Abstract
We have studied signaling mechanisms that stimulate exocytosis and luteinizing hormone secretion in isolated male rat pituitary gonadotropes. As judged by reverse hemolytic plaque assays, phorbol-12-myristate-13-acetate (PMA) stimulates as many gonadotropes to secrete as does gonadotropin-releasing hormone (GnRH). However, PMA and GnRH use different signaling pathways. The secretagogue action of GnRH is not very sensitive to bisindolylmaleimide I, an inhibitor of protein kinase C, but is blocked by loading cells with a calcium chelator, 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. The secretagogue action of PMA is blocked by bisindolylmaleimide I and is not very sensitive to the intracellular calcium chelator. GnRH induces intracellular calcium elevations, whereas PMA does not. As judged by amperometric measurements of quantal catecholamine secretion from dopamine- or serotonin-loaded gonadotropes, the secretagogue action of PMA develops more slowly (in several minutes) than that of GnRH. We conclude that exocytosis of secretory vesicles can be stimulated independently either by calcium elevations or by activation of protein kinase C.
Keywords: phorbol ester, gonadotrope, pituitary, calcium, amperometry
Regulated secretion by exocytosis frequently is triggered by a rise of cytoplasmic Ca2+ concentration ([Ca2+]i). At fast chemical synapses and in chromaffin cells the [Ca2+]i signal is considered necessary and sufficient for rapid release of neurotransmitters. Here we describe in an endocrine cell an example of regulated secretion that normally is mediated by a [Ca2+]i elevation but also can be triggered by another, less Ca2+-dependent, pathway. This suggests cells can use multiple parallel and alternate signals to control exocytosis of the same population of secretory vesicles.
Pituitary gonadotropes respond to their releasing hormone, gonadotropin-releasing hormone (GnRH), by secreting luteinizing hormone (LH) and follicle-stimulating hormone. The releasing hormone activates phosphoinositide turnover (1–3) and initiates cyclic release of Ca2+ from intracellular stores (4, 5). This triggers exocytosis of secretory granules, as is indicated electrically by cyclic increases of membrane capacitance (6, 7). The key importance of the [Ca2+]i signal is clear from three biophysical observations: the fast GnRH-induced exocytosis is synchronous with each [Ca2+]i rise; it can be blocked by loading Ca2+ buffers into the cytoplasm; and in the absence of GnRH, exocytosis can be initiated within milliseconds by rapid stepwise increases of [Ca2+]i using caged Ca2+ compounds (6, 7). Nevertheless, many laboratories have reported that agents that activate protein kinase C (PKC) or increase cellular cAMP can stimulate exocytosis in a variety of cell types (8–16). Indeed, numerous early papers reported that such agents stimulated secretion of LH from gonadotropes (2, 17–20), and therefore PKC and cAMP-dependent protein kinase were suggested as important for the action of the releasing hormone, a hypothesis that has remained controversial. In this paper we return to this issue. Do these agents reliably stimulate LH secretion in male rat gonadotropes and, if so, do they activate a Ca2+-independent secretory pathway or do they act indirectly by raising [Ca2+]i? In addition, because GnRH receptors couple to phospholipase C and hence can activate PKC, we ask if activation of PKC is an essential component of normal hormone-induced LH secretion.
MATERIALS AND METHODS
Chemicals and Solutions.
Phorbol-12-myristate-13-acetate (PMA), 4α-phorbol-12,13-didecanoate (4α-PDD), and ionomycin (Calbiochem) were dissolved in dimethyl sulfoxide (Sigma) at 1 mM and kept at −70°C. Bisindolylmaleimide I hydrochloride (BIS) (synonymous with GF 109203X) was from Calbiochem. 8-(4-chlorophenylthio)-cAMP (CPTcAMP) was from Boehringer Mannheim, and emetine dihydrochloride was from Sigma. These agents were dissolved in water at about 1,000 times their final concentration. GnRH (Peninsula Laboratories) was dissolved in water, aliquoted, lyophilized, and kept at −20°C. Indo-1 acetoxymethyl ester, 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) tetrakis(acetoxymethyl)ester, and pluronic F-127 were from Molecular Probes. Easy Tag protein labeling mix ([35S]methionine) was from DuPont/NEN, and 3-isobutyl-1-methylxanthine (IBMX) and forskolin were from Research Biochemicals (Natick, MA). They were solubilized in dimethyl sulfoxide at 200 mM and 20 mM, respectively. All other chemicals were from Sigma.
External saline contained 150 mM NaCl, 5 mM CaCl2, 2.5 mM KCl, 1 mM MgCl2, 8 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes) (pH 7.4 with NaOH). Incubation medium consisted of Hepes-buffered DMEM (GIBCO) supplemented with 0.1% BSA (Sigma), 100 units/ml penicillin V, and 100 μg/ml streptomycin (GIBCO or Sigma).
Animals, Cell Preparation, and Identification.
All experiments used pituitary gonadotropes from male Sprague–Dawley rats castrated at 3 weeks of age to increase the yield of gonadotropes. Rats were anesthetized with 0.9 ml/kg of a mixture containing 100 mg/ml ketamine (Aveco, Fort Dodge, IA) and 20 mg/ml xylazine (Phoenix Pharmaceuticals, St. Joseph, MO) before castration, given an injection of Bicillin L-A (Wyeth Laboratories, Philadelphia, PA) postoperatively, and closely monitored during surgery and recovery. One to three weeks later, an animal was euthanized, and the pituitary was removed and dissociated into a cell preparation (21).
