Abstract
Estrogens have numerous effects on the brain, both in adulthood and during development. These actions of estrogen are mediated by two distinct estrogen receptor (ER) systems, ER alpha (ERα) and ER beta (ERβ). In brain, ERα plays a critical role in regulating reproductive neuroendocrine function and behavior, however, a definitive role for ERβ in any neurobiological function has been slow in forthcoming. Clues to the function of ERβ in the central nervous system can be gleaned from the neuroanatomical distribution of ERβ and the phenotypes of neurons that express ERβ. ERβ immunoreactivity has been found in populations of GnRH, CRH, vasopressin, oxytocin and prolactin containing neurons in the hypothalamus. Utilizing subtype-selective estrogen receptor agonists can help determine the roles for ERβ in non-reproductive behaviors in rat models. ERβ selective agonists exert potent anxiolytic activity when animals were tested in a number of behavioral paradigms. Consistent with this, ERβ selective agonists also inhibited the ACTH and corticosterone response to stress. In contrast, ERα selective agonists were found to be anxiogenic and correspondingly increased the hormonal stress response. Taken together, our studies implicate ERβ as an important modulator of some non-reproductive neurobiological systems. The molecular and neuroanatomical targets of estrogen that are mediated by ERβ remain to be determined.
A number of splice variants of ERβ mRNA have been reported in brain tissue. Imaging of eGFP labeled chimeric receptor proteins transfected into cell lines show that ERβ splice variation can alter trafficking patterns and function. The originally described ERβ (herein termed ER-β1) is characterized by possessing a high affinity for estradiol. Similar to ERα, it is localized in the nucleus and is trafficked to nuclear sites termed “hyperspeckles” following ligand binding. In contrast, ER-β2 contains an 18 amino acid insert within the ligand binding domain and as a result can be best described as a low affinity form of ERβ. A delta3 (δ3) variant of ERβ has a deletion of the 3rd exon (coding for the second half of the DNA binding domain) and as a result does not bind an estrogen response element in DNA. δ3 variants are trafficked to a unique low abundance and larger nuclear site following ligand binding. A delta4 (δ4) variant lacks exon 4 and as a result is localized to the cytoplasm. The amount of individual splice variant mRNAs varies depending upon brain region. Examination of neuropeptide promoter regulation by ERβ splice variants demonstrate that ERβ functions as a constitutively active transcription factor. Moreover, it appears that splice variation of ERβ alters its ability to regulate transcription in a promoter-dependent and ligand-dependent fashion.
Keywords: Splice Variant, Neuropeptide, Stress, Anxiety, Receptor Trafficking
1. Expression and Function of Estrogen Receptors
The genomic actions of estrogen are mediated by two distinct intracellular receptors that function as ligand-activated transcription factors. These have been termed estrogen receptor alpha (ERα) and beta (ERβ) (Green et al., 1986; Kuiper et al., 1996). For both forms of ER, the binding of estrogen results in receptor dimerization, binding to specific DNA sites in gene promoter regions known as estrogen response elements (ERE), and subsequent modulation of gene transcription (Tsai and O'Malley, 1994). ERα and ERβ share similar DNA binding domains (96% homology), similar ligand binding domains (56% homology), and bind to the same hormone response element on DNA (Figure 1) (Kuiper et al., 1996).
Figure 1.
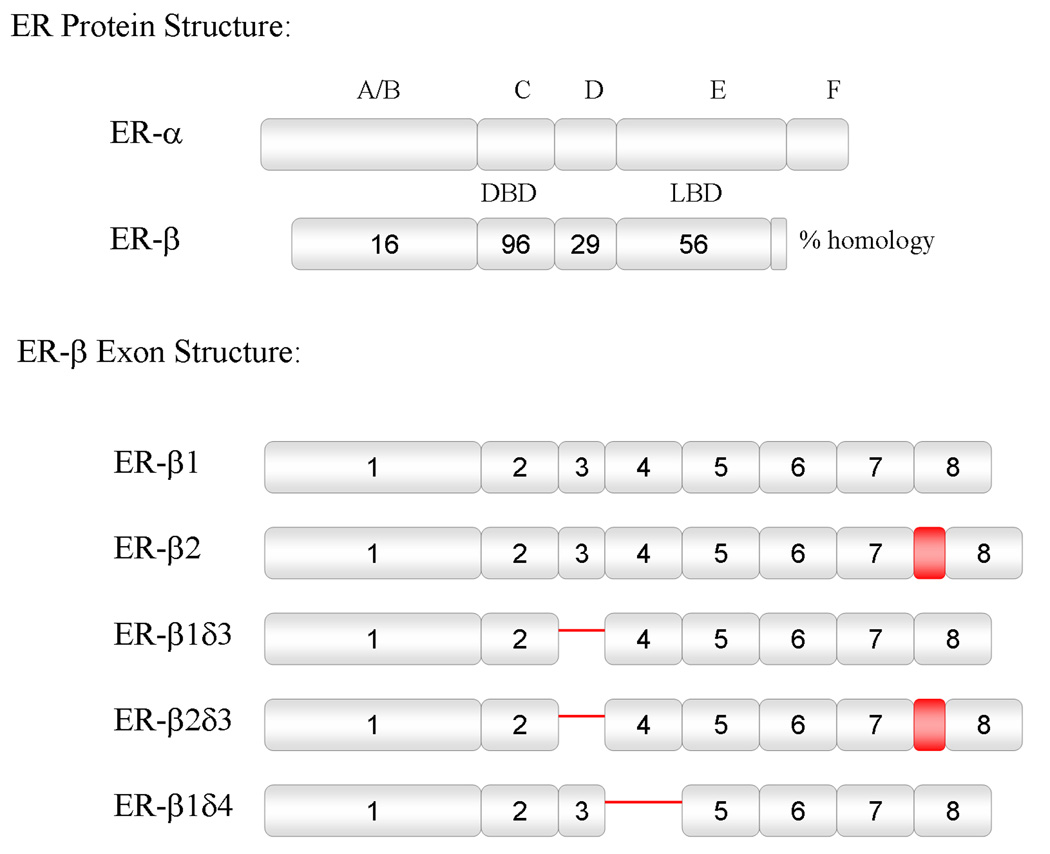
Schematic representation of ERα and ERβ protein structure and relative homology, and ERβ splice variant exon structure. Deletions are indicated by a single line, and insertions are indicated by a shaded box. DBD = DNA binding domain, LBD = ligand binding domain.
