Short abstract
A screen for genes affecting mitochondrial function in Drosophila suggests that many modulators of mitochondrial function act outside the organelle.
Abstract
Genomic and proteomic studies have identified hundreds of proteins from mitochondria. A recent study has added a functional twist to these systematic approaches and identified novel mitochondrial modifiers and regulators.
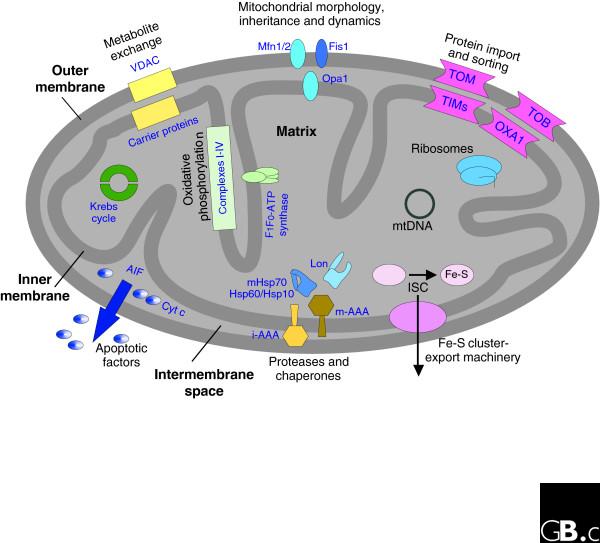
Mitochondria are ubiquitous and essential organelles in all eukaryotes and have many functions other than their main task of producing ATP. Besides the production of energy by cellular respiration, mitochondria host the tricarboxylic acid cycle (TCA cycle) and the β-oxidation of fatty acids. In addition, anabolic processes such as the biosynthesis of heme, amino acids and lipids take place inside the organelle. Mitochondria are also pivotal for the production of iron-sulfur clusters for the entire eukaryotic cell [1]. Altogether, these organelles are involved in a myriad of different processes, as summarized in Figure 1. The shape of the mitochondrial network is sustained by three basic processes - fusion, fission and active transport of the organelle along the cytoskeleton.
Figure 1.
Overview of mitochondria-related processes. A schematic cross-section of a mitochondrion is shown, with the proteins and protein complexes that are involved in various cellular pathways and processes. Complexes I-IV are the complexes of the respiratory chain. AIF, apoptosis-inducing factor; Cyt c, cytochrome c; i-AAA and m-AAA, proteases of the AAA family in the intermembrane space and matrix, respectively; ISC, iron-sulfur cluster assembly complex; Mfn, mitofusin; TIM, translocase of the inner membrane; TOB, topogenesis of mitochondrial outer membrane β-barrel proteins; TOM, translocase of the outer membrane; VDAC, voltage-dependent anion channel; Opa1, Optic Atrophy 1.
To carry out these functions, a mitochondrion is composed of hundreds of different proteins, most of which are encoded by genes in the nucleus. To better understand the complexity of mitochondrial functions we need to identify all the mitochondrial components and to unravel the cytoplasmic signaling pathways that regulate the organelle's activities. To these ends, various systematic approaches to mitochondrial protein identification have been undertaken in the past ten years. While most of these studies aimed at producing inventories of mitochondrial proteins, a recent study by Chen et al. [2] has specifically assayed the proteome of the fruit fly Drosophila melanogaster for proteins that are likely to be involved in the energy-generating functions of mitochondria and has identified several novel mitochondrial regulators.
The central role of mitochondria in both life and the death of the eukaryotic cell emerges not only from their biosynthetic functions but also from their crucial role in cellular signaling. In cooperation with the endoplasmic reticulum, mitochondria play an important role in the cellular homeostasis of Ca2+ [3]. They also have a major role in triggering apoptosis, as several different pro-apoptotic signals are integrated and interpreted at the mitochondrial level. In response to these apoptotic signals, mitochondrial factors - the most prominent being cytochrome c and apoptosis-inducing factor (AIF) - are released to activate downstream signaling proteins and the caspases that execute apoptosis [4]. In the course of these events, dramatic alterations in the mitochondrial network take place. These morphological changes are mediated by the same machineries that control mitochondrial morphology under normal conditions [5].