Gonadotropes, defined as cells that secreted LH in response to GnRH, were identified by the reverse hemolytic plaque assay (RHPA) (22, 23). Briefly, pituitary cells were mixed with protein A-coupled red blood cells and drawn into a low chamber. After 1 hr for attachment, cells were incubated for 1 hr in incubation medium supplemented with 50 nM GnRH and rabbit polyclonal antibody (24) against bovine LH (1:40 dilution; kind gift of J. D. Neill, University of Alabama, Birmingham). Then cells were exposed to complement for 30 min for formation of plaques of red-cell lysis. Finally, the top of the chamber was removed, and the dishes containing pituitary and red blood cells were maintained in culture.
Plaque Measurement of Secretory Response.
The secretory responsiveness of gonadotropes was assessed from the relative density of plaques formed in the RHPA. The assay was conducted as above, except that various test substances were included in the incubation medium and sometimes GnRH was omitted. Dishes with cells then were maintained in culture for 2–4 days to permit disintegration of any cells that may have formed plaques artifactually as a result of lysis during the assay.
In one set of experiments RHPA was used to examine cells loaded with the Ca2+ chelator BAPTA. Dispersed cells were divided into two groups: the control group was resuspended in incubation medium and the test group, in the same medium containing the permeant acetoxymethyl ester form of the chelator BAPTA (BAPTA AM). BAPTA AM was mixed with an equal volume of 20% pluronic F-127 and diluted in incubation medium to a final concentration of 10 μM. Cells were incubated at 37°C for 15 min with agitation, collected, washed twice, mixed with red blood cells, and subjected to RHPA. The acetoxymethyl ester should have been cleaved to regenerate active BAPTA during the 1-hr incubation used for cell attachment before adding secretagogues in the RHPA protocol.
Dishes were viewed under the light microscope (×250), and plaques were tallied, counting only spaces >1.7 times the diameter of the pituitary cell contained in them. The number of plaques in a dish was normalized by the area covered by the cell mixture (around 70 mm2). For each experiment, the mean plaque density was calculated for the set of dishes with GnRH alone, and the plaque densities in all dishes within the experiment were normalized to this mean to give what we will call relative plaque density. This is a measure of the proportion of cells that secrete sufficient LH to form a visible plaque, rather than a measure of the amount of LH secreted by cells. Each experiment included at least four dishes with a given treatment and was repeated two or three times (total n ≥ 12 dishes per treatment). The relative plaque density values for all dishes from all experiments with the same agent were pooled, and mean ± SEM was calculated as an indicator of the agent’s effectiveness. Differences between the normalized plaque densities for dishes with different agents were assessed using the nonparametric Mann–Whitney U test. P < 0.05 was considered significant.
Amperometric Measurement of Secretory Response.
A second method was used to measure exocytotic events in single cells with better frequency response than the plaque assay provides. A micro-amperometric probe polarized to +600 mV was placed next to individual cells to detect release of oxidizable molecules that had been loaded into the secretory vesicles, as has been done previously with pancreatic β cells (25). This technique reports the concentration of monoamine immediately outside a cell and readily detects quantal release of a single vesicle. Gonadotropes are not known to store monoamines normally, so we do not know if the monoamines accumulate in LH-containing granules or other vesicular compartments. Dopamine (1 mM) or a mixture containing 1 mM each of serotonin and 5-OH-tryptophan was added to cell culture medium for 4 or more hr at 37°C. Cells then were studied in monoamine-free solution. A local perfusion system was used to achieve rapid solution exchange. Loading of the transmitters into secretory vesicles was evident from the numerous 5–30-ms spike-like, quantal events seen with the amperometric probe when exocytosis was stimulated with a Ca2+ ionophore (2 μM ionomycin). The 11-μm carbon-fiber amperometric probes were fabricated as described (26), and amperometric current was recorded with a List EPC-5 patch-clamp amplifier.
Single-Cell Ca2+ Photometry.
Single gonadotropes were used for Ca2+ measurements 1–2 days after identification by RHPA. Intracellular free Ca2+ was measured with indo-1 loaded into cells by incubation with its permeant acetoxymethyl ester (27). Cells were incubated in 1 μM indo-1 acetoxymethyl ester for 1 hr in 5% CO2 at 37°C and then washed with external saline. Indo-1 was excited at 365 nm, and the fluorescence at 405 nm (F405) and 500 nm (F500) was measured. Average background fluorescence measured with no cells in the field was subtracted. Fluorescence measurements are reported as the ratio F405/F500, without absolute calibration for [Ca2+]i. Local perfusion was used to achieve rapid solution exchange.
Measurement of Protein Synthesis Rates.
To find an effective concentration of the protein synthesis inhibitor emetine, rates of protein synthesis were assessed by measuring rates of incorporation of [35S]methionine into trichloroacetic acid (TCA)-precipitable total cellular protein. Anterior pituitary cells were suspended in incubation medium with or without 1 or 10 μM emetine. After a 20-min incubation in 5% CO2 at 37°C, [35S]methionine was added at 221 μCi/ml for 60 min. Cells then were collected by centrifugation, resuspended in 8% TCA, and incubated at 90°C for 3 min. The TCA/cell mixtures were spotted on strips of Whatman 3MM filter paper, washed in 7.5% TCA and 95% ethanol at 0°C, dried, and counted.
RESULTS
Preliminary Experiments.
