Abstract
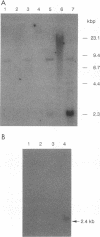
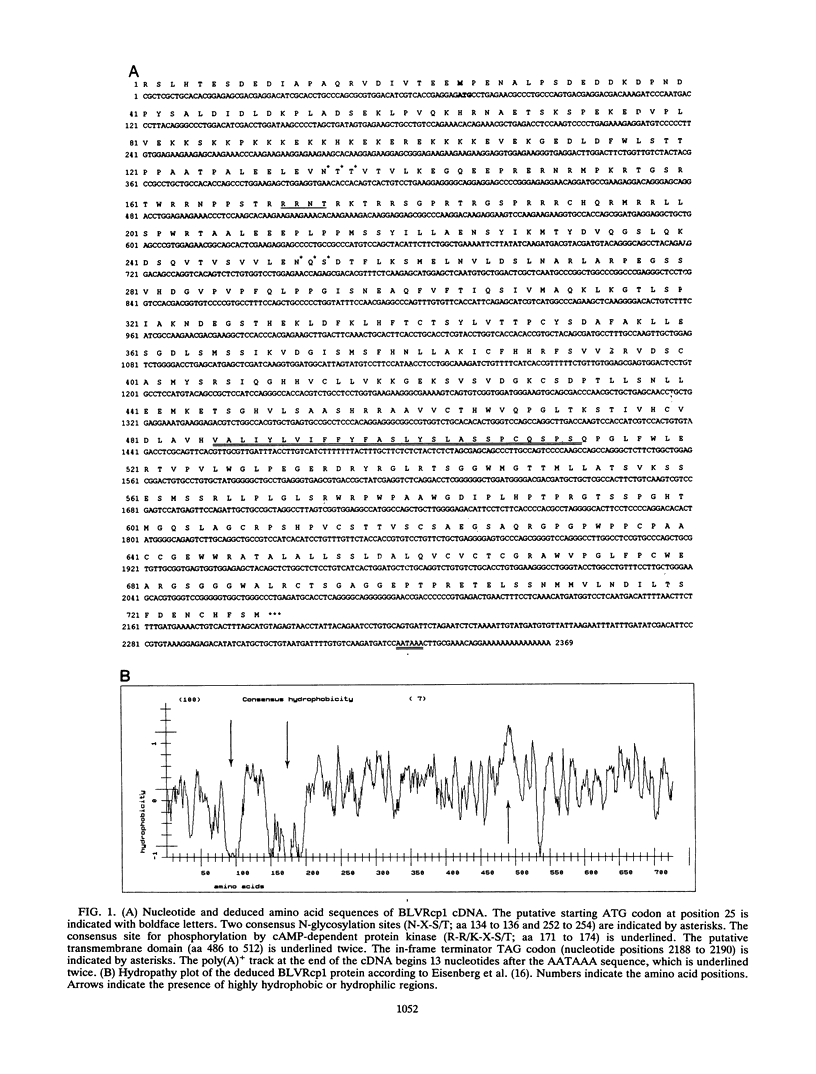
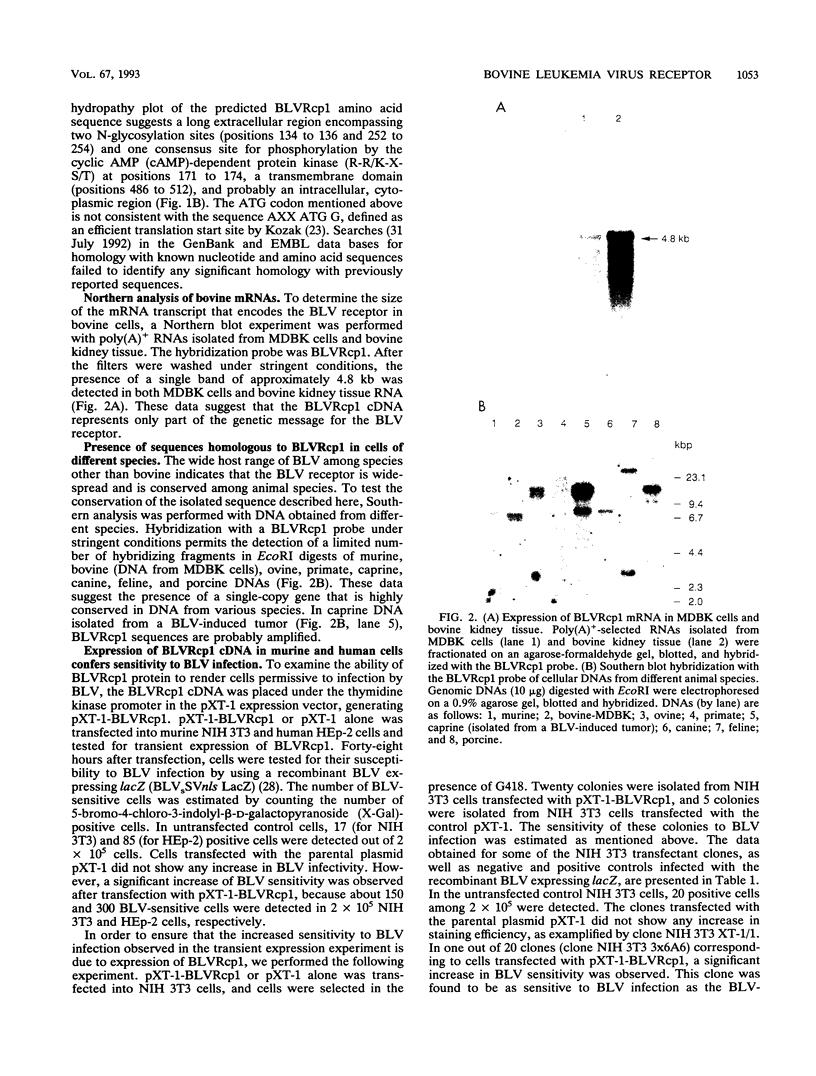
An immunoscreening strategy was used to isolate a cDNA clone encoding the binding domain for the external glycoprotein gp51 of the bovine leukemia virus (BLV). Three recombinant phages demonstrating BLV binding activity and containing 2.3-kbp cDNA inserts with identical nucleotide sequences were isolated from a lambda gt11 cDNA library of bovine kidney cells (MDBK). One clone, BLVRcp1, hybridized with a 4.8-kb mRNA from cells of bovine origin and was also found to be conserved as a single-copy gene in murine, bovine, ovine, primate, canine, feline, and porcine DNAs. The same gene is amplified in caprine DNA isolated from a BLV-induced tumor. The longest open reading frame of BLVRcp1 encodes a protein fragment of 729 amino acids with a putative receptor structure. BLVRcp1 cDNA was cloned in the eucaryotic expression vector pXT-1 and transfected into murine NIH 3T3 and human HEp-2 cells. Cells expressing BLVRcp1 mRNA became susceptible to BLV infection. BLVRcp1 has no known physiological function and has no significant homology with sequences registered in the GenBank and EMBL data libraries (31 July 1992). Expression of deleted constructs of BLVRcp1 indicates that the BLV binding region is encoded at the 5' side of the receptor clone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Altaner C., Altanerová V., Bán J., Niwa O., Yokoro K. Human cells of neural origin are permissive for bovine leukemia virus. Neoplasma. 1989;36(6):691–695. [PubMed] [Google Scholar]
- Altaner C., Bán J., Zajac V., Rössler H., Rosenthal S., Kettmann R., Burny A. Isolation and characterization of cell clones producing various amounts of bovine leukosis virus. Folia Biol (Praha) 1985;31(2):107–114. [PubMed] [Google Scholar]
- Altanerova V., Ban J., Altaner C. Induction of immune deficiency syndrome in rabbits by bovine leukaemia virus. AIDS. 1989 Nov;3(11):755–758. doi: 10.1097/00002030-198911000-00012. [DOI] [PubMed] [Google Scholar]
