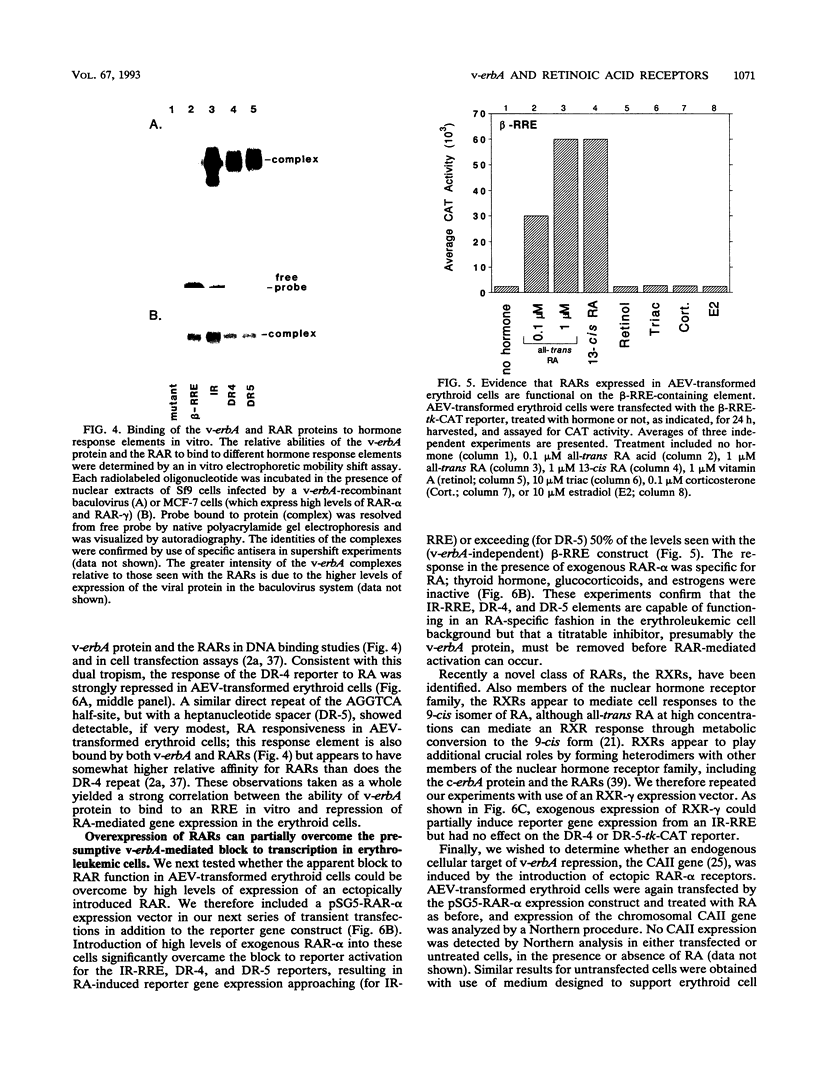
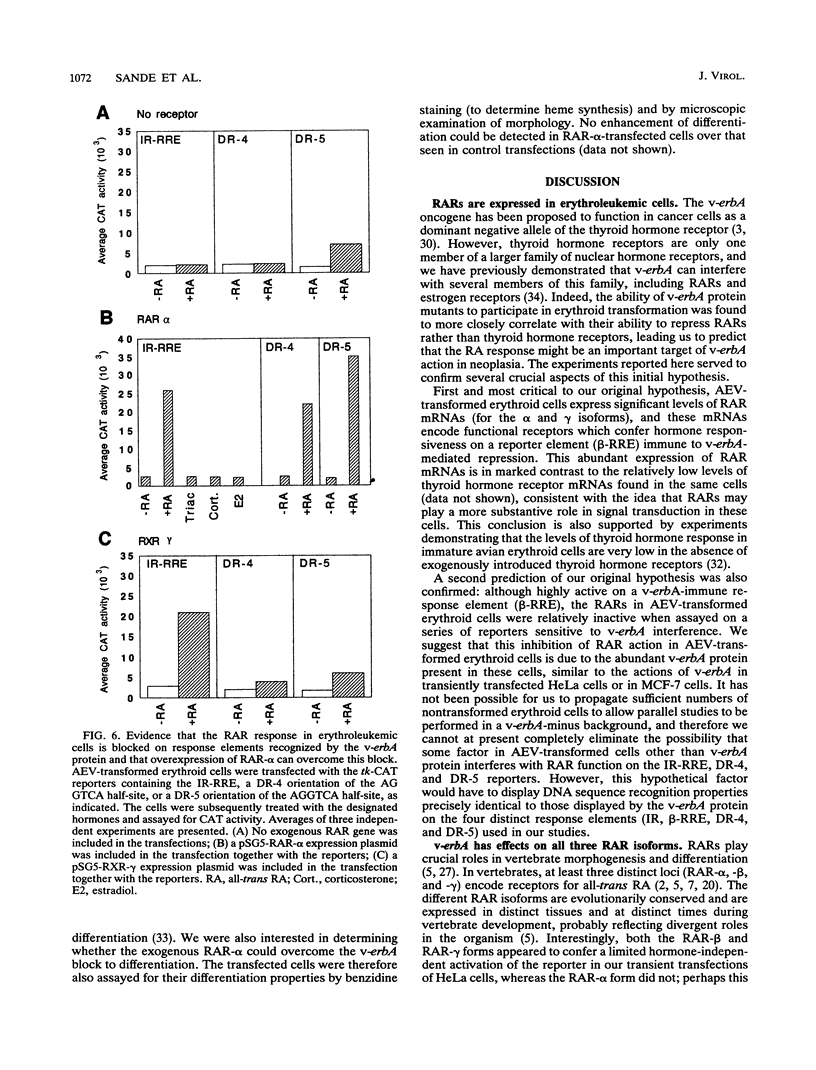
Abstract
The v-erbA oncogene of avian erythroblastosis virus (AEV) encodes an aberrant version of a gene for a thyroid hormone receptor (c-erbA) and functions in neoplasia by blocking erythroid differentiation and altering the growth properties of fibroblasts. The v-erbA gene has been proposed to act as a dominant negative allele, functioning by interfering with the actions of its normal cell homologs, the thyroid hormone receptors. The v-erbA protein can also, however, interfere with the actions of other members of the nuclear hormone receptor family, and it has been proposed that interference with a retinoic acid-mediated response may be a crucial determinant of v-erbA's function in the cancer cell. Here we report that the ability of v-erbA to interfere with retinoic acid receptor (RAR) action extends to the neoplastic erythroid cell and that v-erbA can inhibit transcriptional activation by all three isoforms (alpha, beta, and gamma) of RAR. Overexpression of RAR-alpha was found to partially overcome the presumptive v-erbA block to transcription in the erythroleukemic cell. These results are consistent with our hypothesis that v-erbA can act in neoplasia by interfering with a retinoic acid-mediated signal transduction pathway.