Our previous work with the plaque assay used 2-hr incubations with GnRH. We wanted to focus here on acute effects of potential secretagogues rather than on longer-term effects that might include consequences of changes in protein synthesis. For this reason we first explored how the duration of incubation with secretagogue affected plaque formation. In different experiments, incubating dishes with GnRH for 1, 3, or 17 hr gave approximately the same plaque density, as if a 1-hr incubation suffices to activate most responsive gonadotropes. Shorter incubations (30 min) gave no plaques. Interestingly, the basal plaque density in dishes without secretagogue did increase with duration of incubation. It rose from 8.7 ± 1.7% to 39.4 ± 5% of the GnRH value in the 1- and 3-hr incubations, respectively, and was not significantly different from 100% in the 17-hr incubation. Evidently every gonadotrope has at least one episode of spontaneous activity sometime in a 17-hr period, so short incubation times most efficiently will distinguish between secretagogue-stimulated and basal activity.
Secretion Stimulated by PMA.
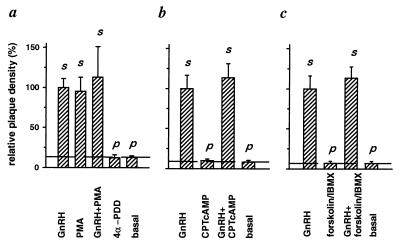
Using the plaque assay, we began by asking if secretion of LH from pituitary cells could be triggered by activating PKC or by raising cAMP and how this secretion compared with that evoked by GnRH. In the experiments of Fig. 1, the basal plaque density obtained in the 1-hr incubation without secretagogue was 6.8–12.6% of that with 50 nM GnRH. Incubation with 100 nM PMA, an active phorbol ester, or with GnRH plus PMA gave plaque densities indistinguishable from those with GnRH alone (Fig. 1a). The inactive phorbol ester, 4α-PDD, did not stimulate secretion above the basal level (Fig. 1a). If the number of plaques evoked by GnRH defines the number of gonadotropes on the dish, we conclude that PMA can stimulate all gonadotropes to secrete enough LH in 1 hr to form plaques.
Figure 1.
Activation of PKC (a) but not elevation of cAMP (b and c) causes LH secretion. Pituitary cells were exposed for 1 hr in RHPA to the indicated compounds or to no secretagogue (basal). Bars indicate the mean (±SEM) number of LH secreting cells (the number of plaques) per area occupied by the cell mixture normalized to the density with GnRH. Dashed line indicates secretion without secretagogue (basal). p designates significant difference from GnRH-induced LH release. s designates significant difference from basal LH release. Compounds were used at the following concentrations: 50 nM GnRH, 100 nM PMA, 1 μM 4α-PDD, 100 μM CPTcAMP, 20 μM forskolin, and 500 μM IBMX.
By contrast, the effects of raising cAMP were minor. Two approaches were tried, incubation with the membrane-permeant cAMP analogue CPTcAMP (100 μM) and incubation with a mixture of the adenylyl cyclase activator forskolin (20 μM) and the phosphodiesterase inhibitor IBMX (500 μM). The results were identical. Incubation with CPTcAMP or with the forskolin/IBMX mixture gave no more plaques than incubation without any secretagogue, and combining either of these treatments with 50 nM GnRH did not increase the number of plaques obtained with GnRH alone (Fig. 1 b and c). Therefore, we did not consider the action of cAMP as a secretagogue further and focused on the actions of PMA.
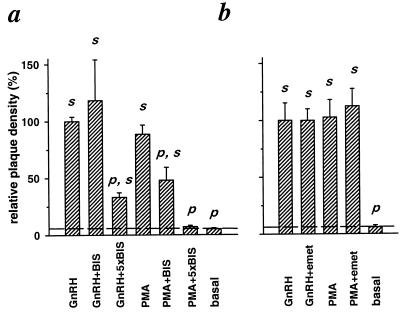
Is PMA acting via activation of PKC, or is it acting nonspecifically on other targets? Fig. 2a shows a test with a bisindolylmaleimide that is a potent and relatively specific inhibitor of many PKC isozymes (28). The inhibitor was applied 10 min before addition of PMA or GnRH and was present through the 1-hr incubation. Inclusion of 100 nM BIS had no effect on GnRH-stimulated plaque formation but significantly depressed the stimulation by PMA. Inclusion of 500 nM BIS reduced the number of GnRH-induced plaques by 67% and completely blocked the PMA effect. Hence the stimulatory action of PMA on LH secretion requires activation of PKC, whereas the stimulatory action of GnRH is only partially depressed when PKC is blocked.
Figure 2.
GnRH- and PMA-evoked secretion differ in their sensitivity to inhibition of PKC (a) and are insensitive to blockade of protein synthesis (b). Where indicated, dissociated pituitary cells were incubated for 10 min with BIS or emetine. Then all cells were exposed for 1 hr in RHPA to the indicated compounds. Compounds were used at the following concentrations: 50 nM GnRH, 100 nM PMA, 100 nM or 500 nM (designated 5×BIS) BIS, and 10 μM emetine.
Activators of PKC can stimulate protein synthesis in a variety of cell types, including pituitary gonadotropes (29). We therefore considered the hypothesis that the PMA-induced increase in relative plaque density was a consequence of enhanced protein synthesis. This was tested by blocking protein synthesis at the level of translation with emetine before and during the plaque assay. In pilot experiments, we verified the effectiveness of emetine by showing that it reduced incorporation of [35S]methionine into total precipitable protein by 70% at 1 μM and by 90% at 10 μM (see Materials and Methods). Emetine (10 μM) did not reduce GnRH-stimulated or PMA-stimulated plaque formation significantly (Fig. 2b). Hence protein synthesis is not needed to obtain the stimulatory effect of a 1-hr exposure to PMA.
