Abstract
Purpose
Telomerase is an attractive target antigen for cancer immunotherapies because it is expressed in >85% of human tumors but is rarely found in normal tissues. A HLA-A*0201-restricted T-cell epitope was previously identified within telomerase reverse transcriptase hTERT:540-548. This peptide was reported to induce CTL that recognized tumor cells and transfectants that endogenously expressed telomerase. Therefore, we initiated a clinical protocol to evaluate the therapeutic and immunological efficacy of this peptide.
Experimental Design
Fourteen patients with metastatic cancers were vaccinated with hTERT:540-548 emulsified in incomplete Freund's adjuvant.
Results
In 7 patients, peripheral blood mononuclear cells collected after immunization recognized hTERT:540-548, whereas those collected before vaccination did not. However, none of these CTLs recognized tumors that endogenously expressed telomerase, and none of the patients had an objective clinical response. Several highly avid T-cell clones were generated that recognized T2 cells pulsed with ≤1 nm hTERT:540-548, but none of these recognized HLA-A*0201+ hTERT+ tumors or cells transduced with the human telomerase reverse transcriptase (hTERT) gene. Also, an antibody specific for hTERT:540-548/HLA-A*0201 complexes stained peptide-pulsed cells but not telomerase+ tumors.
Conclusions
Our results are discordant with previous studies and those of a clinical trial that claimed peripheral blood mononuclear cells from patients vaccinated with peptide-pulsed dendritic cells lysed hTERT+ tumors. However, our findings are consistent with a previous study that demonstrated that the hTERT:540-548 peptide is cleaved in the proteasome. These results suggest that hTERT:540-548 is not presented on the surfaces of tumor cells in the context of HLA-A*0201 and will not be useful for the immunotherapy of patients with cancer.
Introduction
The identification of tumor-associated antigens and epitopes has enabled the development of novel immunotherapies for the treatment of patients with cancer (1). Multiple tumor-associated antigens and specific class I MHC-restricted epitopes have been identified that are capable of being recognized by CTLs, including melanocyte differentiation antigens (e.g., MART-1, gp100, and tyrosinase-related proteins), cancer-testis antigens (e.g., NY-ESO-1 and the MAGE family of proteins), and mutated proteins (e.g., β-catenin, MUM-1, and CDK-4; Refs. 1, 2).
The ribonucleoprotein telomerase has also been suggested to be a tumor-associated antigen (3, 4). This enzyme mediates the RNA-dependent synthesis of telomeric DNA. Telomeres at the distal ends of eukaryotic chromosomes stabilize the chromosomes during cell division and prevent end-to-end fusion (5). The telomerase catalytic subunit, human telomerase reverse transcriptase (hTERT), is the rate-limiting component in the telomerase complex and is most closely correlated with telomerase activity (6, 7). More than 85% of human cancers have telomerase activity and express hTERT, whereas most normal adult human cells do not maintain the lengths of their telomeres (8–11). Therefore, telomerase was considered to be an attractive candidate target antigen for the development of immunotherapies for the treatment of patients with a variety of human cancers. In addition, telomerase expression has been directly linked to the ability of tumor cells to replicate indefinitely (12), and the inhibition of telomerase in tumor cells has been shown to lead to cell death (13, 14). Therefore, if a T-cell response could be directed against peptide epitopes processed from telomerase, it seems likely that any immune escape variants that did not express this protein would not be able to survive.
The hTERT:540-548 peptide (ILAKFLHWL) was previously identified by investigators from two laboratories as an immunodominant HLA-A*0201-restricted T-cell epitope (3, 4). In both studies, it was reported that this peptide could induce CTL in vitro that recognized the peptide as well as cells that endogenously expressed telomerase, including breast, colon, lung, melanoma, and prostate cancers and cells transfected with the full-length hTERT gene. On the basis of these studies and our prior success with immunizing patients against peptides from the melanoma antigens MART-1 and gp100 (15, 16), we initiated a clinical protocol to vaccinate 14 patients with metastatic cancers with the hTERT:540-548 peptide emulsified in incomplete Freund's adjuvant. In 7 patients, peripheral blood mononuclear cells (PBMCs) collected after immunization were able to recognize the hTERT peptide in vitro, whereas those collected before vaccination were not. However, none of these CTLs recognized tumor cells that endogenously expressed HLA-A*0201 and telomerase. We also generated several T-cell lines and clones that were highly avid for the peptide, but these lymphocytes also did not recognize HLA-A*0201+ hTERT+ tumors or cells transduced with the full-length hTERT gene. Furthermore, Fab fragments that specifically bound to the hTERT:540-548/HLA-A*0201 complex (17) stained peptide-pulsed cells but not hTERT+ tumors. These results are consistent with those of Ayyoub et al. (18), who demonstrated that the hTERT:540-548 peptide is cleaved in the proteasome and is not presented in the context of HLA-A*0201 on the surfaces of cells that endogenously express telomerase. Our results thus indicate that the hTERT:540-548 peptide will not be useful for the development of cancer immunotherapies.
Materials and Methods
Patients and Clinical Protocol
Fourteen patients who had confirmed metastatic cancer were enrolled in the hTERT:540-548 peptide vaccination protocol, which was approved by the Institutional Review Board of the National Cancer Institute. Each patient underwent a complete clinical evaluation, including measurements and X-rays of all evaluable tumor sites. All patients were confirmed to express HLA-A*0201 by high-resolution nested sequence PCR subtyping, and all patients signed an informed consent before treatment. No patient had received any treatment in the previous month, nor were any of them receiving immunosuppressive drugs, including steroids. Before treatment, each patient underwent a leukapheresis, and PBMCs were cryopreserved at −180°C after Ficoll-Hypaque separation.
Patients were randomized into one of the following three arms of the study: (a) hTERT:540-548 peptide once a week every week for 4 or 10 cycles followed by a 3-week break and then repeated injections of peptide every week for 4 or 10 cycles (once weekly 4 or 10 cycles); (b) hTERT:540-548 peptide once every 3 weeks; and (c) hTERT:540-548 peptide 4 days in a row every 3 weeks (Monday–Thursday). For each immunization, 1 mg of peptide was injected as an emulsion with incomplete Freund's adjuvant (Montanide ISA-51; Seppic, Paris, France) in two equal volumes (1 ml/injection) into the s.c. tissue of the anterior thigh. Patients underwent leukapheresis 3 weeks after each course of immunization, and PBMCs were cryopreserved at −180°C after Ficoll-Hypaque separation.
Media and Cell Culture
Multiple human cell lines were used in these studies to evaluate hTERT recognition by T lymphocytes. These included T2 cells (HLA-A*0201+ peptide transporter-associated protein-deficient T-B hybrid), melanomas (888mel, 938mel, 624mel, 1861mel, and Sk23mel), renal cells (293), B lymphoblasts (C1R), EBV-transformed B lymphocytes (EBV-B cells: SKW6.4, IM9, and 1978E), myeloma (U266), colon carcinomas (H508 and SW480), breast carcinoma (HBL-100), osteosarcoma (U20S), ovarian carcinoma (SK-OV-3), renal cell carcinomas (N-KH and S-KH), and normal human fibroblasts (WI-38). All of these cell lines were routinely cultured in either RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and 2 mm l-glutamine (Invitrogen, Carlsbad, CA) or DMEM (Invitrogen) containing 10% heat-inactivated fetal bovine serum, 2 mm l-glutamine, and 10 mm HEPES. Human lymphocytes were cultured in complete medium (CM) consisting of RPMI 1640, 2 mm l-glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin (Invitrogen), and 10% heat-inactivated human AB serum (Gemini Bio-Products, Woodland, CA; Valley Biomedical, Winchester, VA).
