a sham plasmid carrying the actin promoter alone were still alive, but cells overexpressing GLaz were protected, showing no significant cell death attributable to this paraquat exposure (Fig. 6B).
Fig. 6.
GLaz protects Drosophila S2 cells against death induced by paraquat and Aβ42. (A) Anti-HA Western blot showing that GLaz-HA is expressed under the actin promoter in Drosophila S2 cells and secreted into the medium. (B) S2 cells were transfected for 48 h and then exposed to 10 mM paraquat or 1 μM Aβ42 for 24 h and assayed for viability. Cells transfected with a control plamid (actin promoter alone, pAc) showed low survival when exposed to paraquat or Aβ42 (35% and 53%, respectively). GLaz overexpression by pAc-GLaz was sufficient to protect the cells against the stress of both paraquat and Aβ42 (94% and 95% viability, respectively, after 24 h; ***, P < 0.005, t test).
Aβ42 overexpression is sufficient to cause AD-like pathology in animal models (33), and it has been shown that Aβ42 can foster the production of the reactive oxygen species hydrogen peroxide and superoxide via Fenton chemistry (21). The oligomeric form of Aβ42 is toxic to neurons and glial cells in culture. It is thought to be the main cytotoxic species in vivo and is found in the cerebrospinal fluid (CSF) of AD patients (34). ApoD is also up-regulated in AD and found in the vicinity of plaques (12). Therefore, we wondered whether GLaz could protect from Aβ42 cytotoxicity. Transfected Drosophila S2 cells were incubated for 24 h with 1 μM Aβ42 preaggregated into its cytotoxic oligomeric form. Remarkably, we found that the overexpression of GLaz is indeed protective against Aβ42 cytotoxicity (Fig. 6C).
Discussion
These observations are in agreement with a conserved regulation and function of ApoD and its homologs under stressful conditions. The results obtained with heat stress point to the well recognized overlap in regulation of the oxidative stress response and the heat-shock pathway (35). More studies are needed to establish to what extent the expression of GLaz is under control of the heat shock or other stress-inducible pathways. Because many of the pathological situations where ApoD is up-regulated in mammals also are thought to involve oxidative stress and induction of the heat-shock pathway, this line of investigation is important. It also is intriguing to ask whether such direct stress responses can be observed in mammalian systems. In a recent study of mouse ApoD in cultured cells, the authors observed the induction of mouse ApoD by similar stress conditions (36), along with a thought-provoking translocation to the nucleus. Up-regulation of the plant ortholog (atTIL) also is seen upon exposure to temperature stress. In a previous study of gene expression, changes after exposure of Drosophila to hyperoxia (37) alterations in GLaz expression were not found. However, a critical difference between that study and ours is that we looked for changes after very short exposures to hyperoxia, whereas they examined the gene expression after 7 days under hyperoxia. Note that the levels of induction of GLaz observed here are similar to the 7-fold overexpression we induced in flies using the UAS/GAL4 system in a previous study (23). That overexpression was beneficial to the organism over its entire life. The inductions we observe occur very early in a process that will eventually lead to death, whether by heat or oxidative stress.
In transgenic flies, we observe a remarkable increase in lifespan and multistress resistance in hApoD overexpressors. Independently of the results obtained for GLaz and the homology that rooted our interest, these results clearly indicate a beneficial function of hApoD and may explain its up-regulation in various disorders. Such an up-regulation may limit cumulative processes, typical of age-related disorders, such as lipid peroxidation, protein carbonylation, or DNA modifications. This may take effect by either accelerating recycling or preventing formation of adducts in the first place. Other lipocalins have been identified as direct scavengers of potentially harmful lipophilic molecules (38). Our results with Drosophila S2 cells support the idea that ApoD homologs also may protect cells in acute stress situations, providing a possible beneficial role for chronic ApoD secretion by astrocytes in the brains of patients with AD or PD and its acute secretion by glia in crushed peripheral nerves. As neurons strive to regenerate damaged neurites, glial cells known to express ApoD could provide local trophic support or sequester arachidonic acid, thereby tempering inflammation. The non-cell-autonomous aspect is exemplified by our cell culture results, where a majority of cells are protected regardless of whether they express the transgenes. In humans, lipoproteins produced outside of the nervous system do not pass the blood–brain barrier, therefore it is likely that glial cells would be the most important reservoir of ApoD in stressful conditions and could modulate the levels of this protein in the CSF.