To fulfill all these functions, mitochondria have to maintain their unique protein composition, which is dynamic and adapts itself to the requirements of the organelle and the rest of the cell. The vast majority of mitochondrial proteins are encoded in the nucleus and synthesized as precursor proteins on cytosolic ribosomes. They are targeted to the mitochondria and become sorted to the correct sub-mitochondrial destination by an elaborate network of translocases and sorting machineries [6]. Reminiscent of their endosymbiotic descent, mitochondria host their own DNA genome, which encodes only a few components of the respiratory-chain complexes (for example, 8 in Saccharomyces cerevisiae and 13 in human). Many more proteins are involved in the intra-organelle transcription and translation of this limited number of genes. Newly synthesized precursor proteins acquire their native conformation with the help of a dedicated set of mitochondrial chaperones [7]. The organelle also harbors an independent quality-control system and misfolded proteins are degraded by mitochondrial proteases such as the AAA-proteases of the matrix and the intermembrane space and by the Lon-protease in the matrix [8].
Various systematic approaches for identifying mitochondrial components and associated signaling proteins have been developed. The proteomes of mitochondria from yeast [9-12], the filamentous fungus Neurospora crassa [13], mouse [14-16], human cells [17] and plants [18] have been systematically analyzed. Collectively, approximately 700 mitochondrial proteins have been identified in yeast and around 500 in rodent and human cells. Interestingly, plant and human mitochondria were found to harbor kinases and other regulatory proteins that are less abundant in yeast mitochondria. This observation is in accordance with the higher diversity of mitochondria in higher eukaryotes compared with the somewhat limited variability in the unicellular S. cerevisiae. In higher eukaryotes, each cell type may have distinct tasks for mitochondria, which are reflected by differing localizations and morphologies of the organelle in different cells, tissues and organs of the same organism. To address the diversity in the mitochondrial composition among different cell types, Mootha and coworkers [15] analyzed the mitochondrial proteomes from different mouse tissues. Surprisingly, the authors propose that about half of the 'complete' mitochondrial proteome is ubiquitously expressed, whereas the expression of the other half is tissue specific.
Screening for proteins with mitochondria-related function
Identifying all proteins localized in or on mitochondria is just one facet of the task. To understand mitochondrial behavior in the cellular context it is also necessary to identify proteins that 'communicate' with mitochondria. Which transcription factors are necessary for the expression of nuclear-encoded mitochondrial proteins? How is import of precursor proteins into mitochondria regulated? How do mitochondria communicate with other compartments of the cell? What are the regulatory mechanisms that shape mitochondria? These and other questions can be answered only by understanding the molecular mechanisms that integrate the organelle into the cell.
The first functional screens were carried out in S. cerevisiae, in which collections of mutant strains are readily available. As S. cerevisiae is a facultative anaerobe, most mitochondrial functions can be eliminated without loss of viability; on the other hand, when grown on nonfermentable carbon sources, yeast cells depend on functional respiring mitochondria. A phenotypic screen of deletion mutants for around 4,800 nonessential genes used this requirement to search for mitochondria-related proteins [19]. This screen identified 341 proteins that are crucial for respiration and/or for the regulatory circuits controlling it. Using the same collection of mutants, the authors also aimed to identify genes essential for mitochondrial fusion, fission and transport. Assaying each knockout strain for defects in the morphology and/or distribution of mitochondria led to the discovery of new genes involved in mitochondrial morphology and cellular pathways important for mitochondrial behavior [19]. This study provided the first hint that mitochondrial fusion is regulated by proteasome-related protein degradation. A similar approach using strains bearing inducible essential genes showed that vesicle transport, protein import, the proteasome machinery and ergosterol biosynthesis have an influence on mitochondrial shape and distribution [20].