Absence of a Calcium Signal.
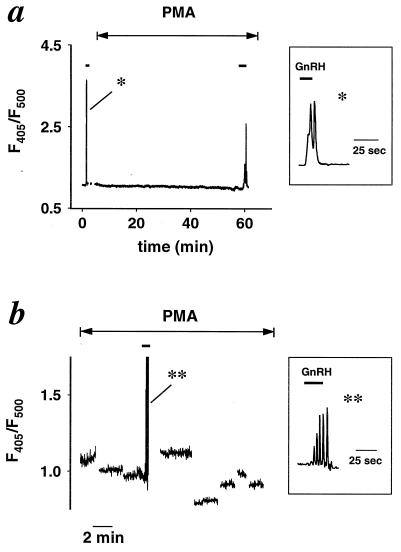
We next tested the hypothesis that PMA might act as a secretagogue that triggers [Ca2+]i elevations in gonadotropes much as GnRH does. The [Ca2+]i was monitored with the Ca2+-sensitive fluorescent dye indo-1 in plaque-identified cells. In the experiment of Fig. 3a, an initial, brief application of GnRH gave a short episode of [Ca2+]i oscillations, verifying the well known response of identified gonadotropes to GnRH. By contrast, during a subsequent 1-hr exposure to PMA, [Ca2+]i never rose above resting levels, although a final application of GnRH showed that the cell still could produce a [Ca2+]i rise in response to releasing hormone. The same result was obtained with five cells in different dishes. In a different paradigm, eight gonadotropes in one dish were sampled in succession for short intervals during one 1.5-hr exposure to PMA (Fig. 3b). None showed a [Ca2+]i elevation with PMA alone, although each of them responded with a [Ca2+]i elevation when tested subsequently with GnRH (shown only for one cell). Thus, stimulation of LH release by PMA, unlike stimulation by GnRH, is accomplished without a detectable [Ca2+]i elevation.
Figure 3.
PMA does not induce a [Ca2+]i rise. Traces show the time course of indo-1 fluorescence ratios (F405/F500) for individual plaque-identified gonadotropes. (a) [Ca2+]i oscillations develop during brief local perfusion of 10 nM GnRH (short solid lines) but not during 1-hr bath application of 100 nM PMA (double-headed arrow). Representative of five similar experiments. (Inset) GnRH-evoked [Ca2+]i oscillations on a faster time scale. (b) Eight different cells monitored for short intervals during a 1.5-hr incubation in PMA (double-headed arrow). Each cell was monitored for about 2 min and then confirmed to be responsive to GnRH applied by brief local perfusion (solid bar, shown for one cell only). Consecutive cells were monitored with 5–15 min intervals in between. Representative of two similar experiments. (Inset) GnRH-evoked oscillations on a faster time scale.
A possible criticism of these fluorescence experiments is that they were done at room temperature whereas the plaque assays were done at 37°C. Therefore, additional plaque assays were done at room temperature to check if PMA still induced LH secretion. In 1-hr incubations, the relative plaque densities were 100 ± 10%, 97 ± 17%, and 13 ± 1% for GnRH, PMA, and secretagogue-free dishes, respectively, (n = 8 for each treatment), showing that the plaque assay works well at room temperature and that PMA is as effective under these conditions as at 37°C.
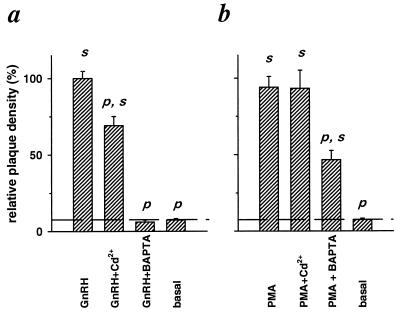
The apparent lack of [Ca2+]i signals during PMA-induced LH secretion contrasted with the obvious [Ca2+]i oscillations evoked by GnRH. This discrepancy seemed worth checking in other ways. Therefore we asked if actions of GnRH and PMA have a different sensitivity to two treatments that should depress Ca2+ signaling; these were blocking of plasma membrane Ca2+ entry pathways by applying 100 μM Cd2+ in the bath and blunting of [Ca2+]i elevations by loading cells with the fast Ca2+ chelator BAPTA. To ensure significant loading with chelator, cells were soaked in 10 μM membrane-permeant BAPTA tetrakis(acetoxymethyl)ester, 10 times the concentration used to load cells with indo-1 acetoxymethyl ester in the experiments of Fig. 3. In the experiments with GnRH, Cd2+ depressed the plaque density only modestly but significantly, whereas BAPTA depressed plaque density to below the basal, unstimulated value (Fig. 4a). These effects on GnRH-induced secretion are consistent with an absolute dependence of GnRH signaling on [Ca2+]i elevations, with much of the Ca2+ coming from intracellular stores. By contrast, when PMA was used as the secretagogue, Cd2+ was without effect on plaque density and BAPTA depressed it by less than 50% (Fig. 4b). The stimulatory effect of PMA clearly depends much less on Ca2+ signaling than does the effect of GnRH.
Figure 4.
GnRH-evoked (a) and PMA-evoked (b) LH release differ in their sensitivity to blockade of Ca2+ entry and to chelation of [Ca2+]i. Dissociated pituitary cells were exposed for 1 hr in RHPA to the indicated compounds except that BAPTA was loaded into the cells by 15-min incubation before RHPA. Compounds were used at the following concentrations: 50 nM GnRH, 100 nM PMA, 100 μM Cd2+, and 10 μM BAPTA.
