Abstract
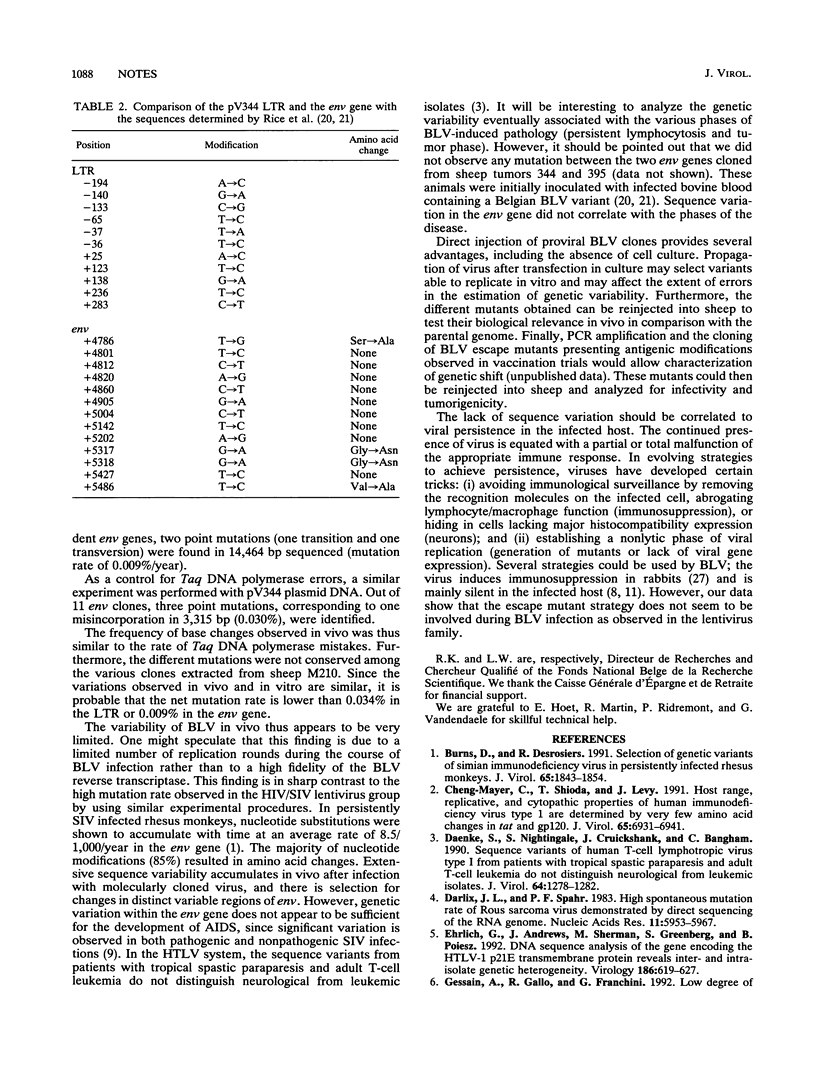
Intradermal injection of a cloned bovine leukemia virus (BLV) provirus (pV344) into sheep allowed direct evaluation of intrastrain variability. A sheep was injected with pV344 DNA mixed with DEAE-dextran and became persistently infected with BLV strain 344. After 18 months, DNA was extracted from peripheral blood leukocytes from a single 0.5-ml blood sample. The long terminal repeat (LTR) and the env gene were amplified by using the polymerase chain reaction, cloned, and sequenced. Nineteen independent LTR clones (0.6-kb inserts) and 16 env clones (1-kb inserts) were analyzed. The in vivo rate of nucleotide change was 0.009%/year (two mutations out of 14,464 bp in 1.5 years), corresponding to only one amino acid change in the env gene. Five point mutations (all transitions), corresponding to a modification rate of 0.034%/year (five mutations out of 9,709 bp in 1.5 years), were identified in the LTR. As a control for Taq DNA polymerase errors, the same procedure using pV344 plasmid DNA was carried out. Out of 9,944 bp sequenced, three point mutations were found (i.e., one misincorporation in 3,315 nucleotides). These data demonstrate the extremely low level (or absence) of intrastrain variability of BLV in vivo. Consequently, BLV persistence in the infected host does not seem to result from an escape mutant strategy, in sharp contrast with the high mutation rates observed in the lentivirus family. The lack of genetic variation supports the possibility of successful vaccine against BLV and probably against the related human T-cell leukemia viruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns D. P., Desrosiers R. C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991 Apr;65(4):1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Shioda T., Levy J. A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991 Dec;65(12):6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenke S., Nightingale S., Cruickshank J. K., Bangham C. R. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J Virol. 1990 Mar;64(3):1278–1282. doi: 10.1128/jvi.64.3.1278-1282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. High spontaneous mutation rate of Rous sarcoma virus demonstrated by direct sequencing of the RNA genome. Nucleic Acids Res. 1983 Sep 10;11(17):5953–5967. doi: 10.1093/nar/11.17.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. D., Andrews J., Sherman M. P., Greenberg S. J., Poiesz B. J. DNA sequence analysis of the gene encoding the HTLV-I p21e transmembrane protein reveals inter- and intraisolate genetic heterogeneity. Virology. 1992 Feb;186(2):619–627. doi: 10.1016/0042-6822(92)90028-n. [DOI] [PubMed] [Google Scholar]
- Gessain A., Gallo R. C., Franchini G. Low degree of human T-cell leukemia/lymphoma virus type I genetic drift in vivo as a means of monitoring viral transmission and movement of ancient human populations. J Virol. 1992 Apr;66(4):2288–2295. doi: 10.1128/jvi.66.4.2288-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessian A., Yanagihara R., Franchini G., Garruto R. M., Jenkins C. L., Ajdukiewicz A. B., Gallo R. C., Gajdusek D. C. Highly divergent molecular variants of human T-lymphotropic virus type I from isolated populations in Papua New Guinea and the Solomon Islands. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7694–7698. doi: 10.1073/pnas.88.17.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W. A., Rovnak J., Cockerell G. L. In vivo transcription of the bovine leukemia virus tax/rex region in normal and neoplastic lymphocytes of cattle and sheep. J Virol. 1991 May;65(5):2484–2490. doi: 10.1128/jvi.65.5.2484-2490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Hirsch V. M. Genetic variation of simian immunodeficiency viruses in nonhuman primates. AIDS Res Hum Retroviruses. 1992 Mar;8(3):367–372. doi: 10.1089/aid.1992.8.367. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Skalka A. M. Generation of diversity in retroviruses. Annu Rev Genet. 1990;24:409–445. doi: 10.1146/annurev.ge.24.120190.002205. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Cleuter Y., Gregoire D., Burny A. Role of the 3' long open reading frame region of bovine leukemia virus in the maintenance of cell transformation. J Virol. 1985 Jun;54(3):899–901. doi: 10.1128/jvi.54.3.899-901.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komurian-Pradel F., Pelloquin F., Sonoda S., Osame M., de The G. Geographical subtypes demonstrated by RFLP following PCR in the LTR region of HTLV-I. AIDS Res Hum Retroviruses. 1992 Apr;8(4):429–434. doi: 10.1089/aid.1992.8.429. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Mamoun R. Z., Morisson M., Rebeyrotte N., Busetta B., Couez D., Kettmann R., Hospital M., Guillemain B. Sequence variability of bovine leukemia virus env gene and its relevance to the structure and antigenicity of the glycoproteins. J Virol. 1990 Sep;64(9):4180–4188. doi: 10.1128/jvi.64.9.4180-4188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M., Papenhausen M. D., Benveniste R. E., Morton W. R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991 Dec;65(12):7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin J. D., Moscona A., Pan W. T., Leider J. M., Palese P. Measurement of the mutation rates of animal viruses: influenza A virus and poliovirus type 1. J Virol. 1986 Aug;59(2):377–383. doi: 10.1128/jvi.59.2.377-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Philpott T., Trowbridge D. B. Nucleotide sequence analysis of isolates of human T-lymphotropic virus type 1 of diverse geographical origins. AIDS Res Hum Retroviruses. 1991 Nov;7(11):923–941. doi: 10.1089/aid.1991.7.923. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Burny A., Gilden R. V. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985 Apr 30;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., de la Torre J. C., Meier E., Holland J. J. Extreme heterogeneity in populations of vesicular stomatitis virus. J Virol. 1989 May;63(5):2072–2080. doi: 10.1128/jvi.63.5.2072-2080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Portetelle D., Kerkhofs P., Chen G., Burny A., Mammerickx M., Kettmann R. In vivo transfection of bovine leukemia provirus into sheep. Virology. 1992 Aug;189(2):775–777. doi: 10.1016/0042-6822(92)90604-n. [DOI] [PubMed] [Google Scholar]
- Wyatt C. R., Wingett D., White J. S., Buck C. D., Knowles D., Reeves R., Magnuson N. S. Persistent infection of rabbits with bovine leukemia virus associated with development of immune dysfunction. J Virol. 1989 Nov;63(11):4498–4506. doi: 10.1128/jvi.63.11.4498-4506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