ERα and ERβ are expressed throughout the rostral-caudal extent of the brain and spinal cord. The receptors have been shown to have overlapping expression patterns with a few exceptions where either ERα or ERβ are not expressed, or one of the receptors is expressed at significantly higher levels compared to the other. Brain regions, including the bed nucleus of the stria terminalis (BNST), medial and cortical amygdaloid nuclei, preoptic area (POA), lateral habenula, periaqueductal gray, parabrachial nucleus, locus ceruleus, nucleus of the solitary tract, spinal trigeminal nucleus and superficial laminae of the spinal cord, express both forms of ER. However there are also striking differences in the expression pattern in certain brain areas. Only ERα is found in the ventromedial hypothalamic nucleus (VMH) and subfornical organ. In contrast, neurons of the olfactory bulb, supraoptic (SON), paraventricular (PVN), suprachiasmatic (SCN), and tuberal hypothalamic nuclei, zona incerta, ventral tegmental area, cerebellum, laminae III–V, VIII, and IX of the spinal cord, and pineal gland contain exclusively ERβ. Although both receptors are expressed by neurons in the arcuate nucleus and hippocampus, ERα is more abundant in the arcuate nucleus, and ERβ is more prevalent in the hippocampus (Shughrue et al., 1996; Chu and Fuller, 1997; Kuiper et al., 1997; Shughrue et al., 1997; Laflamme et al., 1998; Hileman et al., 1999; Mitra et al., 2003). Recent studies have also demonstrated that glia can also express ERα and ERβ (Santagati et al., 1994; Azcoitia et al., 1999; Platania et al., 2003; Zhang et al., 2004; Mhyre and Dorsa, 2006), although the function of glial ERs are not known.
ERβ has been shown to be differentially regulated under a number of physiological conditions. ERβ expression levels in the periventricular preoptic, SON and posterodorsal medial amygdala are strikingly different in pregnant and proestrous females. ERβ mRNA expression in the rat POA and medial basal hypothalamus is highest during the diestrous phase of the estrous cycle (Arteaga-Lopez et al., 2003). In addition, the number of ERβ mRNA expressing cells and ERβ immunoreactive cells are significantly reduced in the external plexiform layer of the olfactory bulb, entorhinal cortex, intermediate part of the lateral septal nucleus, nucleus of the horizontal limb of the diagonal band, lateral, medial and basolateral parts of the amygdala, anteroventral, laterodorsal and lateral posterior parts of the thalamus, medial geniculate nucleus, PVN, medial amygdala, BNST, periventricular preoptic, SCN and Purkinje cells in the cerebellum following estrogen treatment of ovariectomized female rats (Osterlund et al., 1998; Patisaul et al., 1999; Shima et al., 2003). In addition, in primary hippocampal cultures, 17β-estradiol treatment has been shown to increase ERα expression, but decrease ERβ (Prange-Kiel et al., 2003). Likewise, dexamethasone (DEX) and estradiol benzoate (EB) can change the protein and expression levels of ERβ in the PVN and SON of ovariectomized female rats. EB treatment of ovariectomized female rats decreases ERβ immunoreactive cell numbers and mRNA levels in the PVN, whereas DEX treatment increases ERβ expression (Suzuki and Handa, 2004). Similarly, Isgor et al. (Isgor et al., 2003) have shown that removal of endogenous glucocorticoids by adrenalectomy reduces ERβ mRNA levels in the PVN of female rats and that corticosterone replacement reverses this effect. However, in the latter study, upregulation of ERβ mRNA by adrenal steroids was observed only during proestrous when estrogen levels are high. Downregulation of ERβ has also been seen in the SON following hypernatremia stress (Somponpun and Sladek, 2003; Somponpun et al., 2004).
Interestingly, although ERα and ERβ share similar ligand binding domains, ERβ possesses a relative binding affinity (RBA) for several steroid hormones that differs from that of ERα (Kuiper et al., 1998b) (Table 1). Moreover, a number of ER modulators have been developed that selectively bind ERα or ERβ (Veeneman, 2005). Diarylpropionitrile (DPN) is a subtype selective agonist with a 70-fold greater RBA and 170-fold greater relative potency in transcription assays for ERβ than for ERα (Meyers et al., 2001; Sun et al., 2003). In contrast, propylpyrazole triol (PPT) is selective for ERα, with a 400-fold RBA for ERα over ERβ (Stauffer et al., 2000). These subtype selective agonists provide indispensable tools for use in functional assays.
Table 1.
Binding affinities of selected compounds for ERα and ERβ
| Compound | Ki (nm) |
|
|---|---|---|
| ER-α | ER-β | |
| Estradiola | 0.12 | 0.15 |
| DPNa | 195 | 2.5 |
| PPTa | 0.50 | 700 |
| Diethylstilbestrolb | 0.13 | 0.15 |
| Moxestrolb | 0.50 | 2.6 |
| 4-OH-Tamoxifenb | 0.10 | 0.04 |
| Genisteinb | 2.6 | 0.30 |
Binding affinities (Ki) of ER-subtype-selective ligands as compared with estradiol. Values obtained from (Lund et al., 2005)a, and (Kuiper et al., 1997)b.
Results of experiments with ERα and ERβnull mice (αERKO and βERKO, respectively) have indicated that ERα, but not ERβ, is vital to reproductive function (Ogawa et al., 1998b; Hewitt and Korach, 2003). Studies by Herbison and colleagues (Dorling et al., 2003; Wintermantel et al., 2006) have clearly demonstarated that, although ERβ is found in GnRH neurons (Herbison and Pape, 2001), it is not involved in regulating the preovulatory surge of leutinizing hormone (LH) in response to rising levels of estrogen. Rather, based on studies showing modest increases in LH levels in βERKO mice, ERβ may be partially involved in mediating the negative feedback control of anterior pituitary LH secretion (Dorling et al., 2003). Of importance, behavioral studies with βERKO mice indicate that ERβ may be involved in controlling anxiety-like as well as learned helplessness types of behaviors (Krezel et al., 2001; Imwalle et al., 2005; Rocha et al., 2005). Taken together, it appears that ERα is essential for reproductive neuroendocrine function, whereas the function of ERβ in controlling behaviors has yet to be fully elucidated.