- Altanerova V., Ban J., Kettmann R., Altaner C. Induction of leukemia in chicken by bovine leukemia virus due to insertional mutagenesis. Arch Geschwulstforsch. 1990;60(2):89–96. [PubMed] [Google Scholar]
- Altanerova V., Portetelle D., Kettmann R., Altaner C. Infection of rats with bovine leukaemia virus: establishment of a virus-producing rat cell line. J Gen Virol. 1989 Jul;70(Pt 7):1929–1932. doi: 10.1099/0022-1317-70-7-1929. [DOI] [PubMed] [Google Scholar]
- Ban J., Czene S., Altaner C., Callebaut I., Krchnak V., Merza M., Burny A., Kettmann R., Portetelle D. Mapping of sequential epitopes recognized by monoclonal antibodies on the bovine leukaemia virus external glycoproteins expressed in Escherichia coli by means of antipeptide antibodies. J Gen Virol. 1992 Sep;73(Pt 9):2457–2461. doi: 10.1099/0022-1317-73-9-2457. [DOI] [PubMed] [Google Scholar]
- Brodsky M. H., Warton M., Myers R. M., Littman D. R. Analysis of the site in CD4 that binds to the HIV envelope glycoprotein. J Immunol. 1990 Apr 15;144(8):3078–3086. [PubMed] [Google Scholar]
- Bruck C., Mathot S., Portetelle D., Berte C., Franssen J. D., Herion P., Burny A. Monoclonal antibodies define eight independent antigenic regions on the bovine leukemia virus (BLV) envelope glycoprotein gp51. Virology. 1982 Oct 30;122(2):342–352. doi: 10.1016/0042-6822(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- De Loof H., Rosseneu M., Brasseur R., Ruysschaert J. M. Use of hydrophobicity profiles to predict receptor binding domains on apolipoprotein E and the low density lipoprotein apolipoprotein B-E receptor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2295–2299. doi: 10.1073/pnas.83.8.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Johann S. V., Gibbons J. J., O'Hara B. GLVR1, a receptor for gibbon ape leukemia virus, is homologous to a phosphate permease of Neurospora crassa and is expressed at high levels in the brain and thymus. J Virol. 