Abstract
The insulin-like growth factor (IGF) system is comprised of receptors, ligands (IGF-I and IGF-II), and a family of binding proteins (IGFBPs). It plays an important role in growth and development and in the maintenance of normal homeostasis. We present a review of the current laboratory and epidemiologic evidence that suggests an important role of the IGF system in colorectal carcinogenesis. Due to the complexity of this system, we have focused the review on the role of the IGF-1 receptor and its ligands in colorectal carcinogenesis and the strategies to block this pathway as a potential anti-cancer therapy.
Keywords: Anti-IGF therapy, growth factors, acromegaly, epidemiologic studies in cancer
1.0. INTRODUCTION
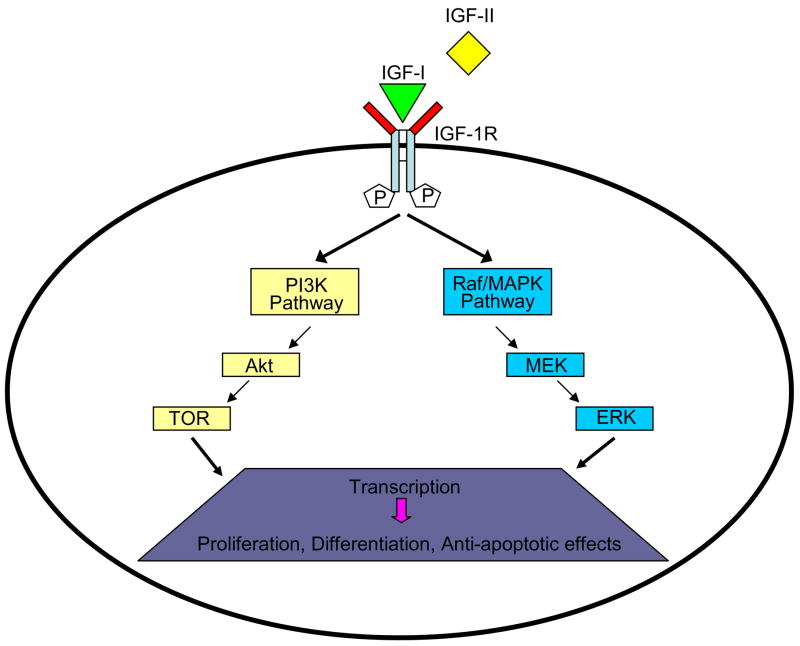
The insulin-like growth factor (IGF) system has been shown to play a critical role in growth and development and in the maintenance of normal homeostasis. It is comprised of receptors, ligands (IGF-I and IGF-II) and a family of binding proteins (IGFBPs) (Figure 1) [1, 2]. IGF-I and IGF-II are single chain polypeptides that share 62% homology with proinsulin. Although liver is the major site of production of IGF-I, which is regulated by growth hormone (GH) levels, it is produced by a number of tissues and exerts paracrine and autocrine effects on cells. Both IGF-I and IGF-II exert their biologic effects through activation of insulin-like growth factor type 1 receptor (IGF-1R). Binding of ligands causes activation of downstream cascades resulting in proliferative, differentiative and anti-apoptotic effects.
Figure 1.
Insulin-like growth factor I receptor (IGF-1R): IGF-1R is a tyrosine kinase cell-surface receptor containing two α and two β subunits joined by disulfide bridges forming a heterotetrameric receptor complex. When ligand binds to IGF-1R, a conformational change occurs, leading to trans-autophosphorylation of the cytoplasmic tyrosine kinase domain, resulting in activation of the PI3K and Raf/MAPK pathways. [PI3K, phosphatidylinositol 3-kinase; TOR, target of rapamycin; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; Raf/MAPK, RAF-mitogen-activated protein kinase]
We present a review of the current evidence, from laboratory and epidemiologic studies, that suggests an important role of the IGF system in colorectal carcinogenesis. Due to the complexity of the effects and interactions of this system, the focus of this review is on the role of IGF-1R and its ligands, IGF-I and -II, in colorectal carcinogenesis and strategies to block this pathway as potential anti-cancer therapy. Data for this review was identified by searches of MEDLINE and PubMed and references from relevant articles using the search terms “insulin-like growth factor”, “cancer”, and “colorectal cancer”. Abstracts and reports from meetings are included. Only papers published in English between 1980 and 2006 are included.
2.0 IGF SYSTEM AND COLORECTAL CANCER: EMERGING EVIDENCE
Increased levels of IGF ligands and/or over-expression of IGF receptor have been observed in many cancers and have been shown to affect proliferation, differentiation, migration and apoptosis of cancer cells [1,3].
2.1 LABORATORY STUDIES
In this section, we discuss data from experimental models of CRC and from analysis of human specimens exploring the role and differential effects of the IGF system in colorectal tumorigenesis.