2. Effects of Estrogen Receptor β on Neuroendocrine Function and Behavior
2.1 Phenotypes of Estrogen Receptor β Containing Neurons
Clues to determining the function of ERβ in the CNS can be gleaned by identifying the phenotype of ERβ expressing neurons. Information from our laboratory and others has indicated that ERβ is expressed within several different phenotypes of neurons within the CNS. ERβ immunoreactivity (IR) has been found in populations of gonadotropin-releasing hormone (GnRH), corticotropin-releasing hormone (CRH), vasopressin (AVP), oxytocin (OXY) and prolactin (PRL) containing neurons in the hypothalamus and in tryptophan hydroxylase containing neurons in the midbrain (Nomura et al., 2005). ERβ-IR is co-localized with OXY-IR within the medial parvocelluar PVN (84% of OXY neurons), and in the SON (60% of OXY neurons) (Hrabovszky et al., 2004; Suzuki and Handa, 2004). Additionally, ERβ-IR is co-localized with CRH-IR within the medial parvocellular PVN (13% of CRH neurons), and ERβ mRNA is found in CRH-IR neurons of the caudolateral PVN (60–80% of ERβ neurons) (Laflamme et al., 1998; Suzuki and Handa, 2004). ERβ-IR has also been observed within AVP-IR in the parvocellular magnocellular PVN (66% of AVP neurons), and in the SON (88% of AVP neurons) (Hrabovszky et al., 2004; Suzuki and Handa, 2004). PRL-IR neurons of the parvocellular magnocellular PVN and the SON also contain ERβ-IR (85% and 88% of PRL neurons, respectively) (Suzuki and Handa, 2004). Coexpression of GnRH and ERβ protein and/or mRNA has now been described in a variety of species (Skynner et al., 1999; Hrabovszky et al., 2000; Hrabovszky et al., 2001; Sharifi et al., 2002; Legan and Tsai, 2003; Skinner and Dufourny, 2005) including the rat, sheep and mouse. In the rat, 75–88% of GnRH-IR neurons of the preoptic area contain ER-β-IR (Hrabovszky et al., 2001). This matches the percentage of GnRH neurons that contain ER-β mRNA in the mouse, but curiously, the ERβ protein has not yet been found in mouse GnRH neurons.
Anatomical evidence supporting a role for ERβ in depressive-like behaviors comes from studies showing that greater than 90% of ERβ-IR neurons in the dorsal raphe and periaqueductal gray also express tryptophan hydroxylase (TPH), the rate limiting enzyme in serotonin synthesis (Nomura et al., 2005). This finding correlates with the behavioral effects seen following treatment of rodents with ERβ selective ligands (see below), while the existence of ERβ in neuropeptidergic neurons provides a potential explanation for the neuroendocrine actions of these ligands. In summary, these findings further support the possibility of direct effects of ERβ on neuropeptide expression and implicate estrogen involvement in the regulation of various aspects of neuroendocrine function.
2.2 Stress and Anxiety-Related Behaviors
Estrogen is a well-known regulator of mood, with reported effects of estradiol treatment ranging from depressant to anti-depressant (Fink et al., 1998; Shors and Leuner, 2003). Major depressive disorder (MDD) is one of the most common psychiatric illnesses with a lifetime prevalence of greater than 17% in the general population (Osterlund et al., 1998; Varghese and Brown, 2001). Several studies have consistently reported that MDD episodes are twice as common in women as compared to men (Angold and Worthman, 1993; Weissman et al., 1993; Kornstein, 1997; Llewellyn et al., 1997). A potential link between this inherent sex difference in MDD susceptibility and the variable effects of estrogen on mood may be explained by opposing actions of estrogen when mediated through ERα or ERβ.
Studies performed on βERKO mice provided an initial clue for a role of ERβ in anxiety- or depressive-like behaviors (Krezel et al., 2001). In the forced swim test (FST), a model for depression (Porsolt et al., 1977), estradiol-treated wild type mice showed less depressive-like behaviors (more time struggling and less time immobile) than controls, however, this effect of estradiol was lost on βERKO mice suggesting that estradiol’s antidepressant actions are mediated through ERβ (Rocha et al., 2005). In the elevated plus maze (EPM), a test to model anxiety-like behaviors (Handley and McBlane, 1993), two separate studies indicate increased anxiety-like behaviors in βERKO mice relative to their wild-type counterparts (Krezel et al., 2001; Imwalle et al., 2005).
Studies performed in our laboratory utilizing subtype-specific ER agonists have concurred with the experiments using knockout animals (Lund et al., 2005). In the open field test, ovariectomized females treated with the ERβ agonist diarylpropionitrile (DPN) spent more time in the middle of the arena, had more novel item interactions and a greater number of rears as compared to controls. The total number of square-crossings remained consistent suggesting an activity-independent decrease in anxiety-type behavior in DPN treated animals. In the light/dark box, DPN treated animals had a significantly longer latency to enter the dark portion of the box. Furthermore, in the elevated plus maze, DPN treated animals had more entries and time spent on the open arms of the maze, a greater number of rears and head dips which are signs of anxiolysis whereas they also exhibited fewer anxiogenic behaviors such as numbers of fecal boli, and time spent grooming as compared to controls (Figure 2). Treatment of gonadectomized males with DPN produced similar effects on anxiety-type behaviors in the EPM. These behavioral effects have since been replicated with the use of other ERβ agonists (Weiser et al., unpublished). The effects of DPN are prevented by concomitant treatment with the ER antagonist tamoxifen, indicating an ER-mediated mechanism. Interestingly, treatment with the ERα agonist propylpyrazoletriol (PPT) was anxiogenic on the EPM (Figure 2). Such data could help explain why estrogen has been reported to have both anxiogenic and anxiolytic effects (Palermo-Neto and Dorce, 1990; Leret et al., 1994).
Figure 2.