Peptides
For clinical administration, hTERT:540-548 (ILAKFLHWL) was purchased from Multiple Peptide Systems (San Diego, CA) as GMP-grade lyophilized powder. For other in vitro studies, HBVc:18–27(23Y) (FLPSDYFPSV), G250:254-262 (HLSTAFARV), gp100:209-217 (ITDQVPFSV), gp100:209-217(210M) (IMDQVPFSV), gp100:280-288 (YLEPGPVTA), and hTERT:540-548 (ILAKFLHWL) were commercially synthesized and purified (>95%) by reversed-phase high-performance liquid chromatography by Macromolecular Resources (Fort Collins, CO) and were resuspended at 1–5 mg/ml in DMSO as stock solutions.
Evaluation of HLA-A*0201, hTERT, and Telomerase Expression in Tumor Cell Lines and Normal Tissues
SW480, HBL-100, SK-OV-3, and U2OS cells were kindly provided by Dr. Robert Vonderheide (Abramson Family Cancer Research Institute, University of Pennsylvania, Philadelphia, PA). These cell lines had previously been evaluated for expression of HLA-A*0201 and telomerase enzyme activity using the telomeric repeat amplification protocol (TRAP) in his laboratory with the following results: SW480 (HLA-A*0201+ hTERT+); HBL-100 (HLA-A*0201+ hTERT+); SK-OV-3 (HLA-A*0201- hTERT+); and U2OS (HLA-A*0201+ hTERT-).
The expression of HLA-A2 was evaluated on all other cell lines by fluorescence-activated cell sorting using an anti-HLA-A2 monoclonal antibody (One Lambda, Canoga Park, CA), and for some cell lines, DNA sequencing confirmed the presence of HLA-A*0201 (HLA Laboratory, NIH). Although 293 and C1R cells did not express HLA-A*0201 (293-UT and C1R-UT), they were stably transfected with cDNA encoding this MHC molecule (293-A2 and C1R-A2).
The presence of hTERT mRNA in cell lines was assessed by reverse transcriptase-PCR using specific intron-spanning primers (forward GCCTGAGCTGTACTTTGTCAA and reverse CGCAAACAGCTTGTTCTCCATGTC). In addition, PCR was performed with these primers on serially diluted cDNAs from 24 normal human tissues (Rapid-Scan; Origene Technologies, Rockville, MD). Telomerase enzyme activity was also measured in cell lines using a commercially available TRAP assay followed by detection of the telomerase products by ELISA (TRAPese ELISA, Intergen Company, Purchase, NY) following the manufacturer's recommended protocol.
In some experiments, cells were transduced with a recombinant adenovirus encoding hTERT. To determine whether this adenovirus induced functional telomerase, human fibroblasts (WI-38) that do not naturally express hTERT were transduced with the hTERT-adenovirus at an multiplicity of infection of either 10:1 or 100:1. Telomerase activity was then measured in the transduced cells using the TRAP assay.
In Vitro Comparison of Peptide and Tumor Reactivities of Pre- and Postvaccination Peripheral Blood Lymphocytes
PBMCs that had been cryopreserved before or after immunization with hTERT:540-548 were stimulated with peptide as described previously (16). Briefly, T-cell cultures were established by plating PBMCs in 24-well plates (1.5 × 106 cells/ml; 2 ml/well) in CM containing 1–10 μg/ml peptide. Two days later, 300 IU/ml recombinant interleukin 2 (rIL-2; Chiron Co., Emeryville, CA) were added, and media were replaced as needed with fresh media containing IL-2. Recognition of hTERT by bulk T-cell cultures was evaluated ∼12 days after culture initiation on the basis of IFN-γ secretion in response to T2 cells preincubated with peptide and HLA-A*0201+ hTERT+ cell lines. T2 cells were incubated with peptide 1–3 h at 37°C and were either used directly (peptide loaded) or were washed twice before use (peptide pulsed). 105 responder T cells were coincubated with 105 stimulator cells (250 μl total) ∼20 h at 37°C, and the concentration of human IFN-γ in coculture supernatants was measured using commercially available ELISA reagents (Endogen, Cambridge, MA).
Generation of Peptide-Reactive T-Cell Lines and Clones
In some experiments, T-cell lines were generated from patients with metastatic cancers that specifically recognized peptides derived from hTERT, gp100, or G250 as described previously (19). Briefly, hTERT:540-548, gp100:209–217(210M), or G250:254-262 was used to stimulate lymphocytes in vitro from HLA-A*0201+ patients with metastatic cancer in either a bulk culture (24-well plates) or microculture (96-well plates) format. For bulk cultures, PBMCs were initially plated in 24-well plates (3 × 106 cells/well; 1.5 × 106 cells/ml; 2 ml/well) in CM containing 1–10 μm peptide. Two days later, 300 IU/ml rIL-2 (Chiron Co.) were added. On day 7 and weekly thereafter, lymphocytes were restimulated with peptide-pulsed autologous-irradiated (4000 rad) PBMCs. Responder lymphocytes were harvested and replated in new 24-well plates (2.5 × 105 cells/ml; 2 ml/well) in CM. Autologous-irradiated PBMCs were incubated with 1–10 μm peptide in 15-ml conical tubes (1–10 × 106 cells/ml; 6–12 ml/tube) 2–4 h at 37°C. Peptide-loaded PBMCs were washed and added to responder lymphocytes at a responder to stimulator ratio of ∼1:10. One day after each restimulation, 300 IU/ml rIL-2 were added, and generally, cultures were split between 1:1 and 1:4 3 days later with CM containing 300 IU/ml rIL-2.
For microcultures, PBMCs were initially plated in 96-well plates (3–5 × 105 cells/well; 1.5–2.5 × 106 cells/ml; 200 μl/well) in CM containing 1–10 μm peptide, and 2 days later, 300 IU/ml rIL-2 (Chiron Co.) were added. On day 7, peptide-pulsed autologous-irradiated PBMCs were prepared as described above and were added to responder lymphocytes in 96-well plates (3–5 × 105 cells/well). One day after the restimulation, 300 IU/ml rIL-2 were added, and generally, cultures were split 1:1 into new 96-well plates 3 days later with CM containing 300 IU/ml rIL-2. On day 14, each microculture was evaluated for specific peptide recognition, and positive microcultures (≥100 pg/ml and at least twice background with an HBVc peptide) were restimulated individually in 24-well plates with ∼5 × 106 peptide-pulsed autologous-irradiated PBMCs/well.
Alternatively, in some experiments, PBMCs were initially stimulated with peptide-loaded autologous dendritic cells (DCs) as antigen-presenting cells. Immature DCs were prepared as described previously (20). Briefly, adherent PBMCs were cultured in CM containing 1000 units/ml granulocyte macrophage colony-stimulating factor and 1000 units/ml IL-4 (Peprotech, Rocky Hill, NJ) for 5–7 days. DCs were then harvested and preloaded with 1–10 μm peptide and plated in 96-well plates (1–5 × 104 cells/well; 100 μl/well). PBMCs were then added to the wells containing peptide-loaded DCs (3–5 × 105 cells/well; 100 μl/well). Two days later, 300 IU/ml rIL-2 (Chiron Co.) were added. On day 7, cultures were restimulated with peptide-pulsed autologous-irradiated PBMCs as described above. On day 14, peptide-reactive microcultures were restimulated individually in 24 well plates with ∼5 × 106 peptide-pulsed autologous-irradiated PBMCs/well. Recognition of hTERT, gp100, or G250 by T-cell cultures was evaluated ∼7 days after each bulk restimulation on the basis of IFN-γ secretion in response to T2 cells preincubated with peptide and HLA-A*0201+ cell lines that endogenously expressed the antigen of interest. In some experiments, melanoma cells were transduced with recombinant adenoviral vectors encoding hTERT or G250 1 day before use as target cells in cytokine release assays. 105 responder T cells were coincubated with 105 stimulator cells (250 μl total) ∼20 h at 37°C, and the concentration of human IFN-γ in coculture supernatants was measured using commercially available ELISA reagents (Endogen, Cambridge, MA). Alternatively, in some experiments, 51Cr release cytotoxicity assays were performed to evaluate specific recognition of peptide and tumor cells as described previously (21).