Jehle et al [4] studied the release, binding, and growth-promoting activity of insulin, IGF-I, and IGF-II in rapidly growing IEC-6 crypt cells as compared with differentiating enterocytes (CaCo-2 cells) to evaluate the effect of the IGF system in intestinal epithelium proliferation and differentiation. During IEC-6 growth, the autocrine release of IGF-I and IGF-II increased, and specific receptors for IGF-I and –II were detectable. During proliferation of CaCo-2 cells, there was no evidence of IGF-I secretion, while basal levels of IGF-II secretion were higher than in IEC-6 cells. At the switch from cell proliferation to differentiation, a marked increase in the secretion of IGF-II was observed [4]. These data indicate the differential modulation of enterocytic cell proliferation and differentiation by the IGF system.
To investigate the role of serum IGF-I levels in the regulation of colon cancer growth and metastasis, Wu et al implanted Colon 38 adenocarcinoma tissue fragments orthotopically to the surface of the cecum of 74 control and 82 liver-specific IGF-I deficient mice [5]. Serum levels of IGF-I in liver-specific IGF-I deficient mice are 25% of that in controls, without a change in the local tissue production of IGF-I. Both groups were randomized to receive either recombinant human IGF-I (rhIGF-I) injection twice a day for 6 weeks or saline injections. In the saline-treated group, the incidence of tumor growth in the cecum and the frequency and number of hepatic metastases were significantly higher in controls compared to liver-specific IGF-I deficient mice. Administration of rhIGF-I significantly increased the frequency of cecal tumor growth, weight of tumor, and frequency and number of hepatic metastases but did not significantly alter IGF-1R mRNA expression in tumors compared to saline treatments [5]. Regulation of VEGF expression by IGF-I has been demonstrated in CRC experimental model systems. Treatment of HT-29 human colon cancer cells with IGF-I resulted in an increase in VEGF mRNA and protein expression within 2 hours, with peak at 24 hours [6]. This could have important implications as angiogenesis is a critical event in colorectal carcinogenesis with therapeutic possibilities. IGF-I may also contribute to higher invasive and metastatic potential of colon cancer cells due to its effects on cell motility and migration. Upon IGF-I stimulation of HT-29 cells, integrins have been shown to reorganize at the leading edge of migrating cells and induction of cell migration through modulation of the E-cadherin/catenins complex function has been demonstrated [7].
IGF-I, IGF-II and insulin have been shown to exert a strong protective effect against tumor necrosis factor-alpha (TNF)-induced apoptosis in interferon-gamma (IFN)-sensitized HT29-D4 human colon carcinoma cells [8]. Inhibition of either NF-κB or mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) or MAPK/p38 partially reversed this protective effect. Combined inhibition of NF-κB and MAPK/ERK or MAPK/p38 resulted in complete reversal of this protective effect [8].
COX-2 and prostaglandins (PGs) have been demonstrated to play a role in colon polyp formation, and COX-2 inhibitors like celecoxib inhibit polyp formation in patients with adenomatous polyposis coli. COX-2 mRNA and PGE2 levels are higher in intestinal cells that constitutively overexpress IGF-II [9]. Up-regulation of COX-2 expression by IGF-II has been shown to be mediated through activation of IGF-1R as: (i) treatment of Caco-2 cells with a blocking antibody to IGF-1R inhibits COX-2 mRNA expression; (ii) transfection of Caco-2 cells with a dominant negative IGF-1R reduces COX-2 expression and activity. Also the blockade of PI3K, which mediates the proliferative effect of IGF-1R in Caco-2 cells, inhibits IGF-II-dependent COX-2 up-regulation and PGE2 synthesis. Moreover, COX-2 expression and activity inversely correlate with the increase of apoptosis in parental, IGF-II and dominant-negative IGF-1R transfected cells [9].
The role of the IGF pathway has also been evaluated in human blood and tissue samples with varying results. In a retrospective study, the expression of IGF-1R in 12 colonic adenomas, 36 primary CRCs, and 27 corresponding metastases was investigated [10]. Moderate to strong cytoplasmic immunostaining with anti-IGF-1R rabbit polyclonal antibody was observed in 96% of carcinoma cases and 93% of metastases. In 83% of adenomas, only faint cytoplasmic staining was identified. Normal mucosa, adjacent to the carcinomas (34 cases), was negative. Strong IGF-1R positivity correlated with higher grade and higher-stage tumors (p<0.01) [10].
Expression of IGF-1R was examined in 40 paired samples of CRC and adjacent normal mucosa in a study by Weber et al [11]. Tissue was obtained immediately after surgical resection of colon carcinoma, and IGF-1R expression was assessed using both reverse transcriptase polymerase chain reaction (RT-PCR) and immunohistochemical (IHC) staining. Mean IGF-1R mRNA level was found to be five-fold higher in tumor tissue compared to adjacent normal mucosa (p<0.0001). Of 33 paired samples analyzed by IHC, 91% of tumors stained positive for IGF-1R, while very faint or no staining was seen in adjacent normal mucosa. Relative overexpression of IGF-II mRNA in carcinomatous versus adjacent normal tissue was documented in 70% of cases by RT-PCR; mean IGF-II expression was 57 times higher as compared to adjacent normal tissue (p<0.05) [11].