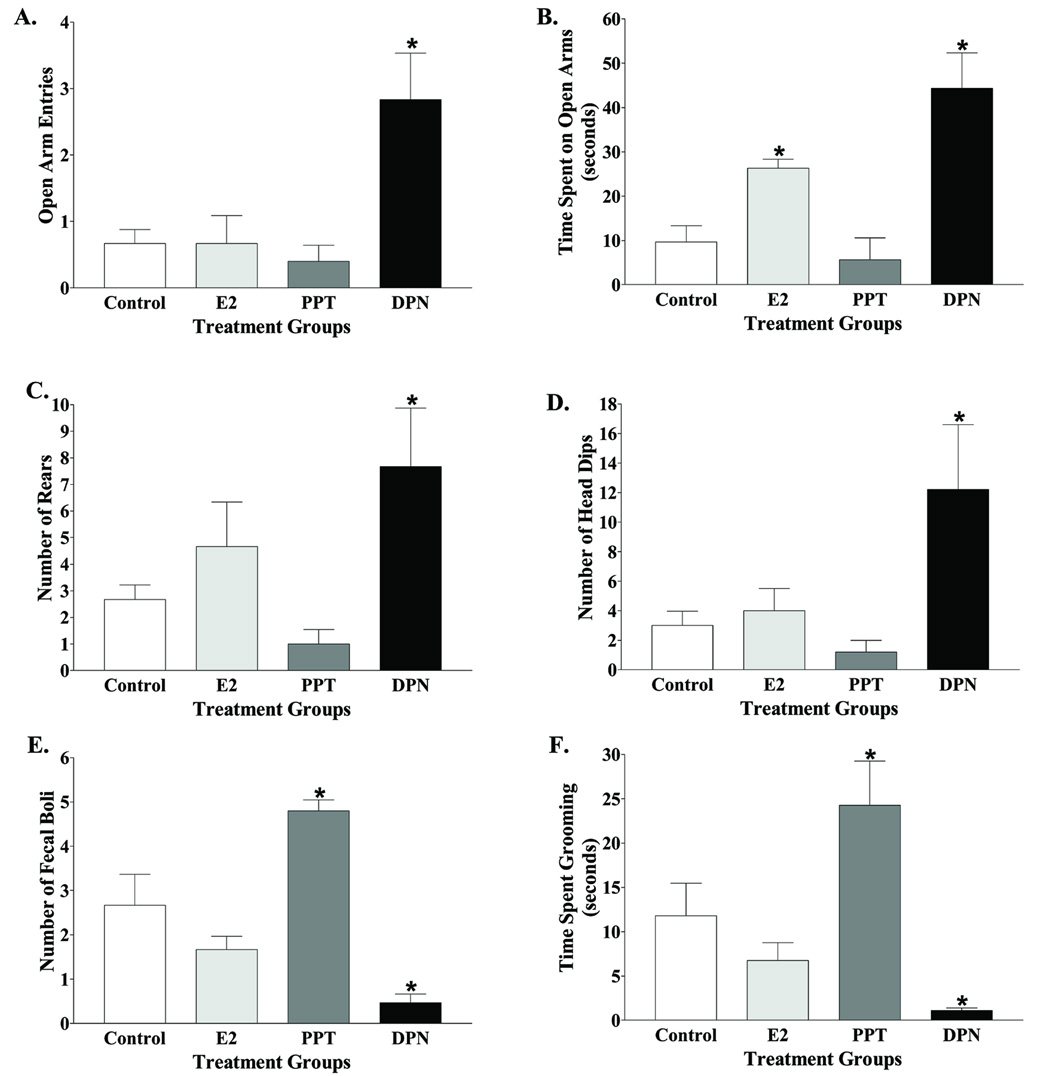
DPN treatment of ovariectomized female rats reduced anxiety-type behavior in the elevated plus maze. Quantification of behaviors included open arm entries (A), time spent on the open arms (B), rears (C), head dips (D), fecal boli (E), and time spent grooming (F). n = 9 animals per group, n = 8 for PPT. *, significant difference (P < 0.01) compared with control treatment. Adapted from (Lund et al., 2005).
Other studies have further confirmed the anxiolytic actions of ERβ agonists DPN treated ovariectomized females exhibit less depressive-like behavior in the FST and horizontal crossing task (Walf et al., 2004), as well as decreased anxiety in the EPM (Walf and Frye, 2005). Administration of ERβ-selective ligands directly to the hippocampus decrease depressive and anxiety-type behaviors, suggesting a possible role for ERβ in the hippocampus (Walf and Frye, 2006). Furthermore, the Flinders Sensitive Line (FSL) of rat, a strain selectively bred for depression, exhibit decreased immobility in the FST and increased social interaction following DPN treatment, both signs of anxiolysis (Overstreet et al., 2006).
Behavioral actions of ERβ activation may be explained by direct effects on regulation of neuropeptides that are involved in the stress response. ERβ has been shown to drive CRH and AVP promoter activity in vitro (Shapiro et al., 2000; Miller et al., 2004; Pak et al., 2007), and subtype selective ligands alter CRH, adrenocorticotropin hormone (ACTH), and corticosterone (CORT) responses to a stressor (see below). Other possible mechanisms by which estradiol regulates mood may include ERβ regulation of oxytocinergic or serotonergic neurotransmission. Oxytocin has powerful anxiolytic properties (Uvnas-Moberg et al., 1994; McCarthy et al., 1996; Windle et al., 1997; Mantella et al., 2003; Amico et al., 2004) that can be augmented by estradiol (McCarthy et al., 1996; Ochedalski et al., 2007), and ERβ has been found in most oxytocinergic neurons (Suzuki and Handa, 2005). Further, ERβ is the predominant estrogen receptor in the midbrain/brainstem raphe nucleus, particularly in the dorsal and ventral divisions of the dorsal raphe nucleus (DRN) (Nomura et al., 2005). Greater than 90% of these ERβ-IR neurons also exhibit TPH immunoreactivity (Nomura et al., 2005), suggesting a potential role for estrogen in regulation of TPH in the DRN. Accordingly, recent studies by Hiroi et al. show that estradiol treatment of ovariectomized female rats significantly increases TPH2, the predominant brain isoform, in the mid-ventromedial and caudal subregions of the DRN (Hiroi et al., 2006). These studies are corroborated by findings in ERβKO mice where serotonin content in the preoptic area and bed nucleus of the stria terminalis, areas of high ERβ expression, is significantly decreased (Imwalle et al., 2005).Furthermore, 5-HT1A receptor levels are significantly increased in the amygdala of ERβKO mice, and estradiol increases serotonin transporter (SERT) expression in the DRN of gonadectomized rats, implicating a role for estrogen and ERβ in serotonin reuptake dynamics (McQueen et al., 1997; McQueen et al., 1999; Krezel et al., 2001).Thus, the positive effects of estrogen on mood appear to be largely via its actions at ERβ and may be a dynamic interplay between the stress and oxytocinergic or serotonergic systems in the brain.
2.3 Hypothalamic-Pituitary-Adrenal Axis Function
Gonadal steroid hormones play a vital role in modulating hypothalamic-pituitary-adrenal (HPA) axis function. It has now been established that basal and stress-induced adrenal steroid secretion is greater in females than in males (Critchlow et al., 1963; Kitay, 1963; Handa et al., 1994a), and that the activational effects of gonadal steroids play an integral role in this sex difference (Sencar-Cupovic and Milkovic, 1976). In females, ovariectomy reduces stress-induced CORT and ACTH, and this is reversed by estrogen treatment (Burgess and Handa, 1992; Handa et al., 1994a; Suzuki et al., 2001). However, this is not always the case as several groups have reported that estrogen can inhibit responses to stress (Young et al., 2001; Figueiredo et al., 2002; Ochedalski et al., 2007).
In males, gonadectomy increases stress-induced CORT and ACTH, and is reversible with testosterone or dihydrotestosterone (DHT) treatment (Bingaman et al., 1994; Handa et al., 1994a; Handa et al., 1994b; Viau and Meaney, 1996; Suzuki et al., 2001; Viau et al., 2003; Viau and Meaney, 2004). Furthermore, treatment of gonadectomized male rats with estrogen increases stress-induced c-fos mRNA, CRH hnRNA, AVP hnRNA, and CORT, whereas DHT treatment inhibits the response when compared to control animals (Lund et al., 2004b). Thus, available evidence suggests that estrogen increases the gain of the HPA axis, while testosterone and its non-aromatizable metabolite DHT decrease the gain of the HPA axis.