We provide evidence that hApoD is more than a neutral bystander, let alone a deleterious actor, in pathological situations in an animal model. This article strongly suggests that induction of ApoD might be worthy of investigation as a possible therapeutic tool for a range of age-related diseases, including AD.
Materials and Methods
Drosophila Strains.
Da-Gal4 (Da) was obtained from the Drosophila Stock Center (Bloomington, IN). The Drosophila strain white1118 was used in all control crosses, as the background for generation of transgenic lines, and to outcross (10 times) the driver line Da. CS flies were used as wild type for the GLaz mRNA-induction experiments. Male flies were used throughout the study.
Histology.
Whole flies were frozen in OCT medium, and horizontal serial 10-μm cryosections were prepared. For VFP detection, the sections were immediately mounted in DAPI-glycerol and imaged by using a Zeiss Axioimager with Apotome (10×). For HA detection, the sections were fixed in 4% paraformaldehyde, washed in PBS, incubated with a rabbit anti-HA primary (1:100), and stained with FITC-conjugated anti-rabbit (1:250). Control sections were stained with the secondary antibody alone.
Lifespans.
All crosses, including controls, were performed at 18°C to minimize the effects of GAL4 during development. After eclosion, the adults were maintained at 25°C. For each lifespan experiment, at least 100 two-day-old males were separated from females while anesthetized in 100% N2. Then 20–30 flies were put in a single vial containing standard food (27) and transferred every 3–4 days to a fresh vial, and the number of dead flies was recorded. Survival curves were analyzed by using the Graphpad Prism 4 software yielding P values from a log-rank test.
Exposure to Hyperoxia.
Two-day-old adult males, 20–30 flies per vial containing standard food, were maintained in a 28 × 28 × 24-inch Plexiglas enclosure at room temperature (22–24°C). O2 (100%) was passed through the box at a constant rate of 300 ml/min.
Paraquat Feeding.
For each experiment, at least 100 two-day-old flies, at 20–30 flies per vial, were transferred daily to fresh medium, and the number of dead flies was recorded approximately every 2 h. For controls, the vials contained 5 ml of 1% agar and 5% sucrose, whereas paraquat vials contained the same food with 20 mM paraquat added.
Generation of Transgenic Flies.
hApoD cDNA was kindly provided by Elizabeth Thomas and J. Gregor Sutcliffe (University of California at San Diego, La Jolla, CA) in a pcDNA3.1 vector and amplified by PCR. The cDNA was then subcloned into an entry vector for the Gateway system (Invitrogen) using the TOPO system. Following the manufacturer's instructions, the cDNA fragments were recombined into a modified pUASt vector (Murphy Laboratory, Carnegie Institution, Washington, DC), putting the hApoD cDNA (without STOP codon) 3′ of UAS repeats in frame with the HA tag motif or VFP sequence. The purified vectors were sequence-verified and injected into w1118 embryos, and the w+ transformants were selected and mapped.
qRT-PCR and RT-PCR.
Total RNA was extracted from 40 wild-type flies (CS) by using TRIzol reagent (Invitrogen). RNA concentration was measured with a Nanodrop spectrophotometer, and sample concentrations were normalized. The Retroscript kit (Ambion) was used according to the manufacturer's instructions by using Oligo-dT primers. For RT-PCR of hApoD and actin, primers were designed and the products were run on a 0.8% agarose gel. For qRT-PCR of GLaz, total cDNA from CS flies exposed to the various test conditions was amplified by using a set of primers specific for GLaz, giving 100-bp amplicon spanning the first intron–exon boundary of the gene. The controls were rp49 and TBPused to normalize the cDNA amounts. SYBR green mix (Bio-Rad) was used to monitor DNA amount during the PCR for 40 cycles by using an iQ5 thermal cycler (Bio-Rad). A fluorescence threshold was chosen in the linear portion of the amplification reaction, and the cycle number (Ct) needed to cross that threshold was recorded for each sample. Analysis of GLaz and control gene values stressed and in normal conditions yielded the ΔΔCt values. By using the Pfaffl method (39) with precalculated primer efficiencies, the ΔΔCt values were converted to mRNA fold changes. Melting curves were established for all conditions to check that no abnormal secondary structures were forming during the PCRs.
Cell Culture.