In some experiments, bulk T-cell populations were cloned by limiting dilution and expanded using a modified version of the previously described rapid expansion protocol (22–24). T lymphocytes were plated at 1, 3, 5, and/or 10 cells/well in U-bottomed 96-well plates in CM containing 30 ng/ml ortho-anti-CD3 (Ortho-Biotech, Raritan, NJ) and 300 IU/ml rIL-2 with 5 × 104 allogeneic irradiated PBMCs/well derived from at least 3 different donors. On day 5 and every 3–4 days thereafter, half of the media in each well was replaced with fresh media containing IL-2. Approximately 2 weeks after culture initiation, wells in which cell growth was visibly apparent were assayed for specific peptide recognition by IFN-γ secretion as described above. Positive wells were expanded with 30 ng/ml ortho-anti-CD3 and 5 × 106 irradiated allogeneic PBMCs in upright 25-cm2 flasks as described previously (22–24).
FACS Analysis of T-Cell Populations Using HLA-A2/hTERT:540-548 Tetramers
Phycoerythrin-conjugated tetrameric complexes consisting of HLA-A*0201 and hTERT:540-548 or HTLV-1 tax peptide L11 (LLFGYPVYV) (as a negative control) were kindly provided by Dr. Robert Vonderheide (Abramson Family Cancer Research Institute) or were purchased commercially (Beckman Coulter Immunomics Operations, San Diego, CA). T-Cell lines were costained with phycoerythrin-labeled tetramers and FITC-conjugated antihuman CD8 (Beckman Coulter Immunomics Operations) and were analyzed by FACS on a FACScan or FACSalidur (Becton Dickinson, Mountain View, CA).
FACS Analysis of Cells Using Fab Fragments against HLA-A*0201/hTERT:540-548 Complexes
Fab fragments that specifically bind to HLA-A*0201/hTERT:540-548 complexes (4G9) were kindly provided by Dr. Yoram Reiter (Technion-Israel Institute of Technology, Technion City, Haifa, Israel) and were used to stain cells as described previously (17). Briefly, T2 cells were incubated overnight with hTERT:540-548 or HBVc:18–27(23Y) (as a negative control) in serum-free media. After washing, T2 cells and tumor cells were incubated with 4G9 Fab antibodies. After washing again, cells were stained with FITC-conjugated antihuman Fab (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and were subsequently analyzed by FACS.
Results
Expression of HLA-A*0201, hTERT, and Telomerase in Cell Lines and Normal Tissues
Expression of HLA-A2 was evaluated by FACS using a FITC-conjugated monoclonal antibody against HLA-A2, and for those that were positive, DNA sequencing confirmed the presence of HLA-A*0201. The presence of hTERT mRNA in melanoma and 293 cell lines as well as in 24 primary human tissues was evaluated by reverse transcriptase-PCR. hTERT mRNA was detected in all of the cell lines tested with the exception of a single melanoma cell line (888mel; Fig. 1A). However, it was not found in any normal human tissue, including brain, muscle, heart, stomach, kidney, testis, spleen, placenta, liver, salivary gland, colon, thyroid, lung, adrenal gland, small intestine, pancreas, ovary, uterus, prostate, skin, peripheral blood lymphocytes, bone marrow, fetal brain, or fetal liver using a semiquantitative Rapid Scan Gene Expression panel (data not shown).
Fig. 1.
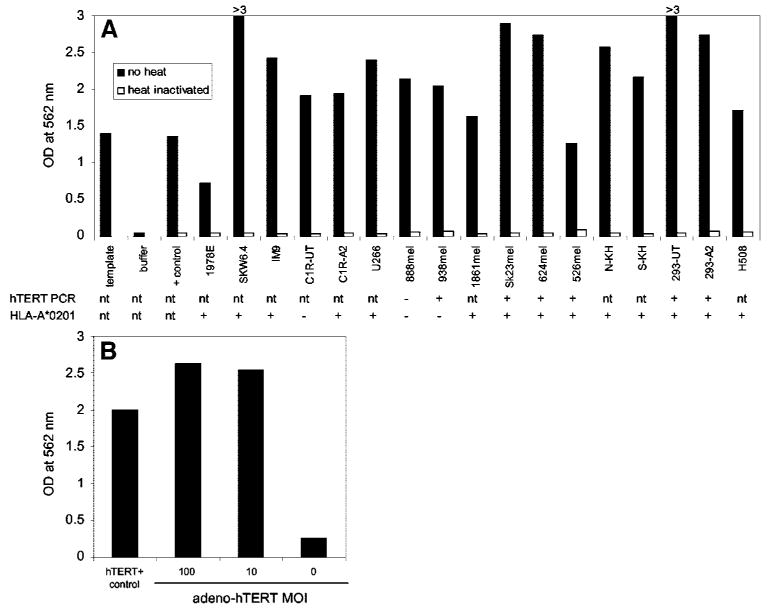
Telomerase enzyme activity in cell lysates as evaluated using a telomeric repeat amplification protocol assay followed by detection of the telomerase products by ELISA. The amount of cell lysate was normalized for the same number of cells in each assay. Also shown is the expression of HLA-A*0201 as evaluated by fluorescence-activated cell sorting and the presence of human telomerase reverse transcriptase (hTERT) mRNA as determined by reverse transcriptase-PCR. A, TSR8 PCR template, negative/contamination PCR control (buffer), positive control cells (+ control), and cell lines. B, WI-38 cells transduced with a recombinant adenovirus encoding hTERT at two different multiplicities of infection (MOI).
Telomerase enzyme activity was also measured in cell lines using a commercially available TRAP assay followed by detection of the telomerase products by ELISA. The assay was performed after normalizing the amount of each cell extract for cell number (Fig. 1) or total protein (data not shown). In addition, because telomerase is a heat-sensitive enzyme, heat-inactivated controls were run for each sample by carrying out a 10-min incubation at 85°C. Absorbance values of all controls, including a PCR/ELISA-positive template, a sample containing no cell extract, and the lysate of a hTERT+ control cell pellet, were all within the limits specified by the kit manufacturer (TSR8 template control and hTERT+ control cell pellet absorbance > 0.8, no sample control absorbance < 0.2, and heat-treated samples absorbance < 0.25). Therefore, all of the cell lines evaluated were positive for telomerase activity because the net increase in absorbance for each sample in comparison to the same heat-treated sample was always >0.15 (Fig. 1A). Similar results were obtained when samples were normalized to total protein content instead of cell number (data not shown).
TRAP assays were also performed on WI-38 cells that did not naturally express telomerase but became positive after transduction with an adenoviral vector encoding hTERT (Fig. 1B). At a multiplicity of infection of 10:1, the absorbance value of the transduced WI-38 cell extracts in the TRAP assay was 2.55 while that of untransduced cells was 0.25, suggesting that the hTERT-adenovirus was capable of inducing telomerase activity.
Immunological Evidence of Immunization in Patients Receiving hTERT:540-548
Because hTERT:540-548 had previously been reported to be an immunodominant HLA-A*0201-restricted peptide that could stimulate T cells in vitro capable of recognizing both peptide and tumor cells endogenously expressing telomerase, we began a clinical immunization trial with this peptide. Fourteen patients who had confirmed metastatic cancer were enrolled in this clinical protocol: 11 had renal cell carcinoma; 2 had colon cancer; and 1 had melanoma (Table 1).
Table 1.