The prognostic value of IGF-1R expression was evaluated in 161 patients with curatively resected Dukes’ C CRC, who had not received neoadjuvant or adjuvant therapy and had at least 5 years follow-up [12]. Membranous and cytoplasmic staining patterns were compared using IHC; only membrane staining of IGF-1R was shown to have prognostic significance. Diffuse membrane staining (high) was detected in 28% and focal staining (low) in 72% of specimens. Recurrence rate was significantly higher in the focal (low) staining group (42% vs 20%, p=0.01) (12). This would indicate an association between low IGF-1R membrane expression and increased risk of metastasis in Dukes’ C CRC [12].
The relationship between IGF-1R expression and aggressiveness of tumor remains unclear, as at least one retrospective study reported a correlation between strong cytoplasmic expression of IGF-1R and higher grade and stage of colorectal tumor [10].
The IGF-II gene has been shown to be over-expressed in CRC as compared with normal colonic epithelium. Over 300,000 transcripts derived from human colorectal epithelium, CRC, or pancreatic cancers were quantified by serial analysis of gene expression (SAGE); of these, 108 transcripts were expressed at higher levels in CRC as compared with normal colon tissue. The IGF-II gene was the single most over-expressed gene in CRC as compared with normal epithelium [13].
In an analysis of IGF-I, IGF-II, IGF-1R, COX-2, and MMP-7 expression in 90 human colorectal tumors (63 adenomas and 27 submucosal pT1 cancers), IGF-II was also found to be the most differentially expressed gene between carcinoma and adenoma lesions [14]. Semiquantitative RT-PCR was used to detect gene expression. Both frequency of IGF-II mRNA expression (70.4% vs 23.8%, p<0.0001) and immunohistochemical IGF-II expression (58.3% vs 25.3%, p<0.001) were significantly higher in pT1 cancers compared to adenomas. IGF-II mRNA expression was undetectable or only faintly detectable in adjacent nontumor tissue. In 6 of 7 patients with carcinomas arising in adenomas, IGF-II mRNA expression in the carcinoma was >10 times higher than in the adenoma portion of the lesion by complimentary DNA (cDNA) array analysis and IHC [14].
In normal cells the IGF-II gene is maternally imprinted so that it is expressed only from the paternal copy of the gene. When loss of imprinting (LOI) occurs, both alleles of the gene are expressed, resulting in over-expression of IGF-II (15). LOI of IGF-II has been reported to be associated with both personal (OR=21.7, 95% CI: 3.48–153.6) and family history (OR=5.15, 95% CI: 1.70–16.96) of CRC. Of 172 subjects evaluated, those with colorectal neoplasia (adenomas/cancer) had a 5.1-fold increase in risk of having LOI of IGF-II in peripheral blood lymphocytes compared to persons without colorectal neoplasia [15].
In summary, there is increasing experimental data on the IGF system playing a significant role in intestinal tumorigenesis. However, the precise role, its effect on other cellular systems and how this relates with other known genetic, dietary or environmental risk factors needs to be further elucidated.
2.2 EPIDEMIOLOGIC STUDIES
2.2.1 STUDIES IN PATIENTS WITH ACROMEGALY (TABLE 1)
Table 1.
Epidemiology Studies in Acromegalic Subjects
| Study Type | Comparison Group | Number of subjects | Findings | Reference |
|---|---|---|---|---|
| Prospective cohort, colonoscopy, St. Bartholomew’s Hospital, London, UK | Screening colonoscopies from 621 asymptomatic subjects (Rex et al, 1993) | 129 | Odds ratio for CRC increased at 13.5 (CI 3.1–75) | Jenkins et al [16] |
| Prospective cohort, colonoscopy, two centers, Manchester and Newcastle, UK | Prevalence in general population(based on 8 autopsy and 4 screening colonoscopy studies) | 122 | No significant increase in colorectal neoplasia
No association between IGF-I or IGF-II levels and presence of adenoma in acromegalic patients |
Renehan et al [17] |
| Nationwide registry-based cohort, Sweden and Denmark | General population of Sweden and Denmark | 1634 | Increased risk of colon cancer(SIR 2.6, CI 1.6–3.8) and rectal cancer (SIR 2.5, CI 1.3–4.2) | Barris et al [18] |
| Retrospective cohort, United Kingdom Acromegaly Group | General population of England and Wales | 1362 | Nonsignificant increase in incidence of colon cancer (SIR1.68, CI 0.87–2.93, p=0.06)
Significant increase in colon cancer mortality ratio (SMR2.47, CI 1.31–4.22, p=0.003) No significant difference in rectal cancer incidence or mortality |
Orme et al [19] |
SIR = standardized incidence ratio, SMR = standardized mortality ratio, CI = 95% confidence interval
Acromegaly is a disease characterized by excessive circulating levels of GH and its mediator, IGF-I. There are several colonoscopy studies that have reported conflicting results on the incidence of colorectal adenomas and cancer in patients with acromegaly compared to various control groups [16, 17]. Two large cohort studies compared the incidences of colon cancer and rectal cancer in acromegalic patients to that in the general population. One using a nationwide registry-based cohort of acromegalic patients reported an increased risk of both colon and rectal cancer [18], while another revealed a significant increase in colon cancer mortality ratio for acromegalic patients but only a nonsignificant increase in colon cancer incidence and no significant difference in rectal cancer incidence or mortality [19].