With the advent of RNA interference (RNAi), systematic downregulation of genes in multicellular eukaryotes such as Caenorhabditis elegans and D. melanogaster became feasible. The recent work of Chen et al. [2] is the first report of a genome-wide screen for genes important for mitochondrial function in a multicellular eukaryote. The authors carried out RNAi using a collection of small interfering RNAs (siRNAs) against the whole genome of D. melanogaster, with an assay of citrate synthase activity as the readout for mitochondrial function. Citrate synthase is located in the mitochondrial matrix and catalyzes the conversion of oxaloacetate and acetyl-CoA to citrate, the first reaction in the TCA cycle. Of the 13,071 genes screened, 152 showed modulation of citrate synthase activity. Obviously, as the activity of only a single enzyme was monitored, this study probably missed many modifiers affecting other mitochondrial activities.
The genes identified [2] are involved in a wide range of pathways and processes. The two largest groups comprise those with functions in transcriptional regulation (22 proteins) and in signaling pathways (17 proteins). Additional biological processes and molecular functions represented were translational regulation, ribosomal proteins, cell-cycle regulation, protein stability and proteolysis, lipid metabolism and defense response. Interestingly, only 17 of the hits were proteins with a clear mitochondrial function, suggesting that most of the modulators of basal mitochondrial function are actually located outside the organelle. The proteins identified in this screen can now serve as a foundation for more intensive work to detect and characterize novel processes and pathways that regulate mitochondrial biogenesis and functions.
In another recent RNAi screen, this time for genes affecting mitochondrial morphology, Ichishita et al. [21] used RNAi to downregulate C. elegans genes encoding mitochondrial proteins. Downregulation of about 80% of the genes identified influenced mitochondrial morphology, indicating that proper mitochondrial function is necessary for the establishment of the mitochondrial network [21].
Mitochondria in aging and disease
In the past couple of years the central role of mitochondria in cellular aging has become apparent. It is well established that mitochondrial DNA (mtDNA) accumulates mutations with aging, but a causal link between such mutations and the early onset of aging was shown only recently. An impressive example of this is the 'mutator mouse', in which an error-prone mitochondrial DNA polymerase leads to early onset of aging, and which shows a clear correlation between aging and increased mutation rate in the mitochondrial genome [22].
Defects in mitochondria in human cells often have devastating consequences for the cell and consequently for the whole human body. Many mitochondrial diseases, such as mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS, a cause of dementia), myoclonic epilepsy associated with ragged-red fibers (MERRF) and Leber's hereditary optic neuropathy (LHON), are caused by mutations in mtDNA. These mutations lead in most cases to dysfunction of the complexes involved in oxidative phosphorylation and thus to inefficient cellular respiration, with a resulting wide range of heterogeneous symptoms [23]. Mutations in genes encoding components of mitochondrial import systems (Mohr-Tranebjaerg syndrome) and mitochondrial morphology (dominant optic atrophy, Charcot-Marie-Tooth neuropathy type 2A, Wolf-Hirschhorn syndrome) also lead to pathological disorders [24,25]. Furthermore, mitochondria have recently been found to interact with many of the proteins specifically implicated in genetic forms of neurodegenerative diseases such as Parkinson's disease or Alzheimer's disease [26].
The accumulation of data from different approaches and various organisms is helping us to get closer to a complete inventory of the mitochondrial proteome. However, despite this recent progress, our understanding of how mitochondria adapt their set of proteins to the requirements of the rest of the eukaryotic cell and how such cross-talk is regulated is only partial, and many loose ends have to be connected. Information on the precise suborganellar location of each mitochondrial protein and its individual function is still missing. This knowledge, together with analysis of the regulatory circuits inside and outside mitochondria, will be necessary to understand how this essential organelle plays its pivotal role in the life and death of eukaryotic cells.
Acknowledgments
Acknowledgements
We thank J Herrmann and L Scorrano for helpful comments. Our work is supported by grants from the Deutsche Forschungsgemeinschaft (SFB 446-A30 and RA 1028/2-1).