Characteristics of patients vaccinated with hTERT:540-548
| Patient | Age (yrs)/sex | Type of cancer | Treatment course |
|---|---|---|---|
| 1 | 64/F | Renal | M-Th Q3 wksa |
| 2 | 66/M | Renal | Once weekly 10 cycles |
| 3 | 29/M | Melanoma | M-Th Q3 wks |
| 4 | 45/M | Renal | Once Q3 wks |
| 5 | 49/M | Renal | Once weekly 10 cycles |
| 6 | 45/M | Renal | M-Th Q3 wks |
| 7 | 63/M | Renal | Once Q3 wks |
| 8 | 77/M | Colon | M-Th Q3 wks |
| 9 | 41/M | Renal | Once Q3 wks |
| 10 | 44/F | Colon | M-Th Q3 wks |
| 11 | 39/F | Renal | Once weekly 4 cycles |
| 12 | 60/F | Renal | Once Q3 wks |
| 13 | 54/M | Renal | Once weekly 4 cycles |
| 14 | 63/F | Renal | Once weekly |
Q3 wks, every 3 weeks.
To evaluate the in vivo immunogenicity of hTERT:540-548, PBMCs before and after immunization were costained with phycoerythrin-conjugated HLA-A2/hTERT:540-548 tetramers and FITC-labeled antihuman CD8. In 3 of 9 patients evaluated, modest increases in the numbers of dually fluorescent cells were observed after immunization in comparison to preimmunization PBMCs. However, the percentages of these cells among total PBMCs were generally <1%, and therefore, we used a more sensitive methodology for evaluating immunological responses in these patients (16). PBMCs before and after immunization were stimulated in vitro for 11–13 days with 1 μm peptide. After this culture period, recognition of peptide was evaluated on the basis of specific IFN-γ secretion in response to T2 cells preloaded with 1 or 0.1 μm hTERT:540-548 in comparison to a control peptide [HBVc:18–27(23Y) or gp100:280-288]. Peptide recognition by PBMCs from 13 of 14 patients is presented in Table 2 (insufficient numbers of lymphocytes from patient 8 precluded this analysis). Before immunization, no PBMCs exhibited specific recognition of hTERT:540-548. However, after immunization, PBMCs from 7 of these 13 patients exhibited specific peptide reactivity. T cells sensitized with hTERT:540-548 recognized highly purified peptide preparations synthesized by two different sources, regardless of the specific preparation used for in vitro stimulation (data not shown). Furthermore, peptide reactive T-cell cultures contained cells that were stained by HLA-A*0201/hTERT:540-548 tetrameric complexes produced by yet an additional source (Fig. 2). Therefore, it is unlikely that the T cells were recognizing a contaminant in the peptide preparation. Despite these in vitro results, no patient had an objective clinical response to treatment.
Table 2.
| Treatment regimen | Patient | Experiment | Before immunization | After immunizationc | ||||
|---|---|---|---|---|---|---|---|---|
| T2 + control peptided | T2 + hTERT:540 (1 μm) | T2 + hTERT:540 (10 nm) | T2 + control peptide | T2 + hTERT:540 (1 μm) | T2 + hTERT:540 (10 nm) | |||
| Once weekly 10 cycles | 2 | 1 | 34 | 32 | 37 | 674 | 2,000e | 1,876 |
| 2 | 12 | 8 | nt | 24 | 532 | nt | ||
| 5 | 1 | 69 | 68 | 77 | 450 | 4,210 | 3,582 | |
| 2 | 25 | 24 | nt | 27 | 380 | nt | ||
| 3 | 36 | 32 | 27 | 39 | 425 | 151 | ||
| Once weekly 4 cycles | 11 | 1 | 243 | 218 | 256 | 153 | 15,004 | 9,858 |
| 13 | 1 | 256 | 123 | 82 | 96 | 98 | 123 | |
| 14 | 1 | 131 | 123 | 151 | 181 | 232 | 279 | |
| Once Q3 wks | 4 | 1 | 144 | 105 | 119 | 88 | 112 | 111 |
| 2 | 42 | 52 | nt | 13 | 16 | nt | ||
| 7 | 1 | 174 | 218 | 176 | 502 | 517 | 390 | |
| 9 | 1 | 149 | 119 | 133 | 409 | 353 | 386 | |
| 3 | 315 | 301 | 315 | 531 | 538 | 507 | ||
| 12 | 1 | 2,065 | 2,076 | 2,105 | 2,245 | 2,852 | 3,108 | |
| 3 | 99 | 96 | 89 | 98 | 98 | 98 | ||
| M-Th Q3 wks | 1 | 1 | 214 | 212 | 235 | 275 | 1,057 | 962 |
| 2 | 315 | 268 | nt | 56 | 408 | nt | ||
| 3 | 1 | 1,016 | 731 | 946 | 1,094 | 1,867 | 1,925 | |
| 2 | 25 | 31 | nt | 62 | 917 | nt | ||
| 6 | 1 | 105 | 84 | 88 | 96 | 886 | 880 | |
| 2 | 34 | 39 | nt | 44 | 182 | nt | ||
| 10 | 1 | 136 | 107 | 136 | 59 | 143 | 157 | |
| 3 | 32 | 32 | 36 | 8 | 156 | 60 | ||
IFN-γ secretion (pg/ml) in 20-h coculture supernatants of T2 cells preloaded with peptide and PBMCs after 11–13 days in culture with 1 μm hTERT:540-548.
PBMC, peripheral blood mononuclear cell; IFA, incomplete Freund's adjuvant; nt, not tested; Q3 wks, every 3 weeks.
Patient 2 (after 10 cycles, 10 mg total); patient 5 (after 10 cycles, 10 mg total); patient 11 (after 16 cycles, 16 mg total); patient 13 (after 8 cycles, 8 mg total); Patient 14 (after 8 cycles, 8 mg total); patient 4 (after 2 cycles, 2 mg total); patient 7 (after 2 cycles, 2 mg total); patient 9 (after 4 cycles, 4 mg total); patient 12 (experiment 1: after 8 cycles, 8 mg total; experiment 2: after 4 cycles, 4 mg total); patient 1 (experiment 1: after 2 cycles, 8 mg total; experiment 2: after 4 cycles, 16 mg total); patient 3 (after 4 cycles, 16 mg total); patient 6 (after 4 cycles, 16 mg total); and patient 10 (post 4 cycles, 16 mg total).
In experiment 1, the control peptide was HBVc:18-27(23Y), and in experiments 2 and 3, the control peptide was gp100:280-288.
Underlined values indicate that IFN-γ secretion in response to T2 cells preincubated with hTERT:540-548 was >50 pg/ml and at least twice background with the control peptide.
Fig. 2.
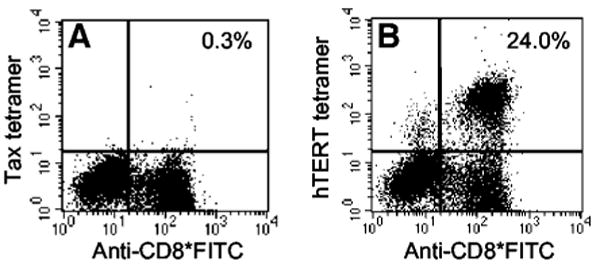
HLA-A*0201/hTERT:540-548 tetramer analysis of peripheral blood mononuclear cells from patient 11 after 16 cycles of immunizations with hTERT:540-548 and after 12 days of in vitro culture with peptide. Cytokine secretion profiles from this T-cell population can be found in Table 2 (patient 11 after immunization), Table 3 (patient 11 post), and Table 4 (patient 11 bulk experiment 1). A, negative control phycoerythrin-conjugated HLA-A*0201/HTLV-1 tax peptide L11 tetramer versus FITC-labeled antihuman CD8. B, phycoerythrin-conjugated HLA-A*0201/hTERT:540-548 tetramer versus FITC-labeled antihuman CD8.
hTERT:540-548 Peptide Reactive CTL Did Not Recognize Cells Endogenously Expressing Telomerase
We next evaluated the ability of peptide-reactive PBMCs from immunized patients to recognize a variety of hTERT+ cell lines (Table 3). After a 12-day in vitro stimulation with hTERT:540-548, PBMCs from 4 patients, in whom evidence of immunization was observed, specifically secreted IFN-γ in response to T2 cells pulsed with 10 ng/ml hTERT:540-548 peptide. In addition, some of these populations contained as many as 24% T cells that were dually fluorescent after staining with FITC-conjugated antihuman CD8 and phycoerythrin-labeled HLA-A*0201/hTERT:540-548 tetrameric complexes (Fig. 2). However, none of these T-cell populations specifically recognized HLA-A*0201+ 293 cells, melanomas, or renal cell carcinomas that endogenously expressed telomerase (Table 3). In contrast, these CTLs did secrete significant amounts of IFN-γ in response to several B-cell lines. However, this cytokine secretion did not correlate with recognition of the hTERT:540-548 peptide because PBMCs from these patients before immunization also secreted IFN-γ in response to these B-cell lines but not in response to hTERT peptide-pulsed T2 cells (Table 3).