The epidemiologic data in patients with acromegaly is conflicting, and most of the studies are either small and lack the power to detect differences as compared with normal controls, are retrospective, or lack age-matched controls.
2.2.2 STUDIES IN HEALTHY INDIVIDUALS (TABLE 2)
Table 2.
Epidemiology Studies in Healthy Subjects
| Study Type | Number of subjects | Findings | Reference |
|---|---|---|---|
| Prospective cohort, colonoscopy | Negative screening colonoscopy - 34
Family hx CRC - 62 Hx of adenomatous polyps or polyps found this exam -78 |
No statistically significant difference in levels of IGF-I between the 3 groups | Paterson et al [20] |
| Nested case- control, Physicians’ Health Study, males only | 193 cases CRC
318 controls |
Increased risk of CRC for highest vs lowest quintile IGF-I, RR 2.1 (CI 1.15–5.46)
No correlation of IGF-II with risk |
Ma et al [21] |
| Nested case- control, NYU | 102 cases CRC
200 controls |
No increase in risk of CRC with higher IGF-I level | Kaaks et al [22] |
| Women’s Health Study, females only | Increased risk of CRC for highest vs lowest quintile IGF-II, OR 2.02 (CI 0.83–4.93) | Hunt et al [23] | |
| Nested case- control, Nurses’ Health Study, females only | 79 cases CRC
158 controls 90 intermediate or late-stage adenomas 107 early adenomas 207 controls |
High tertile of IGF-I with increased risk of intermediate/late-stage adenoma (RR 2.78, CI 0.76–9.76) and CRC (RR 2.18, CI0.94–5.08) | Giovannucci et al[24] |
| Nested case- control, Northern Sweden Health & Disease Cohort | 110 colon cancers
58 rectal cancers 336 controls |
Increased risk of colon cancer with increasing levels of IGF-I (lowest to highest quintile IGF-I OR 1.0, 1.89, 2.3, 2.66, ptrend=0.03) but decreased risk of rectal cancer (lowest to highest quintile IGF-I OR 1.0, 0.45, 0.33, 0.33, ptrend=0.09) | Palmqvist et al[25] |
| Nested case- control, Chinese males | 135 cases CRC
661 controls |
No association between IGF- I and risk of CRC
Positive association between IGF-II levels and risk of CRC (highest vs lowest quintile IGF-II OR 2.74, CI1.67–4.5, ptrend=0.0008) |
Probst-Hensch et al [26] |
| Nested case- control, Honolulu Heart Program, Japanese- American males | 177 colon cancers
105 rectal cancers 282 controls |
Mean IGF-I level higher in colon cancer cases vs controls but OR only 1.8 (CI0.8–4.3) for highest quartile IGF-I vs lowest
No significant difference in IGF-I levels for rectal cancer cases vs controls |
Nomura et al [27] |
| Prospective cohort, Melbourne Collaborative Cohort Study | 443 cases CRC | No association of IGF-I levels with risk of CRC- specific death | Haydon et al [28] |
| Meta-regression analysis of case- control studies | See text | See text | Renehan et al [29] |
CRC = colorectal cancer, OR = odds ratio, RR = relative risk, CI = 95% confidence interval
Epidemiologic studies have been done to investigate the relationship between the IGF system and CRC in healthy individuals [20–28]. While some have documented a correlation between higher levels of IGF-I or –II and increased risk of CRC, others have found no such association.
The association of circulating levels of IGF-I with cancer risk was assessed by a systematic review and meta-regression analysis of case-control studies, which included meta-analysis by cancer site [29]. Five studies were in CRC with a total of 677 patients and 1673 controls. In a meta-analysis of these 5 studies comparing the highest versus the lowest categories of IGF-I levels, there was a positive association between elevated levels of circulating IGF-I and CRC risk, with an odds ratio (OR) of 1.58 and a 95% confidence interval (CI) of 1.11–2.27. Conducting multivariate meta-regression analysis, this positive association remained but was not statistically significant (p=0.09). A dose-response analysis of 4 of the 5 studies, showed no significant dose-response relationship between IGF-I level and risk of CRC (OR 1.18, 95% CI 0.92–1.51, p=0.19) [29].