Time-Resolved Measurement of Exocytosis with PMA.
A 1-hr plaque assay is not suitable to determine the time course of PMA-induced secretion. Much better time resolution is obtained by using amperometry to measure the release of monoamines previously loaded into the pituitary cells. After cells had been exposed to dopamine or serotonin, an amperometric probe placed within a few micrometers of the cell reported occasional brief spikes of released oxidizable material. They appeared very similar to quantal amperometric spikes recorded with sympathetic neurons (26). Most events had a half-width of 2–19 ms and corresponded to oxidation of 2,500 to 70,000 oxidizable molecules. The amperometric spikes were not seen if the probe was moved farther away from the cell. We presume that these events represent quantal release of monoamine from fusion of single secretory vesicles with the plasma membrane. Others have obtained amperometric signals from anterior pituitary cells that had not been exposed to catecholamines (30). In that case unknown oxidizable material appeared for many seconds in the perfusate flowing past populations of cells growing on beads while they were stimulated with GnRH. In our experiments, we saw no spikes if the cell was not previously loaded with monoamine, so presumably the unknown endogenous oxidizable substances are not concentrated enough to be seen at the single-cell level we have used.
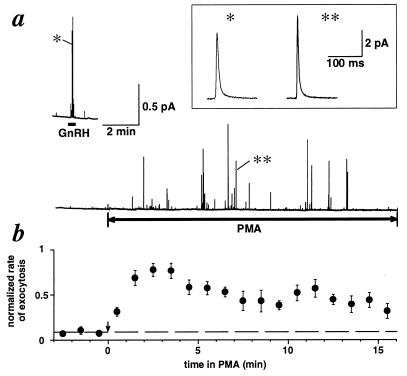
The rate of appearance of amperometric spikes was accelerated by addition of 1–50 nM GnRH (Fig. 5a, Upper). After GnRH was removed, some cells showed a slightly higher level of exocytosis for several minutes. PMA then was applied after exocytosis returned to the basal level. In all 10 GnRH-responsive cells tested, PMA also accelerated the rate of exocytosis (Fig. 5a, Lower). The effect of PMA developed with a short delay, rose to a peak within 2–4 min, and then slowly declined (Fig. 5b). The stimulation was not as brisk as with GnRH, and whenever tested, it persisted for many minutes even after the PMA was removed.
Figure 5.
PMA slowly activates exocytosis. (a) Amperometric signals from an identified gonadotrope. (Upper) Rapid response to application of 10 nM GnRH for the duration of the bar. (Lower) Slower response to 100 nM PMA. Effective plotting bandwidth, 20 Hz. (Inset) The events indicated by ∗ were filtered at 500 Hz and plotted on an expanded time scale. (b) The number of spikes per minute normalized to the maximal number of spikes per minute obtained in each experiment. Average of nine gonadotropes.
DISCUSSION
Secretion Evoked by PKC.
Our observations confirm a long series of earlier reports (2, 17–19, 31) that phorbol esters can stimulate LH release from pituitary gonadotropes by a pathway that involves PKC. In our preparation from male rats, the effect is large and involves every gonadotrope. The stimulation by PMA uses a mechanism that is clearly different from that activated by GnRH. With GnRH, [Ca2+]i oscillates between resting and micromolar levels, providing the final trigger for rapid exocytosis (6, 7). The [Ca2+]i rise is necessary and sufficient, the number of cells responding to GnRH is only partly reduced when PKC is inhibited with BIS or when Ca2+ entry is blocked by extracellular Cd2+, but it is fully depressed by intracellular BAPTA to below the levels seen without any secretagogue. On the other hand, with PMA there is no detectable [Ca2+]i rise, no effect of Cd2+, only a 50% depression with BAPTA, and a full supression with 500 nM BIS. The time courses of GnRH- and PMA-induced secretion also differ. GnRH evokes secretion within tens of seconds, and the response disappears usually within minutes upon removal of the agonist, whereas PMA action has a longer delay and persists for up to 15 min (the duration of our experimental observations) after removal of PMA. Both the delay and persistence of the PMA response observed here at room temperature are in good agreement with previous work by Stojilkovic et al. (32) if one takes into account that their experiments were done at 37°C.
Most previous work with PMA measured how much LH is secreted from populations of pituitary cells rather than how many gonadotropes are stimulated to secrete. Twenty years ago, before the enzyme PKC was known, Vale’s laboratory found an increase in secretion of several pituitary hormones with PMA (17, 31); these included LH, growth hormone, and adrenocorticotropic hormone. Subsequent work showed that the increase could be induced by various phorbol esters (18) and by diacylglycerols; it was blocked by previous down-regulation of PKC with PMA (33) and by kinase inhibitors like staurosporine or retinal (34, 35). The Catt laboratory (19, 32) reported that secretion begins within a fraction of a minute of PMA application. They also reported (30) that in single female rat gonadotropes PMA could induce small transient elevations of [Ca2+]i that were an order of magnitude smaller than those with GnRH. These appeared to be the consequence of a few spontaneous action potentials and could be abolished by removing extracellular Ca2+ or by adding dihydropyridine Ca2+ channel antagonists. Using sheep pituitary cells permeabilized with Staphylococcus aureus α-toxin, Millar’s laboratory showed that PMA stimulated LH release even when applied in a medium strongly buffered to 100 nM free Ca2+ with 30 mM of the Ca2+ buffer EGTA (36). Our results are in basic agreement with all of these findings.