Table 3.
Lack of recognition of tumor cells expressing hTERT and HLA-A*0201 by PBMCs from patients immunized with hTERT:540-548 peptide in IFAa,b
| Target | Cell type | HLA-A2c | hTERTd | Patient 1 | Patient 5 | Patient 6 | Patient 11 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Poste | Pre | Postf | Pre | Postg | Pre | Posth | ||||
| Media | None | − | − | 183 | 138 | 45 | 71 | 57 | 30 | 218 | 116 |
| T2 + 1 μg/ml HBVc:18 | T-B hybrid | + | − | 214 | 275 | 69 | 450 | 105 | 96 | 243 | 153 |
| T2 + 1 μg/ml hTERT:540 | T-B hybrid | + | − | 212 | 1,057i | 68 | 4,210 | 84 | 886 | 218 | 15,004 |
| T2 + 0.01 μg/ml hTERT:540 | T-B hybrid | + | − | 235 | 962 | 77 | 3,582 | 88 | 880 | 256 | 9,858 |
| 293-UT | Renal cell | − | + | 92 | 271 | 47 | 222 | 68 | 47 | 84 | 51 |
| 293-A2 | Renal cell | + | + | 109 | 330 | 49 | 288 | 117 | 91 | 77 | 48 |
| 888mel | Melanoma | − | +/− | 98 | 220 | 51 | 299 | 54 | 38 | 142 | 82 |
| 938mel | Melanoma | − | + | 103 | 119 | 46 | 120 | 159 | 45 | 113 | 64 |
| 1861mel | Melanoma | + | + | 91 | 80 | 45 | 82 | 43 | 19 | 102 | 57 |
| 624mel | Melanoma | + | + | 88 | 69 | 41 | 79 | 44 | 25 | 88 | 56 |
| Sk23mel | Melanoma | + | + | 114 | 147 | 47 | 142 | 96 | 29 | 101 | 59 |
| N-KH | Renal cell cancer | + | + | 74 | 104 | 39 | 75 | 46 | 28 | 56 | 43 |
| S-KH | Renal cell cancer | + | + | 96 | 95 | 48 | 70 | 46 | 26 | 124 | 72 |
| C1R-UT | B lymphoblast | − | + | 529 | 620 | 174 | 247 | 118 | 135 | 541 | 291 |
| C1R-A2 | B lymphoblast | + | + | 1,056 | 1,626 | 458 | 766 | 305 | 324 | 1,139 | 1,270 |
| U266 | Myeloma | + | + | 152 | 225 | 71 | 276 | 92 | 55 | 411 | 178 |
| SKW6.4 | EBV-B cell | + | + | 181 | 384 | 1,004 | 422 | 270 | 107 | 457 | 363 |
| IM-9 | EBV-B cell | + | + | 371 | 856 | 282 | 579 | 666 | 191 | 1,396 | 1,078 |
| 1978E | EBV-B cell | + | + | 848 | 1,554 | 477 | 1,168 | 715 | 441 | 1,212 | 1,274 |
IFN-γ secretion (pg/ml) in 20-h coculture supernatants of T2 cells preloaded with peptide or tumor cells and PBMCs after 11–13 days culture with 1 μm hTERT:540-548.
hTERT, human telomerase reverse transcriptase; PBMC, peripheral blood mononuclear cell; IFA, incomplete Freund's adjuvant.
Determined by fluorescence-activated cell sorting analysis of cells with FITC-conjugated anti-HLA-A2.
Determined by telomeric repeat amplification protocol assay and reverse transcriptase-PCR (Fig. 1).
Patient 1 (after 2 cycles, 8 mg total).
Patient 5 (after 10 cycles, 10 mg total).
Patient 6 (after 4 cycles, 16 mg total).
Patient 11 (after 16 cycles, 16 mg total).
Underlined values indicate that IFN-γ secretion was >50 pg/ml and at least twice background with control target cells: (a) for T2 cells preincubated with hTERT:540-548, the control was T2 cells preincubated with HBVc:18-27(23Y); (b) for C1R-A2 cells, the control was C1R-UT cells; and (c) for other B-cell lines, the control was media.
To test whether the lack of tumor cell recognition was related to the presence of T lymphocytes with low avidity for the peptide, we generated several T-cell lines and clones with high peptide avidity. PBMCs from patients 3 and 11, who had previously been immunized with hTERT:540-548, were stimulated 11–13 days in vitro with peptide and were subsequently cloned by limiting dilution. The frequencies of growth-positive wells were significantly <37%, suggesting that the resulting T cells were clonal according to Poisson statistics. The bulk culture from patient 11 as well as two clones (R1-1 and R2-11) specifically recognized T2 cells pulsed with as little as 0.1 nm hTERT:540-548. However, these cells failed to recognize HLA-A*0201+ telomerase+ melanoma, colon carcinoma, and breast cancer cell lines (Table 4, experiment 1). In a second experiment, the T-cell clones from patient 11 were evaluated for recognition of a larger panel of telomerase+ cell lines, including melanomas, transformed B lymphocytes, renal cell lines, and colon carcinomas. However, none of these cell lines were recognized either (Table 4, experiment 2) despite highly avid recognition of peptide-pulsed T2 cells. A T-cell clone with high peptide avidity was also derived from patient 3 (clone 13-7) that specifically reacted with T2 cells pulsed with 1 nm hTERT:540-548. Similarly, these cells did not specifically recognize HLA-A*0201+ telomerase+ melanomas, B cells, or renal cell lines (Table 4, experiments 3–5).
Table 4.