3.0 THERAPEUTIC STRATEGIES
Based on above evidence, the IGF pathway could be an important target for anti-cancer therapies. The strategies to inhibit IGF-1R signaling include IGF-1R monoclonal antibodies, IGF mimetic peptides that inhibit ligand/receptor interaction, tyrosine kinase inhibitors (TKIs), expression of dominant negative IGF-1R mutants, antisense strategies (antisense oligodeoxynucleotides, antisense RNA, IGF-1R specific small interfering RNAs (siRNAs)), IGF-1R specific peptide aptamers, and purified or synthetic IGF binding proteins [1, 30]. Inhibition of this pathway has been evaluated in pre-clinical models as a single therapeutic modality, in combination with chemotherapy and as a potential radiosensitizer. The potential side effects of blocking this pathway could include effects on glucose tolerance by agents that cross-react with the insulin receptor. Table 3 includes agents currently in preclinical or clinical development, some of which are discussed below.
Table 3.
Agents Targeting the IGF Pathway
| Agent | Development stage | Comments |
|---|---|---|
| Anti-IGF-1R Antibodies | ||
| A12 (IMC-A12) | Phase I | Ref. 31
Fully human monoclonal antibody |
| CP-751,871 | Phase I, II | Ref. 32, 33, 55
Fully humanized monoclonal antibody against IGF-1R |
| AMG 479 | Phase I | Ref 56
A fully human monoclonal antibody against IGF-1R |
| R1507 | Phase I | Ref 57
Human monoclonal antibody IGF-1R antagonist |
| EM164 (AVE1642) | Preclinical | Ref. 34
Antagonistic monoclonal antibody AVE1642 is the humanized version of EM164 |
| hC710 (A2CHM) | Preclinical | Ref. 35 |
| 19D12 | Preclinical | Ref. 36
Fully humanized monoclonal antibody |
| scFv-Fc-IGF-1R | Preclinical | Ref. 37
Chimeric single chain antibody fusing Fc domain of human IgG1 with Fv region of 1H7, an anti-IGF-1R murine monoclonal antibody Transient activation of IGF-1R followed by strong and durable downregulation of IGF-1R |
| Di-diabody | Preclinical | Ref. 38 |
| IGF-1R Kinase inhibitors | ||
| INSM-18 | Phase I | Ref. 30
Also active against HER2 receptor |
| NVP-ADW742 | Preclinical | Ref. 41
>16-fold more potent inhibitory effect against IGF-1R than against insulin receptor in cellular kinase activity assays |
| NVP-AEW541 | Preclinical | Ref. 42
Highly selective for IGF-1R tyrosine kinase as compared to the insulin receptor in vitro |
| Cyclolignan picropodophyllin (PPP) | Preclinical | Ref. 43 |
| AG 538 | Preclinical | Ref. 44
Low molecular weight compound containing two catechol rings sensitive to oxidation in cells |
| BMS-554417 | Preclinical | Ref. 45
Also significantly inhibits insulin receptor; in vivo, high glucose and tenfold increase in insulin level during oral glucose tolerance test |
| BMS-536924 | Preclinical | Ref. 46
Also significantly inhibits insulin receptor |
| OSI-906 | Preclinical | Ref. 47
Orally administered small molecule |
| Other Agents | ||
| IGF-1R/AS ODN | Phase I | Ref. 49 |
| 486/STOP | Preclinical | Ref. 50 |
| IGFBP-3 | Phase I | Ref. 51
Phase I is examining recombinant IGFBP-3 (INSM-120-101) |
| IGFBP-1 | Preclinical | Ref. 52
Short half-life has led to development of a pegylated form |
3.1 IGF-1R ANTIBODIES
Antibodies against the binding domain of IGF-1R block binding of the ligand and subsequent activation of the receptor [31–38]. Binding of these antibodies can trigger receptor internalization and degradation with reduction in the receptor number on the cell surface.
IMC-A12, a fully human monoclonal antibody currently in early phase clinical development, binds to IGF-1R with high affinity and inhibits ligand binding with an IC(50) of 0.6–1 nM [31]. Blockade of ligand binding to IGF-1R inhibits downstream signaling of the two major IGF pathways, MAPK and PI3K/Akt, in MCF7 human breast cancer cells. IMC-A12 does not block insulin binding to the insulin receptor. In xenograft tumor models, IMC-A12 results in significant growth inhibition of breast, pancreatic, and colon tumors [31].
Another fully humanized monoclonal antibody directed against IGF-1R, CP-751,871, is in phase 1 clinical trial in patients with advanced solid tumors multiple myeloma [32]. In experimental models, it inhibits IGF-I binding to cells, inhibits IGF-I-induced receptor phosphorylation, and results in down-regulation of IGF-1R expression at the plasma membrane through internalization of the receptor. Inhibition of tumor growth has been documented in multiple xenograft models, and its combination with standard chemotherapeutic agents enhances its antitumor efficacy. The combination of CP-751,871 with 5-FU in a Colo-205 xenograft model resulted in improved antitumor activity compared to either agent given alone [33].