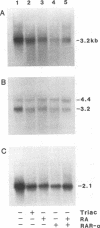
Full text
PDF



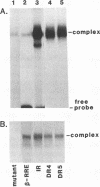
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcalay M., Zangrilli D., Pandolfi P. P., Longo L., Mencarelli A., Giacomucci A., Rocchi M., Biondi A., Rambaldi A., Lo Coco F. Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor alpha locus. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1977–1981. doi: 10.1073/pnas.88.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Dejean A., de Thé H. Hepatitis B virus as an insertional mutagene in a human hepatocellular carcinoma. Mol Biol Med. 1990 Jun;7(3):213–222. [PubMed] [Google Scholar]
- Disela C., Glineur C., Bugge T., Sap J., Stengl G., Dodgson J., Stunnenberg H., Beug H., Zenke M. v-erbA overexpression is required to extinguish c-erbA function in erythroid cell differentiation and regulation of the erbA target gene CAII. Genes Dev. 1991 Nov;5(11):2033–2047. doi: 10.1101/gad.5.11.2033. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein H. G., Burman K. D., Djuh Y. Y., Usala S. J., Bale A. E., Weintraub B. D., Smallridge R. C. Tight linkage of the human c-erbA beta gene with the syndrome of generalized thyroid hormone resistance is present in multiple kindreds. J Endocrinol Invest. 1991 Mar;14(3):219–223. doi: 10.1007/BF03346792. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Casanova J., Raaka B. M., Ghysdael J., Samuels H. H. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol Endocrinol. 1992 Mar;6(3):429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Benchaibi M., Xiao J. H., Ghysdael J., Samarut J. Expression of the v-erbA oncogene in chicken embryo fibroblasts stimulates their proliferation in vitro and enhances tumor growth in vivo. Cell. 1987 Jun 5;49(5):687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Pain B., Desbois C., Madjar J. J., Moscovici M. G., Moscovici C., Samarut J. Expression of the v-erbA product, an altered nuclear hormone receptor, is sufficient to transform erythrocytic cells in vitro. Cell. 1989 Jul 14;58(1):115–121. doi: 10.1016/0092-8674(89)90408-x. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M. Regulation of gene expression by the thyroid hormone receptor. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):157–176. doi: 10.1016/0304-419x(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H., Jr, Umesono K., Warrell R. P., Jr, Frankel S. R., Murty V. V., Dmitrovsky E., Evans R. M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991 Aug 23;66(4):663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Pain B., Melet F., Jurdic P., Samarut J. The carbonic anhydrase II gene, a gene regulated by thyroid hormone and erythropoietin, is repressed by the v-erbA oncogene in erythrocytic cells. New Biol. 1990 Mar;2(3):284–294. [PubMed] [Google Scholar]
- Privalsky M. L. Retinoid and thyroid hormone receptors: ligand-regulated transcription factors as proto-oncogenes. Semin Cell Biol. 1992 Apr;3(2):99–106. doi: 10.1016/s1043-4682(10)80019-4. [DOI] [PubMed] [Google Scholar]
- Rowe A., Eager N. S., Brickell P. M. A member of the RXR nuclear receptor family is expressed in neural-crest-derived cells of the developing chick peripheral nervous system. Development. 1991 Mar;111(3):771–778. doi: 10.1242/dev.111.3.771. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Miyamoto T., Refetoff S., DeGroot L. J. Dominant negative transcriptional regulation by a mutant thyroid hormone receptor-beta in a family with generalized resistance to thyroid hormone. Mol Endocrinol. 1990 Dec;4(12):1988–1994. doi: 10.1210/mend-4-12-1988. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Schroeder C., Gibson L., Beug H. The v-erbA oncogene requires cooperation with tyrosine kinases to arrest erythroid differentiation induced by ligand-activated endogenous c-erbA and retinoic acid receptor. Oncogene. 1992 Feb;7(2):203–216. [PubMed] [Google Scholar]
- Schroeder C., Gibson L., Zenke M., Beug H. Modulation of normal erythroid differentiation by the endogenous thyroid hormone and retinoic acid receptors: a possible target for v-erbA oncogene action. Oncogene. 1992 Feb;7(2):217–227. [PubMed] [Google Scholar]
- Schroeder C., Raynoschek C., Fuhrmann U., Damm K., Vennström B., Beug H. The v-erb A oncogene causes repression of erythrocyte-specific genes and an immature, aberrant differentiation phenotype in normal erythroid progenitors. Oncogene. 1990 Oct;5(10):1445–1453. [PubMed] [Google Scholar]
- Sharif M., Privalsky M. L. V-erbA and c-erbA proteins enhance transcriptional activation by c-jun. Oncogene. 1992 May;7(5):953–960. [PubMed] [Google Scholar]
- Sharif M., Privalsky M. L. v-erbA oncogene function in neoplasia correlates with its ability to repress retinoic acid receptor action. Cell. 1991 Sep 6;66(5):885–893. doi: 10.1016/0092-8674(91)90435-2. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Murakami K. K., Evans R. M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]