Lack of recognition of tumor cells expressing hTERT and HLA-A*0201 by T-cell clones or lines that recognize hTERT:540-548 at concentrations < 10-8 ma,b
| Target | Cell type | HLA-A2 | hTERT | Patient 11 | ||||
|---|---|---|---|---|---|---|---|---|
| Bulk Experiment 1 | R1-1 Experiment 1 | R2-11 Experiment 1 | R1-1 Experiment 2 | R2-11 Experiment 2 | ||||
| Media | None | − | − | 16 | 0 | 4 | 14 | 11 |
| 10-6 M control | T-B hybrid | + | − | 13 | 0 | 8 | 18 | 42 |
| 10-6 M hTERT:540 | T-B hybrid | + | − | 12,562c | 7,931 | 4,585 | >2,000 | 1,693 |
| 10-7 M hTERT:540 | T-B hybrid | + | − | 11,494 | 5,503 | 3,849 | >2,000 | 1,172 |
| 10-8 M hTERT:540 | T-B hybrid | + | − | 6,364 | 1,916 | 1,946 | 1,073 | 308 |
| 10-9 M hTERT:540 | T-B hybrid | + | − | 3,488 | 974 | 630 | 149 | 57 |
| 10-10 M hTERT:540 | T-B hybrid | + | − | 1,598 | 381 | 123 | 13 | 2 |
| 10-8 M gp100:209 | T-B hybrid | + | − | |||||
| 888mel | Melanoma | − | +/− | 66 | 0 | 0 | 12 | 44 |
| 938mel | Melanoma | − | + | 58 | 44 | |||
| 1861mel | Melanoma | + | + | 16 | 27 | |||
| 624mel | Melanoma | + | + | 56 | 0 | 0 | 0 | 18 |
| Sk23mel | Melanoma | + | + | 1 | 24 | |||
| C1R-UT | B lymphoblast | − | + | 16 | 16 | |||
| C1R-A2 | B lymphoblast | + | + | 34 | 3 | |||
| SKW6.4 | EBV-B cell | + | + | 2 | 8 | |||
| 1978E | EBV-B cell | + | + | 7 | 0 | |||
| IM9 | EBV-B cell | + | + | 14 | 8 | |||
| U266 | Myeloma | + | + | 17 | 14 | |||
| 293-UT | Renal cell cancer | − | + | 9 | 7 | |||
| 293-A2 | Renal cell cancer | + | + | 21 | 11 | |||
| N-KH | Renal cell cancer | + | + | 22 | 21 | |||
| S-KH | Renal cell cancer | + | + | 33 | 21 | |||
| U2OS | Osteosarcoma | + | − | 10 | 3 | 18 | ||
| SK-OV-3 | Ovarian cancer | − | + | 11 | 174 | 16 | ||
| H508 | Colon cancer | + | + | 45 | 42 | |||
| SW480 | Colon cancer | + | + | 8 | 11 | 22 | ||
| HBL-100 | Breast cancer | + | + | 11 | 1 | 0 | ||
| Target | Cell type | HLA-A2 | hTERT | Patient 3 | Patient 15 | |||
|---|---|---|---|---|---|---|---|---|
| 13-7 Experiment 3 | 13-7 Experiment 4 | 13-7 Experiment 5 | mc14 Experiment 6 | mcD11 Experiment 6 | ||||
| Media | None | − | − | 0 | 55 | 44 | ||
| 10−6 M control | T-B hybrid | + | − | 0 | 10 | 4 | 49 | 44 |
| 10−6 M hTERT:540 | T-B hybrid | + | − | 4,181 | 229,300 | 214,400 | 6,615 | |
| 10−7 M hTERT:540 | T-B hybrid | + | − | 3,803 | 180,700 | 182,000 | 4,385 | |
| 10−8 M hTERT:540 | T-B hybrid | + | − | 3,196 | 63,670 | 23,260 | 1,779 | |
| 10−9 M hTERT:540 | T-B hybrid | + | − | 1,328 | 8,099 | 4,450 | 762 | |
| 10−10 M hTERT:540 | T-B hybrid | + | − | 110 | 44 | 25 | 197 | |
| 10−8 M gp100:217 | T-B hybrid | + | − | 7,754 | ||||
| 888mel | Melanoma | − | +/− | 2 | 48 | 0 | ||
| 939mel | Melanoma | − | + | |||||
| 1861mel | Melanoma | + | + | 49 | >2,000 | |||
| 624mel | Melanoma | + | + | 1 | 55 | |||
| Sk23mel | Melanoma | + | + | 50 | >2,000 | |||
| CIR-UT | B lymphoblast | − | + | 17 | ||||
| CIR-A2 | Melanoma | + | + | 12 | ||||
| SKW6.4 | EBV-B cell | + | + | |||||
| 1978E | EBV-B cell | + | + | 15 | ||||
| IM9 | EBV-B cell | + | + | |||||
| U266 | Myeloma | + | + | |||||
| 293-UT | Renal cell cancer | − | + | 10 | ||||
| 293-A2 | Renal cell cancer | + | + | 12 | ||||
| N-KH | Renal cell cancer | + | + | |||||
| S-KH | Renal cell cancer | + | + | |||||
| U20S | Osteosarcoma | + | − | |||||
| SK-OV3 | Ovarian cancer | − | + | |||||
| H508 | Colon cancer | + | + | |||||
| SW480 | Colon cancer | + | + | |||||
| HBL-100 | Breast cancer | + | + | |||||
FN-γ secretion (pg/ml) in 20-h coculture supernatants of T2 cells preloaded with peptide or tumor cells and T-cell clones or lines.
hTERT, human telomerase reverse transcriptase.
Underlined values indicate that IFNg secretion in response to T2 cells preincubated with hTERT:540-548 was >50 pg/ml and at least twice background with the control peptide.
We also generated a highly avid T-cell line from a nonimmunized patient with metastatic melanoma by repeated stimulation of PBMCs with the hTERT:540-548 peptide in vitro. PBMCs from patient 15 were initially plated in 96-well plate microcultures with peptide and were restimulated with peptide-pulsed autologous-irradiated PBMCs as least three times as described in “Materials and Methods.” One microculture from patient 15 (mc14) specifically recognized T2 cells pulsed with as little as 0.1 nm hTERT:540-548 but failed to secrete IFN-γ in response to melanomas expressing HLA-A*0201 and telomerase. In contrast, T cells from the same patient, stimulated in the same microculture format, with gp100:209-217(210M) clearly recognized HLA-A*0201+ melanomas, suggesting that these target cells were capable of antigen processing and epitope presentation (Table 4, experiment 6).
In other experiments, we used DCs pulsed with hTERT:540-548 as antigen-presenting cells in our stimulation protocol to sensitize PBMCs from nonimmunized HLA-A*0201+ patients with melanoma. PBMCs were initially plated in 96-well plate microcultures with peptide or peptide-pulsed DCs and were restimulated multiple times with peptide-pulsed autologous-irradiated PBMCs. Three peptide reactive microcultures were selected that were initially stimulated with peptide alone, and three were selected that had been stimulated with peptide-pulsed DCs. However, none of these T-cell populations specifically recognized HLA-A*0201+ melanoma cells that naturally expressed telomerase, nor did they secrete IFN-γ in response to melanoma cells that had been transduced with an adenoviral vector encoding the hTERT gene (Table 5). In the same experiment, PBMCs from another patient were initially stimulated with DCs pulsed with an HLA-A*0201-restricted peptide derived from the renal cell carcinoma antigen G250 (G250:254-262; Ref. 25). In contrast to the cells stimulated with hTERT:540-548, these CTLs specifically recognized the G250 peptide as well as HLA-A*0201+ melanoma cells that had been transduced with an adenovirus encoding the full-length G250 gene.
Table 5.
Lack of recognition of tumor cells expressing hTERT and HLA-A*0201 by T-cell microcultures stimulated with hTERT:540-548-loaded PBMC or DCsa,b
| Target | HLA-A2 expression | hTERT expression | Adenoviral transduction | Patient 16 | Patient 17 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBMC D9 | PBMC B7 | PBMC E9 | DC F12 | DC G7 | DC F8 | DC E4 | ||||
| Media | − | − | None | 227 | 51 | 195 | 140 | 62 | 36 | 70 |
| T2 + HBVc:18 | + | na | None | 419 | 53 | 200 | 110 | 67 | 28 | 42 |
| T2 + hTERT:540 | + | na | None | >10,000c | 8,016 | >10,000 | 7,093 | >10,000 | 6,760 | |
| T2 + G250:254 | + | na | None | >10,000 | ||||||
| 888mel | + | +/− | None | 189 | 39 | 148 | 103 | 51 | 31 | |
| hTERT | 254 | 48 | 171 | 162 | 70 | 34 | 73 | |||
| G250 | 234 | 48 | 171 | 146 | 58 | 28 | 66 | |||
| 938mel | − | + | None | 182 | 36 | 140 | 104 | 51 | 32 | |
| hTERT | 228 | 41 | 167 | 123 | 58 | 28 | 61 | |||
| G250 | 224 | 46 | 163 | 118 | 58 | 31 | 64 | |||
| Sk23mel | + | + | None | 256 | 50 | 161 | 113 | 59 | 35 | |
| hTERT | 294 | 43 | 173 | 127 | 57 | 33 | 58 | |||
| G250 | 228 | 40 | 162 | 145 | 56 | 27 | 444 | |||
| 526mel | + | + | None | 206 | 38 | 159 | 123 | 71 | 28 | |
| hTERT | 346 | 45 | 264 | 136 | 53 | 27 | 53 | |||
| G250 | 250 | 47 | 199 | 130 | 70 | 32 | 276 | |||
IFN-γ secretion (pg/ml) in 20 h coculture supernatants of T2 cells preloaded with peptide or tumor cells and T cells stimulated 4–5 times with 1 μm hTERT:540-548.
hTERT, human telomerase reverse transcriptase; PBMC, peripheral blood mononuclear cell; na, not applicable.