In order to define the role of anti-IGF-1R therapy as a radiosentitizer, SW 480 colon cancer cells were cultured to semiconfluent conditions with dose titrations performed for 5-FU to determine the IC(50) [39]. Colon cancer cells were treated with 5-FU, external beam radiation, with or without IGF-I and alpha-IR-3 (mouse monoclonal antibody targeting IGF-1R). The addition of IGF-I 1 h prior to 5-FU or radiation significantly blunted the expected cytotoxicity, resulting in a 10-fold increase in the IC(50). Addition of alpha-IR-3 produced a dose-dependent increase in cytotoxicity compared with 5-FU alone. The addition of radiation produced synergistic amplification of this response. The investigators concluded that blocking IGF-1R activation increased the cytotoxic response to chemoradiation therapy [39].
A recombinant human bispecific antibody, also termed a Di-diabody, targeting IGF-1R and EGFR has been created using the variable regions from two monoclonal antibodies, IMC-A12 which targets IGF-1R and IMC-11F8 which targets EGFR. This Di-diabody has been shown to block tumor cell proliferation in vitro, and in vivo antitumor efficacy was demonstrated using HT29 colorectal carcinoma xenografts [38]. This may be a promising agent, as a retrospective study by Cunningham et al documented coexpression of IGF-1R and EGFR in ≥75% of 87 Duke’s C colorectal tumors [40].
3.2 KINASE INHIBITORS (TABLE 3)
Small molecules that selectively inhibit the tyrosine kinase domain of IGF-1R without significant effect on the insulin receptor are under development [30, 41–47].
NVP-AEW541, a kinase inhibitor, has shown induction of apoptosis and cell cycle arrest in two CRC cell lines, HT29 and HCT-116, resulting in dose dependent inhibition of proliferation. Combining this agent with either 5-fluorouracil or cetuximab resulted in additive growth inhibition. NVP-AEW541 alone inhibited proliferation in primary cancer cell cultures of tumors from 8 patients with primary CRC [42].
Cyclolignan picropodophyllin (PPP), another IGF-1R kinase inhibitor, blocks IGF-1R activity, probably by inhibiting IGF-1R autophosphorylation at the substrate level, without affecting the insulin receptor [43]. PPP caused complete tumor regressions in xenografted and allografted mice [43].
Another class of IGF-1R kinase inhibitors are a family of bioisostere inhibitors, based on the structure of AG 538 a substrate-competitive inhibitor of IGF-IR. Catechol bioisosteres of AG 538 inhibit IGF-1R kinase activity and IGF-I induced IGF-1R autophosphorylation and block the formation of colonies in soft agar by cancer cells. IRS-1 phosphorylation and protein kinase B activation are inhibited when applied to intact cells [44].
3.3 ANTISENSE AGENTS, DOMINANT NEGATIVE VARIANTS, AND OTHER AGENTS
Resnicoff reported that C6 rat glioblastoma cells expressing an antisense IGF-1R RNA implanted for 24 h in the subcutaneous tissue of rats were able to elicit an anti-tumor response in the brain, leading to complete brain tumor regression and long-term survival of the rats [48]. Based on this, a human pilot safety and feasibility study used an antisense oligodeoxynucleotide directed against IGF-1R (IGF-1R/AS ODN) in patients with malignant astrocytoma. Autologous glioma cells collected at surgery were treated ex vivo with IGF-1R/AS ODN encapsulated in diffusion chambers, reimplanted in the rectus sheath within 24 hours of craniotomy, and retrieved after 24-hours of in situ incubation. At follow-up, clinical and radiographic improvements were observed in eight of 12 patients, including 2 complete responses [49].
Reiss et al [50] transfected a human colon cancer cell line with plasmids expressing the dominant negative mutant of IGF-1R, 486/STOP, which has a frameshift mutation resulting in a stop codon at residue 486. The stable expression of 486/STOP inhibited colony formation in soft agar as well as tumor growth in nude mice. Also, co-injection of cells expressing 486/STOP with wild-type tumor cells inhibited the growth of wild-type tumor cells secondary to a bystander effect [50].
IGF binding proteins (IGFBP-1 and IGFBP-3) are also being investigated as possible anticancer agents [51, 52].