S2 cells were maintained as adherent cultures at room temperature in Schneider's medium supplemented with 10% FBS. For viability experiments, cells were transfected with either a sham pAHW plasmid (Murphy Laboratory) containing the actin promoter and gateway cassette alone or pAHW, in which the Glaz cDNA had been subcloned by using gateway recombination, fusing the protein with a C-terminal 3xHA tag. Transfection was performed with the Fugene HD (Roche) reagent in a 9:2 ratio per the manufacturer's instructions. All plasmids were cotransfected with pAc-BFP to assess transfection efficiency. Cells were allowed to recover and express for 48 h before paraquat or Aβ42 were added to serum-free medium at respective final concentrations of 10 mM and 1 μM. Viability was assessed by using the live/dead assay (Invitrogen) per the manufacturer's instructions. Live cells appeared green under a fluorescence microscope (Zeiss axioimager Z1), whereas dead cells appeared red. Experiments were done in 96-well plates in experimental triplicates. Counting was done on aliquots of each experimental well by an experimenter blind to the assay conditions. Results were expressed as the percentage of cells alive after transfection and treatment.
Lipid Peroxidation Assay.
We used the LPO-586 assay (Oxis Research) per the manufacturer's instructions. Each sample consisted of 50 flies of each genotype and age in triplicates. Flies were homogenized in PBS with added butylated hydroxy-toluene (preventing additional lipid peroxidation during sample preparation). After centrifuging insoluble components (13,000 × g for 10 min), the clear supernatant was incubated with the assay reagents, and a blank was prepared incubating the same sample with acetonitrile instead. Again the incubated samples were spun down, and triplicate 250-μl aliquots of each sample were loaded onto a 96-well plate. Absorbance was read at 586 nm. Absorbance values of the blanks were subtracted from each sample value. A standard provided by the manufacturer was used to generate a standard curve and give a nanomolar concentration of malonyldialedhyde and 4-hydroxynonenal corresponding to the absorbances. All amounts were subsequently normalized to protein concentrations of each sample established by using a Bradford assay (Bio-Rad), yielding values in nm/mg of total protein.
Supplementary Material
Acknowledgments.
We thank Rosalind Young, Noelle De La Rosa, and Viveca Sapin for expert technical assistance; E. A. Thomas and J. G. Sutcliffe (Scripps Research Institute, La Jolla, CA) for providing hApoD cDNA; the Murphy Laboratory for providing the gateway-modified expression vectors through the Drosophila Genetic Resource Center; Michael Reid and Carl Parker for help with cell cultures; and Diego Sanchez, Maria Ganfornina, Eric Rassart, and Fathey Sarhan for help with the article and collegial sharing of concurrent data. This work was supported by McKnight Endowment Fund for Neuroscience, Ellison Medical Foundation, National Institutes of Health, and National Science Foundation grants (to S.B.) and a Muscular Dystrophy Association development grant (to D.W.W.). J.M. is a joint Ph.D. candidate of the Biology Program at California Institute of Technology and the Brain, Cognition, and Behavior (3C) doctoral school at the University of Paris VI. J.M. and D.W.W. are forever indebted to their mentor Seymour Benzer and dedicate this article to his memory.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800896105/DCSupplemental.
References
- 1.Rassart E, et al. Apolipoprotein D. Biochim Biophys Acta. 2000;1482:185–198. doi: 10.1016/s0167-4838(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 2.Provost PR, et al. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J Lipid Res. 1991;32:1959–1970. [PubMed] [Google Scholar]
- 3.Provost PR, Weech PK, Tremblay NM, Marcel YL, Rassart E. Molecular characterization and differential mRNA tissue distribution of rabbit apolipoprotein D. J Lipid Res. 1990;31:2057–2065. [PubMed] [Google Scholar]
- 4.Weech PK, et al. Apolipoprotein D: An atypical apolipoprotein. Prog Lipid Res. 1991;30:259–266. doi: 10.1016/0163-7827(91)90023-x. [DOI] [PubMed] [Google Scholar]
- 5.Terrisse L, et al. Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer's patients. J Neurochem. 1998;71:1643–1650. doi: 10.1046/j.1471-4159.1998.71041643.x. [DOI] [PubMed] [Google Scholar]
- 6.Navarro A, Del Valle E, Astudillo A, Gonzalez del Rey C, Tolivia J. Immunohistochemical study of distribution of apolipoproteins E and D in human cerebral beta amyloid deposits. Exp Neurol. 2003;184:697–704. doi: 10.1016/S0014-4886(03)00315-7. [DOI] [PubMed] [Google Scholar]
- 7.Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG. Apolipoprotein D in the aging brain and in Alzheimer's dementia. Neurol Res. 2000;22:330–336. doi: 10.1080/01616412.2000.11740678. [DOI] [PubMed] [Google Scholar]