Underlined values indicate that IFN-γ secretion in response to T2 cells preincubated with the relevant peptide and/or tumor cells was >50 pg/ml and at least twice background with the control peptide and/or tumor cells.
PBMCs that had been stimulated multiple times with hTERT:540-548 in vitro were also simultaneously evaluated for recognition of peptide and hTERT+ cell lines in both cytolysis and cytokine secretion assays (Fig. 3). Results from both types of assays were similar in that T-cell lines specifically recognized hTERT:540-548 peptide but failed to lyse or secrete IFN-γ in response to HLA-A*0201+ telomerase+ melanoma cell lines.
Fig. 3.
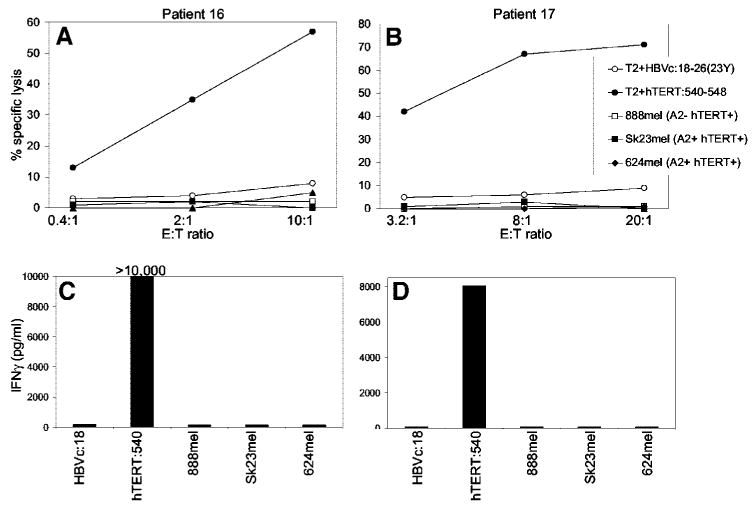
Correlation of cytolysis and cytokine secretion by hTERT:540-548-reactive T-cell lines. T-Cell lines from 2 patients with metastatic melanoma were stimulated with hTERT:540-548 in the microculture format. After one restimulation (∼ day 14), the microcultures were evaluated for specific peptide recognition via cytokine secretion, and those that were positive were restimulated at least two additional times in 24-well plates. Seven to 14 days after the last restimulation, the T-cell populations were evaluated for recognition of peptide and hTERT+ cell lines in both cytolysis (A and B) and IFN-γ secretion assays (C and D).
Fab Fragments against HLA-A*0201/hTERT:540-548 Complexes Did Not Stain Cells Endogenously Expressing Telomerase
As an alternate technique to determine whether the hTERT:540-548 peptide was displayed on the surface of tumor cells in the context of HLA-A*0201, we used FACS to analyze cells stained with Fab fragments that specifically bound to HLA-A*0201/hTERT:540-548 complexes (4G9) (Fig. 4). All FACS analysis profiles were near-normal distributions with no apparent shoulders, and therefore, mean fluorescence intensities were used as statistically relevant data for comparison. T2 cells pulsed with 10 μg/ml hTERT:540-548 were specifically stained with 4G9 Fabs. However, mean fluorescence intensities were significantly decreased when lower peptide concentrations were used, and mean fluorescence intensities of T2 cells pulsed with hTERT:540-548 at concentrations < 0.1 μg/ml were comparable with those of T2 cells pulsed with an irrelevant control peptide (Fig. 4A). In addition, no specific staining above background levels was observed for B-cell lines, melanomas, or 293 cells that endogenously expressed HLA-A*0201 and telomerase (Fig. 4, B and C). Thus, we did not visualize HLA-A*0201/hTERT:540-548 complexes on cell surfaces using this methodology.
Fig. 4.
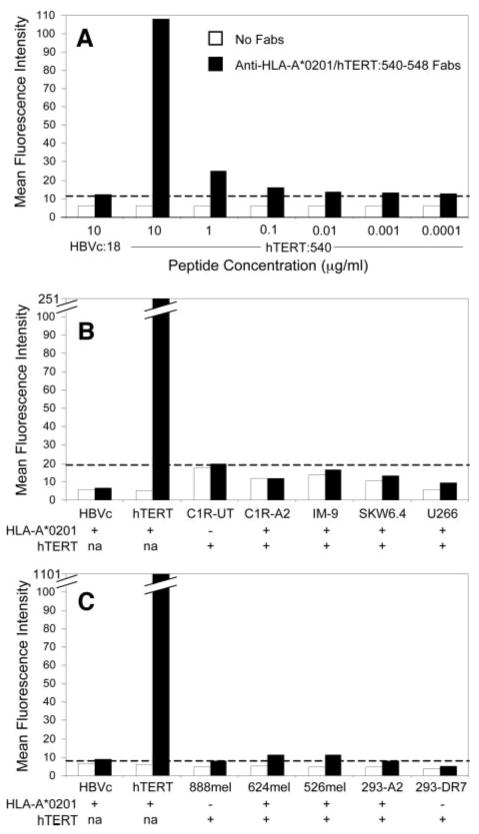
Staining of cell lines with Fab fragments (4G9) against HLA-A*0201/hTERT:540-548 complexes. A, T2 cells were pulsed with various concentrations of hTERT:540-548 and were subsequently stained with 4G9 Fabs. The dashed line indicates the mean fluorescence intensity (MFI) of background staining of 4G9 on T2 cells pulsed with an irrelevant control peptide [HBVc:18-27(23Y)]. B, T2 cells pulsed with 10 μg/ml hTERT:540-548 or HBVc:18-27(23Y) and B-cell lines were stained with 4G9 Fabs. The dashed line indicates background MFI of 4G9 staining of HLA-A*0201- C1R cells. C, T2 cells pulsed with 100 μg/ml hTERT:540-548 or HBVc:18-27(23Y), melanoma cell lines, and 293 cells were stained with 4G9 Fabs. The dashed line indicates background MFI of 4G9 staining of T2 cells pulsed with HBVc:18-27(23Y).
Discussion
Telomerase was thought to be an attractive candidate tumor-associated antigen to be targeted in cancer immunotherapies because it is overexpressed in >85% of human cancers, whereas its expression in normal adult tissues is extremely limited. Investigators from two different laboratories previously reported that sensitized T cells could recognize an epitope derived from the catalytic subunit of telomerase hTERT:540-548 on the surfaces of tumor cells in the context of HLA-A*0201 (3, 4). Therefore, we initiated a clinical protocol to vaccinate patients with metastatic cancers with this peptide. Fourteen patients were enrolled in the study, and in vitro evidence of immunization against the hTERT peptide was observed in 7 of these patients. However, recognition of hTERT:540-548 by CTL was not associated with that of telomerase+ tumors or cells transduced with a recombinant adenovirus encoding the full-length hTERT gene using either 51Cr release cytotoxicity or cytokine secretion assays. Furthermore, none of these patients underwent an objective clinical response to treatment.