As a working hypothesis, we propose that short-term secretion stimulated by GnRH depends only on a Ca2+ signal and not on activation of PKC and conversely that secretion stimulated by PMA depends only on PKC and not on a Ca2+ signal. Some of our findings might seem in conflict with this hypothesis and need further explanation: Thus, Cd2+ blocked GnRH action by only 30% (Fig. 4a); the PKC inhibitor BIS at 500 nM blocked the action of GnRH by 67% (Fig. 2a); and BAPTA depressed the action of PMA somewhat (Fig. 4b). The small size of the block of GnRH action by Cd2+ is expected because the Ca2+ for the initial [Ca2+]i oscillation with GnRH comes entirely from intracellular stores (37) and only later does Ca2+ entry from the outside begin to contribute (5). The partial inhibition of GnRH action by 500 nM BIS indicates that during 1-hr GnRH incubation, activity of PKC does boost the number of LH-secreting cells above that seen with the Ca2+ signal alone, perhaps both by stimulating secretion directly and by some action on the replenishment of the pool of releasable vesicles. We propose that the PKC-dependent component of GnRH action becomes evident only during prolonged incubations, whereas short-term secretion relies solely on Ca2+ (6, 7). In the gonadotrope literature, there are also observations that long incubations with the protein-kinase inhibitor staurosporine reduce GnRH-induced secretion as much as they reduce PMA-induced secretion (34). Such results have been taken as evidence that PKC provides the immediate signal for LH secretion stimulated by GnRH, but they may rather mean that during continuous secretion some of the many cellular kinases blocked by staurosporine are necessary to maintain the secretory machinery and the supply of LH-containing vesicles. The partial depression of secretion with PMA when BAPTA is loaded in the cytoplasm might be because of a lowering of [Ca2+]i below its usual resting level, which could be lower than optimal for the activity of PKC and of other molecules governing the supply, docking, and release of secretory granules.
Weak Actions of cAMP.
With our rat preparation, we did not find a stimulation of LH release by cAMP analogues or by agents that should raise cellular cAMP. The Millar laboratory did find some stimulation of LH secretion when 100 μM cAMP was added to permeabilized sheep pituitary cells, but the effect was considerably less than obtained with elevated Ca2+ (20). If cAMP can stimulate LH release in rat, the effect is much weaker than that of PKC.
PKC in the Action of GnRH.
Because GnRH receptor activation stimulates PKC, would this not contribute to normal secretion? The answer is likely to be complex. We found that in 500 nM BIS, 33% of the gonadotropes still formed plaques. Hence PKC is not essential for GnRH stimulation during our 1-hr assay, but its activity does facilitate secretion. Because the assay does not report the amount of LH secreted per cell, it is possible that BIS depressed secretion even in the responding cells. Miller’s laboratory reported that PMA alone or combined with GnRH can augment LH secretion well above that with GnRH alone in sheep pituitary cells (18). This would imply both that nanomolar GnRH does not give maximal stimulation of PKC and that the PKC-dependent component of secretion when GnRH is added alone must be much lower than that achieved here and in previous work when PMA is added alone. Other experiments from our laboratory also suggest that physiological concentrations of GnRH do not activate PKC as fully as PMA does. We have reported that adding PMA to gonadotropes while they are responding to GnRH greatly augments the current in an apamin-sensitive potassium channel and also decreases the frequency but not the amplitude of the ongoing [Ca2+]i oscillations (38). Both actions are blocked by staurosporine and mimicked by phorbol dibutyrate but not by 4α-PDD. Again these experiments show that PMA can activate PKC much more strongly than GnRH alone. Perhaps PMA is able to recruit additional PKC subtypes that are not well coupled to GnRH receptors.
In conclusion, physiological concentrations of GnRH may activate PKC too weakly to have strong acute effects on secretion in short-term assays, and Ca2+ therefore is the dominant intracellular signal for rapid exocytosis. On the other hand, these arguments do not detract from clear observations that GnRH does initiate PKC-dependent actions in gonadotropes. Thus GnRH causes translocation of several PKC isoforms to the membrane and, through a mechanism that is sensitive to BIS and mimicked by PMA, raises messenger-RNA levels for LH and follicle stimulating hormone α- and β-chains and for PKC-β within minutes (39–41).
Alternate Controlling Signals for Regulated Exocytosis.
This kind of work raises interesting questions for the cell biology of exocytosis. How widespread is the ability of PKC to induce exocytosis? Are there physiological stimuli that normally use this mechanism in preference to the better-studied Ca2+-signaling pathway? Are there yet other second-messenger systems that trigger exocytosis? Do these signals act on different subpopulations of secretory vesicles? What is the mechanism of stimulation?
Such questions have been considered for some time but most remain only poorly answered, partially because tools are lacking to control and assay kinase activities and other second messengers continuously with the high temporal and spatial resolution that can be achieved with [Ca2+]i measurements. In addition to pituitary cells, systems studied include several epithelia and macrophages where exocytotic insertion of transporters and the secretion of mucus and lysosomal enzymes can be initiated by activating PKC or cAMP-dependent protein kinase (10–15), platelets where secretion of serotonin is stimulated by diacylglycerols and phorbol esters (16), and mast cells where degranulation can be initiated by intracellular GTP analogues (42, 43). Only for a few cases has it been determined that the PKC or cAMP-dependent protein kinase activation do not produce a secondary elevation of [Ca2+]i that suffices to explain the exocytosis (16, 43). Sometimes activation of PKC or cAMP-dependent protein kinase seems to be the physiological signal. For example, the natural stimuli for exocytotic insertion of water channels in epithelial cell membranes are agonists that elevate cAMP (13, 14) or activate PKC (44, 45).