References
- Scheffler IE. A century of mitochondrial research: achievements and perspectives. Mitochondrion. 2001;1:3–31. doi: 10.1016/S1567-7249(00)00002-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Shi X, Padmanabhan R, Wang Q, Wu Z, Stevenson SC, Hild M, Garza D, Li H. Identification of novel modulators of mitochondrial function by a genome-wide RNAi screen in Drosophila melanogaster. Genome Res. 2008;18:123–136. doi: 10.1101/gr.6940108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli A, Aguiari P, De Stefani D, Leo S, Marchi S, Rimessi A, Zecchini E, Pinton P, Rizzuto R. Endoplasmic reticulum/mitochondria calcium cross-talk. Novartis Found Symp. 2007;287:122–139. [PubMed] [Google Scholar]
- Keeble JA, Gilmore AP. Apoptosis commitment - translating survival signals into decisions on mitochondria. Cell Res. 2007;17:976–984. doi: 10.1038/cr.2007.101. [DOI] [PubMed] [Google Scholar]
- Dimmer KS, Scorrano L. (De)constructing mitochondria: what for? Physiology. 2006;21:233–241. doi: 10.1152/physiol.00010.2006. [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- Martinus RD, Ryan MT, Naylor DJ, Herd SM, Hoogenraad NJ, Hoj PB. Role of chaperones in the biogenesis and maintenance of the mitochondrion. FASEB J. 1995;9:371–378. doi: 10.1096/fasebj.9.5.7896006. [DOI] [PubMed] [Google Scholar]
- Koppen M, Langer T. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol. 2007;42:221–242. doi: 10.1080/10409230701380452. [DOI] [PubMed] [Google Scholar]
- Prokisch H, Scharfe C, Camp DG, 2nd, Xiao W, David L, Andreoli C, Monroe ME, Moore RJ, Gritsenko MA, Kozany C, Hixson KK, Mottaz HM, Zischka H, Ueffing M, Herman ZS, Davis RW, Meitinger T, Oefner PJ, Smith RD, Steinmetz LM. Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2004;2:795–804. doi: 10.1371/journal.pbio.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schonfisch B, Guiard B, Pfanner N, Meisinger C. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol Biol Cell. 2006;17:1436–1450. doi: 10.1091/mbc.E05-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Kumar A, Agarwal S, Heyman JA, Matson S, Heidtman M, Piccirillo S, Umansky L, Drawid A, Jansen R, Liu Y, Cheung KH, Miller P, Gerstein M, Roeder GS, Snyder M. Subcellular localization of the yeast proteome. Genes Dev. 2002;16:707–719. doi: 10.1101/gad.970902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Prokisch H, Schlunck T, Camp DG, 2nd, Ahting U, Waizenegger T, Scharfe C, Meitinger T, Imhof A, Neupert W, Oefner PJ, Rapaport D. Proteome analysis of mitochondrial outer membrane from Neurospora crassa. Proteomics. 2006;6:72–80. doi: 10.1002/pmic.200402084. [DOI] [PubMed] [Google Scholar]
- Da Cruz S, Xenarios I, Langridge J, Vilbois F, Parone PA, Martinou JC. Proteomic analysis of the mouse liver mitochondrial inner membrane. J Biol Chem. 2003;278:41566–41571. doi: 10.1074/jbc.M304940200. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/S0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Sako Y, Sato M, Kitamura T, Umezawa Y. A genetic approach to identifying mitochondrial proteins. Nat Biotechnol. 2003;21:287–293. doi: 10.1038/nbt791. [DOI] [PubMed] [Google Scholar]
- Gaucher SP, Taylor SW, Fahy E, Zhang B, Warnock DE, Ghosh SS, Gibson BW. Expanded coverage of the human heart mitochondrial proteome using multidimensional liquid chromatography coupled with tandem mass spectrometry. J Proteome Res. 2004;3:495–505. doi: 10.1021/pr034102a. [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Tonti-Filippini JS, Gout AM, Day DA, Whelan J, Millar AH. Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell. 2004;16:241–256. doi: 10.1105/tpc.016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W, Westermann B. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:847–853. doi: 10.1091/mbc.01-12-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann K, Westermann B. Role of essential genes in mitochondrial morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell. 2005;16:5410–5417. doi: 10.1091/mbc.E05-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichishita R, Tanaka K, Sugiura Y, Sayano T, Mihara K, Oka T. An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in C. elegans. J Biochem. 2008 doi: 10.1093/jb/mvm245. doi:10.1093/jb/mvm245. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest. 2003;111:303–312. doi: 10.1172/JCI200317741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]