In previous studies that demonstrated a correlation between hTERT peptide and tumor reactivities (3, 4), the bulk T-cell populations that were used may have contained distinct CTL clones, some of which were responsible for peptide recognition and others that accounted for the apparent tumor reactivities (26). We observed this phenomenon in bulk T-cell cultures stimulated with the hTERT:540-548 peptide as shown in Table 3. PBMCs from 4 patients after immunization secreted IFN-γ in response to peptide-pulsed T2 cells as well as several B-cell lines. However, these reactivities were apparently unrelated because T cells before immunization treated similarly in vitro also secreted cytokine after coculture with the B-cell lines but not in response to the hTERT:540-548 peptide. Therefore, in the absence of a T-cell clone, as defined by a single T-cell receptor, that specifically recognizes both peptide and cells that endogenously express HLA-A*0201 and telomerase, it is not possible to conclude that CTL can recognize hTERT:540-548 on the surfaces of tumor cells.
Another potential explanation for the lack of tumor recognition may have been that peptide reactive T cells did not have sufficient avidity to recognize the low concentrations of peptides on target cell surfaces in the context of HLA-A*0201 molecules after processing and presentation of the full-length hTERT protein. To address this issue, we generated several highly avid T-cell lines and clones that specifically recognized hTERT:540-548 at concentrations of 0.1–1 nm when pulsed onto T2 cells (Table 2). These highly avid T cells also did not recognize HLA-A*0201+ hTERT+ cell lines. Correlations between peptide avidity and tumor cell recognition have previously been made for several melanoma-associated epitopes, including MART-1:27-35 (18), tyrosinase:8-17 (27), gp100:209-217 (28), and TRP-2:180-188 (21). In these studies, recognition of 1 nm peptide was sufficient to enable recognition of melanoma cells. Although many of the peptide reactive T-cell lines and clones described here met that criteria, it is possible that the avidity threshold for recognition of hTERT:540-548 on tumor cells was still lower than we were able to achieve. However, if this is the case, it seems unlikely that such cells could be routinely generated in vivo after immunization.
We also investigated the possibility that professional APCs were required to stimulate CTL that could recognize cells endogenously expressing hTERT. In some experiments, we initially stimulated PBMCs either with peptide alone or with peptide-pulsed autologous DCs. Although peptide recognition was generated using both protocols, none of these cultures recognized telomerase+ cell lines (Table 4).
One explanation for the lack of tumor recognition by hTERT:540-548 reactive lymphocytes was suggested by Ayyoub et al. (18). In that study, a peptide precursor, hTERT:534-554, was digested by purified proteasomes and immunoproteasomes in vitro. The digestion products were analyzed by mass spectrometry and were evaluated for recognition by a hTERT:540-548 reactive T-cell clone. hTERT:540-548 could not be detected in the digestion products by either methodology, and several mass spectrometric peaks were identified that corresponded to fragments generated by proteasomal cleavage within the epitope. These authors thus suggested that the hTERT:540-548 epitope was digested in the proteasome and was not presented on the surfaces of tumor cells in the context of HLA-A*0201. Although it is possible that other HLA-A*0201-restricted epitopes may exist within telomerase that are presented on the surfaces of tumor cells, our data, together with that of Ayyoub et al. (18), suggest that the HLA-A*0201-restricted peptide hTERT:540-548 will not be useful for the immunotherapy of patients with cancer.
The results of the work presented here cannot confirm those of a recently published clinical trial in which patients with advanced breast or prostate cancers were immunized with autologous DCs pulsed with hTERT:540-548 and keyhole limpet hemocyanin (29). Vonderheide et al. (29) reported that peptide-reactive CTLs were induced in 4 of 7 patients who received the vaccine, and recognition of the hTERT:540-548 peptide was correlated with the ability of T cells to lyse tumor cells endogenously expressing HLA-A*0201 and telomerase. In that study, enriched HLA-A*0201/hTERT:540-548 tetramer+ T cells specifically lysed two HLA-A*0201+ telomerase+ tumor lines, but only a single HLA-A*0201- cell was included as a negative control. A different interpretation of data from that investigation may have resulted if a larger panel of target cell lines had been used, including HLA-A*0201+ telomerase- tumor lines and cells transfected or transduced with the full-length hTERT gene.
In summary, although we found evidence of immunization against the peptide in 7 of 14 patients, recognition of the hTERT:540-548 peptide was not associated with that of telomerase+ tumors or cells transduced with a recombinant adenovirus encoding the full-length hTERT gene.
Acknowledgments
We thank Dr. Robert Vonderheide and Dr. Yoram Reiter for helpful discussions, reagents, and cell lines. We also thank Dr. Gang Zeng for performing reverse transcriptase-PCR on the Rapid-Scan panel of cDNAs from Origene Technologies. We thank Arnold Mixon and Shawn Farid for performing FACS analyses.
References
- 1.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature (Lond) 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Antigens recognized by T-lymphocytes on human tumours. Biochem Soc Trans. 1997;25:544–8. doi: 10.1042/bst0250544. [DOI] [PubMed] [Google Scholar]
- 3.Vonderheide RH, Hahn WC, Schultze JL, et al. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–9. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 4.Minev B, Hipp J, Firat H, et al. Cytotoxic T-cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci USA. 2000;97:4796–801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–65. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 6.Counter CM, Meyerson M, Eaton EN, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–22. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science (Wash DC) 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 8.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science (Wash DC) 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 9.Kim NW. Clinical implications of telomerase in cancer. Eur J Cancer. 1997;33:781–6. doi: 10.1016/S0959-8049(97)00057-9. [DOI] [PubMed] [Google Scholar]
- 10.Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–95. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 11.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 12.Hahn WC, Counter CM, Lundberg AS, et al. Creation of human tumour cells with defined genetic elements. Nature (Lond) 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 13.Herbert B, Pitts AE, Baker SI, et al. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–81. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn WC, Stewart SA, Brooks MW, et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–70. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 15.Cormier JN, Salgaller ML, Prevette T, et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J Sci Am. 1997;3:37–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lev A, Denkberg G, Cohen CJ, et al. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–94. [PubMed] [Google Scholar]
- 18.Ayyoub M, Migliaccio M, Guillaume P, et al. Lack of tumor recognition by hTERT peptide 540–548-specific CD8(+) T cells from melanoma patients reveals inefficient antigen processing. Eur J Immunol. 2001;31:2642–51. doi: 10.1002/1521-4141(200109)31:9<2642::aid-immu2642>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Riley JP, Rosenberg SA, Parkhurst MR. Stimulation of tumor-reactive T lymphocytes using mixtures of synthetic peptides derived from tumor-associated antigens with diverse MHC binding affinities. J Immunol Methods. 2003;276:103–19. doi: 10.1016/s0022-1759(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down-regulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhurst MR, Fitzgerald EB, Southwood S, et al. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–901. [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Yee C, Gilbert MJ, Riddell SR, et al. Isolation of tyrosinase-specific CD8+ and CD4+ T-cell clones from the peripheral blood of melanoma patients following in vitro stimulation with recombinant vaccinia virus. J Immunol. 1996;157:4079–86. [PubMed] [Google Scholar]
- 24.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 25.Vissers JL, DeVries IJ, Schreurs MW, et al. The renal cell carcinoma-associated antigen G250 encodes a human leukocyte antigen (HLA)-A2.1-restricted epitope recognized by cytotoxic T lymphocytes. Cancer Res. 1999;59:5554–9. [PubMed] [Google Scholar]
- 26.Speiser DE, Cerottini JC, Romero P. Can hTERT peptide (540-548)-specific CD8 T cells recognize and kill tumor cells? Cancer Immun. 2002;2:14. [PubMed] [Google Scholar]
- 27.Riley JP, Rosenberg SA, Parkhurst MR. Identification of a new shared HLA-A2.1-restricted epitope from the melanoma antigen tyrosinase. J Immunother. 2001;24:212–20. [PubMed] [Google Scholar]
- 28.Dudley ME, Nishimura MI, Holt AK, et al. Antitumor immunization with a minimal peptide epitope (G9-209-2M) leads to a functionally heterogeneous CTL response. J Immunother. 1999;22:288–98. doi: 10.1097/00002371-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Vonderheide RH, Domchek SM, Schultze JL, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–39. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]