If we accept that exocytosis can be initiated by signals other than a [Ca2+]i rise, it becomes important to consider the underlying mechanisms. Here one should distinguish different time scales. If a secretory response develops in 1 s, the sites of action are likely at the final stages of priming and release of secretory vesicles in the readily releasable pool. If it takes a few minutes, it also may involve actions on the vesicle cycle at the level of recruiting stored pools of vesicles or even endocytosis. If it takes more than 15 min, it may include effects of gene expression and protein synthesis, sorting, and turnover. Although our experiments do not demonstrate an action of PMA within 1 s, it still seems likely that PKC does act at a final stage of secretion because it can bypass the Ca2+ signal. Current dogma suggests that regulated exocytosis is arrested at a step after vesicles dock and prime to form a complex that includes the plasma membrane proteins syntaxin and SNAP-25, the vesicle membrane proteins synaptobrevin and synaptotagmin, and numerous cytoplasmic proteins (46). Most molecules of this complex are capable of being phosphorylated (47–49). In synapses, this complex apparently is poised to initiate membrane fusion within 100 μs of binding of Ca2+ to one or several components. We suggest as working hypotheses that such Ca2+-sensitive complexes can give a similar response to other allosteric ligands and to covalent modification (hypothesis of signal convergence) and that different regulated secretory pathways in the cell may use different homologues of, for example, synaptotagmin to favor differential control of secretion by different intracellular signals (hypothesis of regulatory specialization). This would allow secretion from a single population of vesicles to be initiated by alternate stimuli, and it would allow secretion from different pools of vesicles to be controlled differentially by different stimuli.
Acknowledgments
We thank Drs. W. Almers, A. Tse, and F. W. Tse for comments on the manuscript, Dr. M. B. Hille for help with the assay of protein synthesis rates, and L. Miller and D. Anderson for valuable technical help. This work was supported by National Institutes of Health Grants P50HD 12629 and 5T32GM 07270 and by a grant from the W. M. Keck Foundation.
ABBREVIATIONS
- PKC
protein kinase C
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- PMA
phorbol-12-myristate-13-acetate
- 4α-PDD
4α-phorbol-12,13-didecanoate
- BIS
bisindolylmaleimide I
- BAPTA
1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- IBMX
3-isobutyl-1-methylxanthine
- RHPA
reverse hemolytic plaque assay
- [Ca2+]i
intracellular free Ca2+ concentration
- CPTcAMP
8-(4-chlorophenylthio)-cAMP
References
- 1.Snyder G, Bleasdale J E. Mol Cell Endocrinol. 1982;28:55–63. doi: 10.1016/0303-7207(82)90040-5. [DOI] [PubMed] [Google Scholar]
- 2.Andrews W V, Conn P M. Endocrinology. 1986;118:1148–1158. doi: 10.1210/endo-118-3-1148. [DOI] [PubMed] [Google Scholar]
- 3.Naor Z. Endocr Rev. 1990;11:326–353. doi: 10.1210/edrv-11-2-326. [DOI] [PubMed] [Google Scholar]
- 4.Hille B, Tse A, Tse F W, Bosma M M. Recent Prog Horm Res. 1995;50:75–95. doi: 10.1016/b978-0-12-571150-0.50008-1. [DOI] [PubMed] [Google Scholar]
- 5.Stojilkovic S S, Tomic M. Trends Endocrinol Metab. 1996;7:379–384. doi: 10.1016/s1043-2760(96)00189-0. [DOI] [PubMed] [Google Scholar]
- 6.Tse A, Tse F W, Almers W, Hille B. Science. 1993;260:82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- 7.Tse F W, Tse A, Hille B, Horstmann H, Almers W. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- 8.Hong D H, Forstner J F, Forstner G G. Am J Physiol. 1997;272:G31–G37. doi: 10.1152/ajpgi.1997.272.1.G31. [DOI] [PubMed] [Google Scholar]
- 9.Bruck R, Nathanson M H, Roelfsen H, Boyer J L. Hepatology. 1994;20:1032–1040. doi: 10.1002/hep.1840200436. [DOI] [PubMed] [Google Scholar]
- 10.Kai H, Yoshitake K, Isohama Y, Hamamura I, Takahama K, Miyata T. Am J Physiol. 1994;267:L526–L530. doi: 10.1152/ajplung.1994.267.5.L526. [DOI] [PubMed] [Google Scholar]
- 11.Dray-Charier N, Paul A, Combettes L, Bouin M, Mergey M, Balladur P, Capeau J, Housset C. Gastroenterology. 1997;112:978–990. doi: 10.1053/gast.1997.v112.pm9041261. [DOI] [PubMed] [Google Scholar]
- 12.Wright E M, Hirsch J R, Loo D D, Zampighi G A. J Exp Biol. 1997;200:287–293. doi: 10.1242/jeb.200.2.287. [DOI] [PubMed] [Google Scholar]
- 13.Katsura T, Ausiello D A, Brown D A. J Physiol. 1996;270:F548–F553. doi: 10.1152/ajprenal.1996.270.3.F548. [DOI] [PubMed] [Google Scholar]