4.0 CONCLUSION
The IGF system plays an important role in tumorigenesis and has been shown to be an absolute requirement for the establishment and maintenance of the transformed phenotype [53]. The effect of down-regulating the IGF system is more profound on cells growing in anchorage independent conditions as opposed to cells growing in a monolayer [54]. This may provide relative selectivity for agents that target this pathway for the treatment of cancer. Early clinical trials with agents targeting the IGF system are ongoing and will hopefully validate this pathway as a therapeutic target.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, Bethesda, MD. CONFLICTS OF INTEREST: The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 REFERENCES
- 1.Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 3.Durai R, Yang W, Gupta S, et al. The role of the insulin-like growth factor system in colorectal cancer: review of current knowledge. Int J Colorectal Dis. 2005;20:203–220. doi: 10.1007/s00384-004-0675-4. [DOI] [PubMed] [Google Scholar]
- 4.Jehle PM, Fussgaenger RD, Blum WF, et al. Differential autocrine regulation of intestine epithelial cell proliferation and differentiation by insulin-like growth factor (IGF) system components. Horm Metab Res. 1999;31:97–102. doi: 10.1055/s-2007-978705. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Yakar S, Zhao L, et al. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 6.Akagi Y, Liu W, Zebrowski B, et al. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998;58:4008–4014. [PubMed] [Google Scholar]
- 7.Andre F, Rigot V, Thimonier J, et al. Integrins and E-cadherin cooperate with IGF-I to induce migration of epithelial colonic cells. Int J Cancer. 1999;83:497–505. doi: 10.1002/(sici)1097-0215(19991112)83:4<497::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Garrouste F, Remacle-Bonnet M, Fauriat C, et al. Prevention of cytokine-induced apoptosis by insulin-like growth factor-I is independent of cell adhesion molecules in HT29-D4 colon carcinoma cells-evidence for a NF-kappaB-dependent survival mechanism. Cell Death Differ. 2002;9:768–779. doi: 10.1038/sj.cdd.4401022. [DOI] [PubMed] [Google Scholar]
- 9.Di Popolo A, Memoli A, Apicella A, et al. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene. 2000;19:5517–5524. doi: 10.1038/sj.onc.1203952. [DOI] [PubMed] [Google Scholar]
- 10.Hakam A, Yeatman TJ, Lu L, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 11.Weber MM, Fottner C, Liu SB, et al. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer. 2002;95:2086–2095. doi: 10.1002/cncr.10945. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Miyamoto S, Maeda H, et al. Low levels of insulin-like growth factor type 1 receptor expression at cancer cell membrane predict liver metastasis in Dukes’ C human colorectal cancers. Clin Cancer Res. 2004;10:8434–8441. doi: 10.1158/1078-0432.CCR-04-0430. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhou W, Velculescu VE, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 14.Nosho K, Yamamoto H, Taniguchi H, et al. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin Cancer Res. 2004;10:7950–7957. doi: 10.1158/1078-0432.CCR-04-0875. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Correa M, Cui H, Giardiello FM, et al. Loss of imprinting of insulin growth factor II gene: a potential heritable biomarker for colon neoplasia predisposition. Gastroenterology. 2004;126:964–970. doi: 10.1053/j.gastro.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins PJ, Fairclough PD, Richards T, et al. Acromegaly, colonic polyps and carcinoma. Clin Endocrinol (Oxf) 1997;47:17–22. doi: 10.1046/j.1365-2265.1997.1911029.x. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Bhaskar P, Painter JE, et al. The prevalence and characteristics of colorectal neoplasia in acromegaly. J Clin Endocrinol Metab. 2000;85:3417–3424. doi: 10.1210/jcem.85.9.6775. [DOI] [PubMed] [Google Scholar]
- 18.Baris D, Gridley G, Ron E, et al. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control. 2002;13:395–400. doi: 10.1023/a:1015713732717. [DOI] [PubMed] [Google Scholar]
- 19.Orme SM, McNally RJ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 20.Paterson AC, Leeding KS, Bach LA, et al. More about: prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 2000;92:1947–1950. doi: 10.1093/jnci/92.23.1947-a. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 22.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 23.Hunt KJ, Toniolo P, Akhmedkhanov A, et al. Insulin-like growth factor II and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2002;11:901–905. [PubMed] [Google Scholar]
- 24.Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–349. [PubMed] [Google Scholar]
- 25.Palmqvist R, Hallmans G, Rinaldi S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–646. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Probst-Hensch NM, Yuan JM, Stanczyk FZ, et al. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer. 2001;85:1695–1699. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura AM, Stemmermann GN, Lee J, et al. Serum insulin-like growth factor I and subsequent risk of colorectal cancer among Japanese-American men. Am J Epidemiol. 2003;158:424–431. doi: 10.1093/aje/kwg176. [DOI] [PubMed] [Google Scholar]