- 8.Thomas EA, Dean B, Pavey G, Sutcliffe JG. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: Implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci USA. 2001;98:4066–4071. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutcliffe JG, Thomas EA. The neurobiology of apolipoproteins in psychiatric disorders. Mol Neurobiol. 2002;26:369–388. doi: 10.1385/mn:26:2-3:369. [DOI] [PubMed] [Google Scholar]
- 10.Thomas EA, Copolov DL, Sutcliffe JG. From pharmacotherapy to pathophysiology: Emerging mechanisms of apolipoprotein D in psychiatric disorders. Curr Mol Med. 2003;3:408–418. doi: 10.2174/1566524033479681. [DOI] [PubMed] [Google Scholar]
- 11.Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: Influence of enriched environment. J Cereb Blood Flow Metab. 2007;28:551–562. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- 12.Desai PP, et al. Apolipoprotein D is a component of compact but not diffuse amyloid-beta plaques in Alzheimer's disease temporal cortex. Neurobiol Dis. 2005;20:574–582. doi: 10.1016/j.nbd.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Boyles JK, Notterpek LM, Anderson LJ. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J Biol Chem. 1990;265:17805–17815. [PubMed] [Google Scholar]
- 14.Boyles JK, Notterpek LM, Wardell MR, Rall SC., Jr Identification, characterization, and tissue distribution of apolipoprotein D in the rat. J Lipid Res. 1990;31:2243–2256. [PubMed] [Google Scholar]
- 15.Morais Cabral JH, et al. Arachidonic acid binds to apolipoprotein D: Implications for the protein's function. FEBS Lett. 1995;366:53–56. doi: 10.1016/0014-5793(95)00484-q. [DOI] [PubMed] [Google Scholar]
- 16.Vogt M, Skerra A. Bacterially produced apolipoprotein D binds progesterone and arachidonic acid, but not bilirubin or E-3M2H. J Mol Recognit. 2001;14:79–86. doi: 10.1002/1099-1352(200101/02)14:1<79::AID-JMR521>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Eichinger A, Nasreen A, Kim HJ, Skerra A. Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D. J Biol Chem. 2007;282:31068–31075. doi: 10.1074/jbc.M703552200. [DOI] [PubMed] [Google Scholar]
- 18.Nasreen A, Vogt M, Kim HJ, Eichinger A, Skerra A. Solubility engineering and crystallization of human apolipoprotein D. Protein Sci. 2006;15:190–199. doi: 10.1110/ps.051775606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;(10) Suppl:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 22.Melov S, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker DW, Muffat J, Rundel C, Benzer S. Overexpression of a Drosophila homolog of apolipoprotein d leads to increased stress resistance and extended lifespan. Curr Biol. 2006;16:674–679. doi: 10.1016/j.cub.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez G, Ganfornina MD, Sanchez D. Evolution of the lipocalin family as inferred from a protein sequence phylogeny. Biochim Biophys Acta. 2000;1482:35–45. doi: 10.1016/s0167-4838(00)00151-5. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez D, Ganfornina MD, Gutierrez G, Marin A. Exon–intron structure and evolution of the Lipocalin gene family. Mol Biol Evol. 2003;20:775–783. doi: 10.1093/molbev/msg079. [DOI] [PubMed] [Google Scholar]
- 26.Duffy JB. GAL4 system in Drosophila: A fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 27.Lewis EB. A new standard food medium. Drosophila Information Service. 1960;34:118–119. [Google Scholar]
- 28.Walker DW, Benzer S. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc Natl Acad Sci USA. 2004;101:10290–10295. doi: 10.1073/pnas.0403767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neu-ron death in the substantial nigra: implications for Parkinson disease. J Biol Chem. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. discussion 724S–725S. [DOI] [PubMed] [Google Scholar]
- 31.Toroser D, Orr WC, Sohal RS. Carbonylation of mitochondrial proteins in Drosophila melanogaster during aging. Biochem Biophys Res Commun. 2007;363:418–424. doi: 10.1016/j.bbrc.2007.08.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez D, et al. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Curr Biol. 2006;16:680–686. doi: 10.1016/j.cub.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Oakley H, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: Potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh DM, Selkoe DJ. A beta oligomers: A decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do Carmo S, Levros LC, Jr, Rassart E. Modulation of apolipoprotein D expression and translocation under specific stress conditions. Biochim Biophys Acta. 2007;1773:954–969. doi: 10.1016/j.bbamcr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Landis GN, et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechner M, Wojnar P, Redl B. Human tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture system. Biochem J. 2001;356:129–135. doi: 10.1042/0264-6021:3560129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.