- 14.Katsura T, Verbavatz J M, Farinas J, Ma T, Ausiello D A, Verkman A S, Brown D. Proc Natl Acad Sci USA. 1995;92:7212–7216. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapper H, Sundler R. J Leukocyte Biol. 1995;58:485–494. doi: 10.1002/jlb.58.4.485. [DOI] [PubMed] [Google Scholar]
- 16.Rink T J, Sanchez A, Hallam T J. Nature (London) 1983;305:317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- 17.Vale W, Rivier C, Rivier J, Brown M. In: Medicinal Chemistry V. Mathieu J, editor. Amsterdam: Elsevier; 1977. pp. 25–62. [Google Scholar]
- 18.Beggs M J, Miller W L. Endocrinology. 1989;124:667–674. doi: 10.1210/endo-124-2-667. [DOI] [PubMed] [Google Scholar]
- 19.Izumi S, Stojilkovic S S, Iida T, Krsmanovic L Z, Omeljaniuk R J, Catt K J. Biochem Biophys Res Commun. 1990;170:359–367. doi: 10.1016/0006-291x(90)91282-w. [DOI] [PubMed] [Google Scholar]
- 20.Macrae B M, Davidson J S, Millar R P, van der Merwe P A. Biochem J. 1990;271:635–639. doi: 10.1042/bj2710635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas P, Suprenant A, Almers W. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- 22.Tse A, Hille B. Endocrinology. 1993;132:1475–1481. doi: 10.1210/endo.132.4.8384989. [DOI] [PubMed] [Google Scholar]
- 23.Tse A, Hille B. Methods Neurosci. 1994;20:85–98. [Google Scholar]
- 24.Freeman M E, Reichert L E, Jr, Neill J D. Endocrinology. 1972;90:232–238. doi: 10.1210/endo-90-1-232. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z, Misler S. J Biol Chem. 1996;217:270–277. doi: 10.1074/jbc.271.1.270. [DOI] [PubMed] [Google Scholar]
- 26.Koh D S, Hille B. Proc Natl Acad Sci USA. 1997;94:1506–1511. doi: 10.1073/pnas.94.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrington J, Hille B. Endocrinology. 1994;135:1100–1108. doi: 10.1210/endo.135.3.8070352. [DOI] [PubMed] [Google Scholar]
- 28.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Lorielle F, Duhamel L, Charon D, Kirilovsky J. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 29.Starzec A, Jutisz M, Counis R. Mol Endocrinol. 1989;3:618–624. doi: 10.1210/mend-3-4-618. [DOI] [PubMed] [Google Scholar]
- 30.Cantor H C, Padmanabhan V, Favreau P A, Midgley A R., Jr Endocrinology. 1996;137:2782–2790. doi: 10.1210/endo.137.7.8770898. [DOI] [PubMed] [Google Scholar]
- 31.Smith M A, Vale W. Endocrinology. 1981;108:752–759. doi: 10.1210/endo-108-3-752. [DOI] [PubMed] [Google Scholar]
- 32.Stojilkovic S S, Iida T, Merelli F, Torsello A, Krsmanovic L Z, Catt K J. J Biol Chem. 1991;266:10377–10384. [PubMed] [Google Scholar]
- 33.Stojilkovic S S, Chang J P, Ngo D, Catt K J. J Biol Chem. 1988;263:17307–17311. [PubMed] [Google Scholar]
- 34.Dan-Cohen H, Naor Z. Mol Cell Endocrinol. 1990;69:135–144. doi: 10.1016/0303-7207(90)90007-u. [DOI] [PubMed] [Google Scholar]
- 35.Chang J P, Graeter J, Catt K J. Biochem Biophys Res Commun. 1986;134:134–139. doi: 10.1016/0006-291x(86)90537-1. [DOI] [PubMed] [Google Scholar]
- 36.van der Merwe P A, Millar R P, Wakefield I K, Davidson J S. Biochem J. 1989;264:901–908. doi: 10.1042/bj2640901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tse A, Hille B. Science. 1992;255:462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- 38.Tse A, Tse F W, Hille B. Pflügers Arch. 1995;430:645–652. doi: 10.1007/BF00386158. [DOI] [PubMed] [Google Scholar]
- 39.Hirota K, Hirota T, Aguilera G, Catt K J. J Biol Chem. 1985;260:3243–3246. [PubMed] [Google Scholar]
- 40.Ben-Menahem D, Naor Z. Biochemistry. 1994;33:3698–3704. doi: 10.1021/bi00178a029. [DOI] [PubMed] [Google Scholar]
- 41.Shraga-Levine Z, Ben-Menahem D, Naor Z. J Biol Chem. 1994;269:31028–31033. [PubMed] [Google Scholar]
- 42.Lillie T H, Gomperts B D. CIBA Found Symp. 1993;176:164–179. doi: 10.1002/9780470514450.ch11. [DOI] [PubMed] [Google Scholar]
- 43.Neher E. J Physiol. 1988;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klinkspoor J H, Tytgat G N J, Lee S P, Groen A K. Biochem J. 1996;316:873–877. doi: 10.1042/bj3160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dray-Chrier N, Paul A, Combettes L, Bouin M, Mergey M, Balladur P, Capeau J, Housset C. Gastroenterology. 1997;112:978–990. doi: 10.1053/gast.1997.v112.pm9041261. [DOI] [PubMed] [Google Scholar]
- 46.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 47.Südhof T C, Rizo J. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 48.Oyler G A, Higgins G A, Hart R A, Battenberg E, Billingsley M, Bloom F E, Wilson M C. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiteheart S W, Griff I C, Brunner M, Clary D O, Mayer T, Buhrow S A, Rothman J E. Nature (London) 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]