- 28.Haydon AM, Macinnis RJ, English DR, et al. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–694. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann F, Garcia-Echeverria C. Blocking the insulin-like growth factorI receptor as a strategy for targeting cancer. Drug Discov Today. 2005;10:1041–1047. doi: 10.1016/S1359-6446(05)03512-9. [DOI] [PubMed] [Google Scholar]
- 31.Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 32.Lacy M, Alsina M, Melvin CL, et al. Phase 1 first-in-human dose escalation study of cp-751,871, a specific monoclonal antibody against the insulin like growth factor 1 receptor. Proc ASCO. 2006;24:448s. [Google Scholar]; Haluska P, Shaw H, Batzel GN, et al. Phase 1 dose escalation study of the anti-IGF-1R monoclonal antibody CP-751,871 in patients with refractory solid tumors. J Clin Oncol; ASCO Annual Meeting Proceedings Part I: Vol 25; 2007; 2007. p. 3586. [Google Scholar]
- 33.Cohen BD, Baker DA, Soderstrom C, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 34.Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073–5083. [PubMed] [Google Scholar]
- 35.Goetsch L, Gonzalez A, Leger O, et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113:316–328. doi: 10.1002/ijc.20543. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Hailey J, Williams D, et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol Cancer Ther. 2005;4:1214–1221. doi: 10.1158/1535-7163.MCT-05-0048. [DOI] [PubMed] [Google Scholar]
- 37.Sachdev D, Li SL, Hartell JS, et al. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–635. [PubMed] [Google Scholar]
- 38.Lu D, Zhang H, Koo H, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–19672. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 39.Perer ES, Madan AK, Shurin A, et al. Insulin-like growth factor I receptor antagonism augments response to chemoradiation therapy in colon cancer cells. J Surg Res. 2000;94:1–5. doi: 10.1006/jsre.2000.5923. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham MP, Essapen S, Thomas H, et al. Coexpression of the IGF-IR, EGFR and HER-2 is common in colorectal cancer patients. Int J Oncol. 2006;28:329–335. [PubMed] [Google Scholar]
- 41.Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221–230. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- 42.Hopfner M, Sutter AP, Huether A, et al. Tyrosine kinase of insulin-like growth factor receptor as target for novel treatment and prevention strategies of colorectal cancer. World J Gastroenterol. 2006;12:5635–5643. doi: 10.3748/wjg.v12.i35.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girnita A, Girnita L, del Prete F, et al. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 44.Blum G, Gazit A, Levitzki A. Development of new insulin-like growth factor-1 receptor kinase inhibitors using catechol mimics. J Biol Chem. 2003;278:40442–40454. doi: 10.1074/jbc.M305490200. [DOI] [PubMed] [Google Scholar]
- 45.Haluska P, Carboni JM, Loegering DA, et al. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- 46.Carboni JM, Lee AV, Hadsell DL, et al. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 2005;65:3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- 47.Ji Q-S, Mulvihill M, Franklin M, et al. A novel, potent and selective IGF-1R small molecule inhibitor blocks activation of IGF-1R signaling in vitro and inhibits IGF-1R dependent tumor growth in vivo. Proc Amer Assoc Cancer Res. 2006;47:LB-281. [Google Scholar]
- 48.Resnicoff M, Tjuvajev J, Rotman HL, et al. Regression of C6 rat brain tumors by cells expressing an antisense insulin-like growth factor I receptor RNA. J Exp Ther Oncol. 1996;1:385–389. [PubMed] [Google Scholar]
- 49.Andrews DW, Resnicoff M, Flanders AE, et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol. 2001;19:2189–2200. doi: 10.1200/JCO.2001.19.8.2189. [DOI] [PubMed] [Google Scholar]
- 50.Reiss K, D’Ambrosio C, Tu X, et al. Inhibition of tumor growth by a dominant negative mutant of the insulin-like growth factor I receptor with a bystander effect. Clin Cancer Res. 1998;4:2647–2655. [PubMed] [Google Scholar]
- 51.Yu Q, Banerjee K, Paterson J, et al. Insulin-like growth factor-binding protein 3: single-agent and synergistic effects with chemotherapeutic drugs on solid tumour models. Proc Amer Assoc Cancer Res. 2003 [Google Scholar]
- 52.Van den Berg CL, Cox GN, Stroh CA, et al. Polyethylene glycol conjugated insulin-like growth factor binding protein-1 (IGFBP-1) inhibits growth of breast cancer in athymic mice. Eur J Cancer. 1997;33:1108–1113. doi: 10.1016/s0959-8049(97)00071-3. [DOI] [PubMed] [Google Scholar]
- 53.Sell C, Rubini M, Rubin R, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 55.Karp DD, Paz-Ares LG, Blakely LJ, et al. Efficacy of the anti-insulin like growth factor 1 receptor (IGF-1R) antibody CP-751871 in combination with paclitaxel and carboplatin as first-line treatment for advanced non-small cell lung cancer (NSCLC). J Clin Oncol; ASCO Annual Meeting Proceedings Part I: Vol 25; 2007; 2007. p. 7506. [Google Scholar]
- 56.Tolcher AW, Rothenberg ML, Rodon J, et al. A phase 1 pharmacokinetic and pharmacodynamic study of AMG 479, a afully human monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-1R), in advanced solid tumors. J Clin Oncol; ASCO Annual Meeting Proceedings Part I: Vol 25; 2007; 2007. p. 3002. [Google Scholar]
- 57.Rodon J, Patnaik A, Stein M, et al. A phase 1 study of q3W R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol; ASCO Annual Meeting Proceedings Part I: Vol 25; 2007; 2007. p. 3590. [Google Scholar]