The role of ERβ in HPA axis regulation has been explored with the use of ER subtype-selective compounds. For example, studies from our laboratory show that treatment of ovariectomized females with the ERβ selective agonist DPN causes a significant decrease, while treatment with the ERα selective agonist PPT results in a significant increase in stress-induced ACTH and CORT (Lund et al., 2005). This is consistent with the behavioral effects seen following treatment with these compounds. Interestingly, treatment with the DHT metabolite, 5α-androstan-3β, 17-β-diol (3β-diol) has a similar effect in significantly decreasing stress-induced CORT secretion (Lund et al., 2004a). This may be explained by the fact that 3β-diol is not androgenic, and does not bind the androgen receptor, but rather binds ERβ with relatively high affinity and selectivity (Kuiper et al., 1997; Weihua et al., 2002). Since the actions of 3 β-diol can be completely blocked by the ER antagonist, tamoxifen, but not the androgen receptor (AR) antagonist, flutamide, such data suggest that DHT’s inhibition of corticosterone secretion may be through the actions of its metabolite 3β-diol at ERβ (Lund et al., 2004a; Lund et al., 2006).
The results of additional studies examining the distribution of ERβ in the brain suggest that its role in HPA axis regulation may be indirect through alteration of glucocorticoid dependent HPA axis negative feedback (Weiser et al., unpublished), in addition to a direct action upon CRH and AVP neurosecretory neurons of the PVN. To test this hypothesis, we implanted wax pellets containing the ERβ agonist DPN, ERα agonist PPT, or estradiol near the PVN of gonadectomized male rats and measured stressinduced c-fos mRNA and plasma CORT and ACTH levels. Similar to what was observed following peripheral administration, DPN decreased while PPT and estradiol increased stress-induced c-fos mRNA and serum CORT levels, and these effects could be blocked with concomitant treatment with tamoxifen (Lund et al., 2006). These results suggest that attenuation of HPA reactivity via ERβ or augmentation via ERα is mediated via neuronal populations in and/or around the PVN. While it has been well established that ERβ is the dominant ER expressed by neurons within the PVN, recent studies have indicated that ERα transcript and immunoreactivity are present near the PVN (peri-PVN) and sparsely within the PVN (Laflamme et al., 1998; Suzuki and Handa, 2005). Thus, the augmentation of the HPA axis seen with systemic and local treatment with estradiol and PPT may be via ERα around the PVN.
Interestingly, implants of DHT and 3β-diol also cause a significant decrease in stress-induced c-fos mRNA and CORT, and these effects can be blocked with concomitant tamoxifen in the pellet (Lund et al., 2006). Local 3β-diol in the brain is produced by 5α-reduction of testosterone, and subsequent metabolism of DHT by the actions of the enzymes 3α-HSD, 3β-HSD, and 17β-HSD (Krieger et al., 1983; Gangloff et al., 2003; Torn et al., 2003; Steckelbroeck et al., 2004). We have determined that transcripts for 3α-HSD and 17β-HSD are present in the PVN, and provide these neurons the ability to locally convert gonadal androgens to endogenous ERβ ligands (Lund et al., 2006). Therefore, it appears that at one level, the inhibitory effects of DHT on the HPA axis are mediated by its conversion to 3β-diol and subsequent actions on ERβ. This is not to say that DHT does not act elsewhere to inhibit HPA function. Studies by Viau et al.(Viau and Meaney, 1996; Viau et al., 2001) have clearly demonstrated an action of DHT through the AR in BnST/POA in reducing HPA function as well.
The influence of gonadal steroids upon the HPA axis appears to be a delicate interplay between estrogen’s actions at ERβ and ERα, as well as testosterone’s actions at ERβ (through its metabolites DHT and 3β-diol) and AR. Our findings implicate ERs particularly around and within the PVN as a site of action for estrogen and DHT on HPA axis output. Further studies investigating the mechanisms of ERα and ERβ action on neurosecretory neurons of the PVN is certainly warranted.
3. Estrogen Receptor β Splice Variants
3.1 ERβ Splice Variant Characterization and Localization in Brain
There are five splice variants of ER-β mRNA described to date, including the originally described wild-type form ERβ (ER-β1), that are thought to arise from alternative splicing of the eight exons which encode ER-β (ER-β1, ER-β2, ER-β1δ3, ER-β2δ3, ER-β1δ4) (Figure 1). Transcripts designated ER-β2 possess an in-frame insertion between exons 5 and 6 that encodes an additional 18 amino acids (AAs) in the ligand binding domain (Chu and Fuller, 1997); (Maruyama et al., 1998). A deletion of exon 3, which encodes 39 AAs in the carboxyl-terminal half of the DNA binding domain, has been termed ER-β1δ3. ER-β2δ3 is characterized by the addition of 18 AAs inserted between exons 5 and 6 and a deletion of exon 3 (Petersen et al., 1998). ER-β1δ4 encodes an ERβ that is missing exon 4 and does not appear to bind estrogen (Price et al., 2001).
We (Price and Handa, 2000; Price et al., 2000) and others (Petersen et al., 1998) have shown that splice variants of ERβ mRNA are expressed in multiple tissues and in some cases at levels equivalent to or exceeding those of the full-length mRNA. The high expression level of some of the ERβ mRNA splice variants suggests that if corresponding proteins are expressed, they too would be abundant. Sharma et al. (1999) demonstrated that multiple ERβ variants can be seen with ERβ-specific antisera and Western blot analysis of proteins derived from ovary, a tissue known to express ERβ at high levels (Fitzpatrick et al., 1999; O'Brien et al., 1999). The distribution of at least one of these splice variants, ER-β2, has been shown in rat brain using anti-peptide antibodies directed against the unique sequence of the insert in the ligand binding domain. These studies have shown a distribution that largely matches that of ER-β1 and indicate high amounts of ER-β2 in SON, cortex and raphe (Chung et al., 2005). Therefore, the splice variants of ERβ must be considered when assessing receptor function.
ER-β1 is by far the most abundant of the splice variants mRNAs with expression in the lateral septum, SCN, PVN, medial amygdala, hippocampus, cortex and cerebellum with the one exception being the hippocampus. The relative expression levels of ER-β2 and ER-β1δ3 were similar to one another in all ERβ positive brain regions, though both were expressed at a significantly lower level than ER-β1. The isoform that is expressed consistently at the lowest level is ER-β2δ3. In general, the β2 variants are less abundantly expressed than their β1 counterparts (β2 vs. β1 and β2δ3 vs. β1δ3). In the hippocampus, all variants except ER-β1δ4 are expressed at relatively low levels. ER-β1δ4 is also expressed at higher levels in LS and CTX than in the SON, PVN or MA (Petersen et al., 1998; Price and Handa, 2000; Price et al., 2000). The respective levels of the others (β1 is the highest; β1δ3; β2δ3 is the lowest) are maintained, similar to the other brain regions.