1992 Mar;66(3):1635–1640. doi: 10.1128/jvi.66.3.1635-1640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kewalramani V. N., Panganiban A. T., Emerman M. Spleen necrosis virus, an avian immunosuppressive retrovirus, shares a receptor with the type D simian retroviruses. J Virol. 1992 May;66(5):3026–3031. doi: 10.1128/jvi.66.5.3026-3031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krchnák V., Vágner J. Color-monitored solid-phase multiple peptide synthesis under low-pressure continuous-flow conditions. Pept Res. 1990 Jul-Aug;3(4):182–193. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mammerickx M., Portetelle D., de Clercq K., Burny A. Experimental transmission of enzootic bovine leukosis to cattle, sheep and goats: infectious doses of blood and incubation period of the disease. Leuk Res. 1987;11(4):353–358. doi: 10.1016/0145-2126(87)90180-9. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Milan D., Nicolas J. F. Activator-dependent and activator-independent defective recombinant retroviruses from bovine leukemia virus. J Virol. 1991 Apr;65(4):1938–1945. doi: 10.1128/jvi.65.4.1938-1945.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990 Mar;1(3):119–127. [PubMed] [Google Scholar]
- Olshevsky U., Helseth E., Furman C., Li J., Haseltine W., Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990 Dec;64(12):5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portetelle D., Limbach K., Burny A., Mammerickx M., Desmettre P., Riviere M., Zavada J., Paoletti E. Recombinant vaccinia virus expression of the bovine leukaemia virus envelope gene and protection of immunized sheep against infection. Vaccine. 1991 Mar;9(3):194–200. doi: 10.1016/0264-410x(91)90153-w. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Vile R. G., Simpson G., O'Hara B., Collins M. K., Weiss R. A. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992 Feb;66(2):1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]