Binding studies utilizing 3H-estradiol and in vitro transcribed ERβ splice variants have demonstrated that splice variation can provide distinct characteristics to the subsequent forms of ERβ. As shown in Table 2, the relatively high affinity of ER-β1 for estradiol (approx 0.1 nM) is reduced 10 fold by the β2 insertion in the ligand binding domain. In addition, this results in a slower association, and more rapid dissociation of estradiol with ER-β2. Removal of the 3rd exon (δ3 variants), does not seem to have much effect on binding kinetics. Given the changes in estradiol affinity of the β2 splice variant, we have proposed that ER-β2 represents a low affinity form of ERβ that would help extend cellular sensitivity to estradiol with rising levels of estrogen. Such a dual receptor system has been previously proposed for glucocorticoid and mineralocorticoid receptors and their inhibitory feedback on the HPA axis (Reul and de Kloet, 1985; Reul et al., 1987).
Table 2.
Binding affinities of estradiol for ERα and selected ERβ isoforms
| ER Isoform | Kd (nM) | T1/2 association (min) | T1/2 dissociation (min) |
|---|---|---|---|
| ER-α | 0.13 | 354 | 12 |
| ER-β1 | 0.15 ± 0.02 | <45 | >60 |
| ER-β2 | 1.84 ± 0.19 | 165 | 8 |
| ER-β1δ3 | 0.41 ± 0.15 | <45 | ND |
| ER-β2δ3 | 1.44 ± 0.82 | 165 | ND |
Dissociation constant (Kd ± SEM), association half-life (T1/2 association), and dissociation half-life (T1/2 dissociation) of 3[H] for ERα and selected ERβ isoforms. ND = not determined.
3.2 ERβ Splice Variant Transcriptional Activity
The ERβ protein has been shown to form homodimers in vitro and bind to consensus ERE oligonucleotides with an affinity similar to that of ERα (Pettersson et al., 1997). In addition, ERα and ERβ form heterodimeric complexes with EREs in vitro, as well as within intact cells (Cowley et al., 1997; Pace et al., 1997; Pettersson et al., 1997; Ogawa et al., 1998a; Ogawa et al., 1998c). The discovery of ERβ and the formation of heterodimers suggest the existence of previously unrecognized pathways of estrogen signaling; via ERβ in cells that exclusively express this receptor and via the formation of heterodimers in cells expressing both ERα and the ERβ splice variants.
In vitro studies show that co-transfection of different isoforms of ERβ can influence estradiol-induced gene transcription (Paech et al., 1997; Watanabe et al., 1997; Hyder et al., 1999). Homo- and heterodimeric forms can bind to a consensus ERE and activate transcription of a reporter gene (Cowley et al., 1997). Both ERα and ERβ are able to stimulate the transcription of an ERE-driven reporter gene in a dose-dependent manner (Kuiper et al., 1996; Mosselman et al., 1996; Cowley et al., 1997; Pettersson et al., 1997; Tremblay et al., 1997; Watanabe et al., 1997; Kuiper et al., 1998a). Furthermore, 5α-androstane- 3β, 17β–diol, (3β-diol) a metabolite of the potent androgen DHT, has been shown to activate ER-β1 induced transcription mediated by an ERE equivalent to that of 17β-estradiol in the neuronal cell line, HT22. Of importance, ER-β1 exhibits constitutive, or ligand independent, regulation of transcription by activating reporter gene expression through binding an ERE and inhibiting reporter gene activity through an AP-1 site (Pak et al., 2005).
Although the classically described mechanism for ER regulation of transcription involves the binding of ERs to an ERE, non-classical mechanisms have also been described. ERs can enhance transcription by modulating the activity of the activator protein complex-1 (AP-1) (Webb et al., 1995; Pak et al., 2005), and it is this non-classical mechanism that diversifies many of the actions of ERs in regulating endogenous promoters. For example, an important difference exists between ERα and ERβ concerning activation through AP-1 sites. ERα is able to activate AP-1 containing promoters in the presence of agonists, such as estradiol or diethylstilbestrol (DES), and the partial agonist/antagonist tamoxifen. In contrast, ERβ is only able to activate transcription from AP-1 sites in the presence of antagonists (Paech et al., 1997). Moreover, we have shown that δ3 variants of ERβ, which cannot bind the consensus ERE, exhibit ligand-dependent activation at AP-1-responsive reporters (Price et al., 2001; Pak et al., 2005). The AP-1 regulation has been shown to be ER-sensitive in a manner that does not require DNA binding (Webb et al., 1995; Webb et al., 1999), but rather through protein:protein interactions with c-fos, one of the endogenous factors that bind AP-1 elements. Interestingly, the δ3 variants activate at AP-1 sites in the presence of ER agonists unlike the full-length ERβ, which activates at AP-1 sites only in the presence of ER antagonists such as tamoxifen (Paech et al., 1997; Webb et al., 1999).
These functional interactions have been exemplified in our work to determine the estrogen regulation of several neuropeptides through ERβ splice variants. To investigate whether ERs could regulate CRH, AVP, and GnRH promoter activity, cells were cotransfected with the respective promoter constructs and either ERα or individual ERβ isoforms. ERα weakly stimulated CRH promoter transcriptional activity in a ligandindependent manner. Conversely, all ERβ isoforms tested stimulated CRH promoter activity with different ligand profiles. ER-β1 and ER-β2δ3 displayed constitutive activity (ER-β1 more than ER-β2δ3). Ligand-dependent activity of β isoforms 1 and 2 was altered by an Exon3 splice variant (δ3) or by the additional 18 amino acids in the ligand-binding domain of ER-β2 isoforms (Miller et al., 2004). Likewise, cells cotransfected with the AVP promoter and individual ERβ isoforms demonstrated a constitutive ligand independent activity of ER-β1 and ER-β2 (Shapiro et al., 2000; Pak et al., 2007). Both of these isoforms were found to further increase expression in the presence of estradiol. No ligand independent or dependent activity was found in cells transfected with the δ3-variants (Pak et al., 2007). In investigating ERβ variants in GnRH protmoter activity, Pak et al. found a robust increase in GnRH-luciferase activity by all ERβ splice variants in the absence of hormone. Furthermore, estradiol treatment abolished this response for ER-β1 and ER-β2, but not ER-β1δ3 (Pak et al., 2006). The examination of neuropeptide promoter regulation by ERβ and its splice variants demonstrate that in all cases ERβ functions as a constitutively active transcription factor. Moreover, it appears that splice variation of ERβ alters its ability to regulate transcription in a promoter-dependent and ligand-dependent fashion.
The ability of ERβto act in a ligand-independent fashion to regulate transcription may provide evidence of its ancient evolutionary roots. Indeed, estrogen receptors have been found in invertebrate species such as Aplysia californica and Octopus vulgaris. However, although the Octopus synthesizes estrogens, such invertebrate ERs do not bind estrogen and are not responsive to estrogens or other steroid hormones. In such cases, ERs are constitutive activators of transcription at an ERE (Thornton et al., 2003; Keay et al., 2006). It appears that like other nuclear receptors, only later in evolution did the ER attain the ability to bind ligand and exploit the ability to function in a ligand dependent fashion (Escriva et al., 1997; Thornton, 2001). Thus, unlike ERα, ERβ may represent a transitional protein that retains many of the ancient characteristics of the family, such as ligand independence, but has also developed functional versatility by the addition of ligand-dependent activation properties as well.
3.3 Cellular Trafficking of ERβ Splice Variants
Additional evidence that splice variation of ERβ provides functional diversity comes from studies examining the intracellular localization patterns of ERβ splice variants. By constructing chimeric proteins in which the five known ERβ splice variants are tagged with eGFP and overexpressed in cell lines, it was demonstrated that splice variation can drastically alter cellular trafficking patterns (Figure 3). ERα (Stenoien et al., 2000) and ERβ(Price et al., 2001) are both present in the nucleus even in the absence of ligand, thus supporting the idea that ER (α and β forms) are predominantly nuclear proteins. Similar to ERα (Htun et al., 1999; Stenoien et al., 2000), ER-β1 and ER-β2 are distributed in a reticular pattern within the nucleus in the absence of ligand. In the presence of ER agonists this distribution becomes “hyperspeckled” within the nucleus, distinguishing it from the diffuse distribution seen in the absence of any hormone. In contrast, ER-β1δ3 and ER-β2δ3, two variants that have a deletion of the 3rd exon coding for the DNA binding domain, localize to different yet discrete foci within the nucleus in the presence of ER agonists. These foci are larger and less numerous than the hyperspeckles seen when full-length ERβ is bound by ligand. The assembly of δ3 variants to these intranuclear foci is enhanced by ER agonists and disrupted by antagonists. Real-time imaging has revealed that this redistribution process is rapid and reversible. Likely, the forces of attraction for these two variants are determined by protein:protein interactions rather than DNA binding. Indeed, coactivator proteins of ER (co-transfected GFP-GRIP1 and endogenous CBP) colocalize with δ3 variants in the spots in the presence of agonists (Schaufele et al., 2000). This lends strength to the ligand-dependent interaction between δ3 variants and coactivators being at least partially responsible for -δ3 localization to these foci. Lastly, the ER-β1δ4 variant lacks exon 4, a region that contains the nuclear localization signal but not the ligand binding domain. As a result, this variant is localized to the cytoplasm and our binding studies have shown that it does not bind estrogen well. The loss of estrogen binding capacity may result from an altered ability of the ligand binding domain to fold normally (Price et al., 2000). It also may result from the loss of crucial sites for the heat shock protein association, which is known to greatly aid in the folding of a receptive ER ligand-binding domain (Pratt and Toft, 1997).
Figure 3.
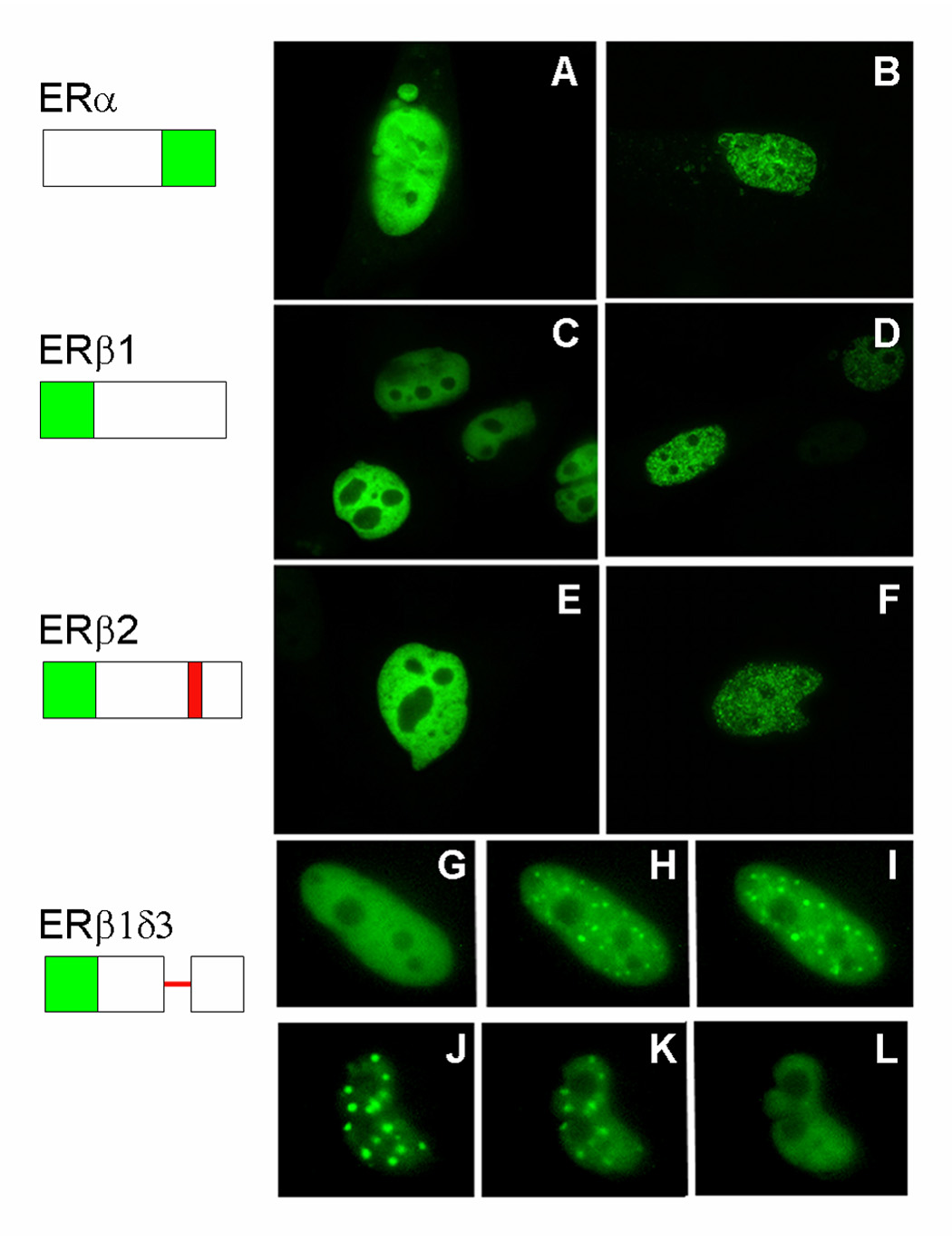
GFP-ERβ splice variants differentially localize within the nucleus in transiently transfected CHO cells. Cells were transfected with constructs coding for chimeric receptor proteins containing eGFP coupled to the various receptors. Schematic diagrams at the left show the protein-encoding regions (boxes; deletions are indicated by a single line, and insertions are shaded red). Panels A, C, E show cell nuclei in cells maintained in medium containing charcoal-stripped FBS. Panels B, D, F show cell nuclei after exposure to 100 nm estradiol for 20 min. Panels G – I show a time sequence of an individual nucleus following treatment with 100 nM estradiol (G = 0 min, H = 10 min, I = 15 min). Panels J–K show a time sequence of an individual nucleus following treatment with 100 nm tamoxifen (J = 0 min, K = 2 min, L = 10 min). The foci in G–L are larger and less abundant than the hyperspeckles shown in panels B, D, F, and the appearance and disappearance of receptor from foci are rapid.
In summary, it has been demonstrated that estrogen receptor beta is crucially involved in the hormonal and behavioral responses associated with stress. ERβ appears to represent an ancient receptor isoform that retains properties of constitutive regulation of transcription as well as ligand-dependent functions. In addition, ERβ presents itself in brain in numerous isoforms which alter the trafficking and functional properties of the receptor. As a result of the splicing events which alter the structure of the mature protein, increased functional diversity of ERβ can be attained.
Abbreviations
- 3β-diol
5α-androstane-3β,17β-diol
- αERKO
estrogen receptor alpha knockout
- βERKO
estrogen receptor beta knockout
- AA
amino acid
- ACTH
adrenocorticotropin hormone
- AP-1
activator protein complex-1
- AR
androgen receptor
- AVP
arginine vasopressin
- BNST
bed nucleus of the stria terminalis
- CBP
cAMP response element binding protein
- CORT
corticosterone
- CRH
corticotropin-releasing hormone
- CTX
cortex
- DEX
dexamethasone
- DHT
dihydrotestosterone
- DPN
diarylpropionitrile
- DRN
dorsal raphe nucleus
- eGFP
enhanced green fluorescent protein
- EPM
elevated plus maze
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- ERE
estrogen response element
- FSL
flinders sensitive line
- FST
forced swim test
- GnRH
gonadotropin-releasing hormone
- GRIP-1
glucocorticoid receptor interacting protein-1
- HPA
hypothalamic pituitary adrenal axis
- HSD
hydroxysteroid dehydrogenase
- IR
immunoreactivity
- LH
luetinizing hormone
- LS
lateral septum
- MA
medial amygdala
- MDD
major depressive dissorder
- OXY
oxytocin
- POA
preoptic area
- PPT
propylpyrazoletriol
- PRL
prolactin
- PVN
paraventricular nucleus
- RBA
relative binding affinity
- SCN
suprachiasmatic nucleus
- SERT
serotonin transporter
- SON
supraoptic nucleus
- TPH
tryptophan hydroxylase
- VMH
ventromedial hypothalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Arteaga-Lopez PR, Dominguez R, Cerbon MA, Mendoza-Rodriguez CA, Cruz ME. Differential mRNA expression of alpha and beta estrogen receptor isoforms and GnRH in the left and right side of the preoptic and anterior hypothalamic area during the estrous cycle of the rat. Endocrine. 2003;21:251–260. doi: 10.1385/ENDO:21:3:251. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Chu S, Fuller PJ. Identification of a splice variant of the rat estrogen receptor beta gene. Mol Cell Endocrinol. 1997;132:195–199. doi: 10.1016/s0303-7207(97)00133-0. [DOI] [PubMed] [Google Scholar]
- Chung WCJ, Pak TR, Andersen ME, Handa RJ. The Distribution of Estrogen Receptor Beta 2 in the Rat Brain; Society for Neuroscience 35th Annual Meeting; Washington, DC.. 2005. Abstract 632.11. [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- Escriva H, Safi R, Hanni C, Langlois MC, Saumitou-Laprade P, Stehelin D, Capron A, Pierce R, Laudet V. Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci U S A. 1997;94:6803–6808. doi: 10.1073/pnas.94.13.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Funkhouser JM, Sindoni DM, Stevis PE, Deecher DC, Bapat AR, Merchenthaler I, Frail DE. Expression of estrogen receptor-beta protein in rodent ovary. Endocrinology. 1999;140:2581–2591. doi: 10.1210/endo.140.6.6928. [DOI] [PubMed] [Google Scholar]
- Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17beta-hydroxysteroid dehydrogenase. Faseb J. 2003;17:274–276. doi: 10.1096/fj.02-0397fje. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994a;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994b;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125:143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Handa RJ, Jackson GL. Distribution of estrogen receptor-beta messenger ribonucleic acid in the male sheep hypothalamus. Biol Reprod. 1999;60:1279–1284. doi: 10.1095/biolreprod60.6.1279. [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct sub regions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Steinhauser A, Merchenthaler I, Coen CW, Petersen SL, Liposits Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. doi: 10.1002/cne.20127. [DOI] [PubMed] [Google Scholar]
- Htun H, Holth LT, Walker D, Davie JR, Hager GL. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell. 1999;10:471–486. doi: 10.1091/mbc.10.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder SM, Chiappetta C, Stancel GM. Interaction of human estrogen receptors alpha and beta with the same naturally occurring estrogen response elements. Biochem Pharmacol. 1999;57:597–601. doi: 10.1016/s0006-2952(98)00355-4. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Keay J, Bridgham JT, Thornton JW. The Octopus vulgaris estrogen receptor is a constitutive transcriptional activator: evolutionary and functional implications. Endocrinology. 2006;147:3861–3869. doi: 10.1210/en.2006-0363. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Pituitary-Adrenal Function in the Rat after Gonadectomy and Gonadal Hormone Replacement. Endocrinology. 1963;73:253–260. doi: 10.1210/endo-73-2-253. [DOI] [PubMed] [Google Scholar]
- Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry. 1997;58 Suppl 15:12–18. [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger NR, Scott RG, Jurman ME. Testosterone 5 alpha-reductase in rat brain. J Neurochem. 1983;40:1460–1464. doi: 10.1111/j.1471-4159.1983.tb13591.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998a;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998b;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Tsai HW. Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J Neuroendocrinol. 2003;15:1164–1170. doi: 10.1111/j.1365-2826.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- Leret ML, Molina-Holgado F, Gonzalez MI. The effect of perinatal exposure to estrogens on the sexually dimorphic response to novelty. Physiol Behav. 1994;55:371–373. doi: 10.1016/0031-9384(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Llewellyn AM, Stowe ZN, Nemeroff CB. Depression during pregnancy and the puerperium. J Clin Psychiatry. 1997;58 Suppl 15:26–32. [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitaryadrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004a;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004b;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Endoh H, Sasaki-Iwaoka H, Kanou H, Shimaya E, Hashimoto S, Kato S, Kawashima H. A novel isoform of rat estrogen receptor beta with 18 amino acid insertion in the ligand binding domain as a putative dominant negative regular of estrogen action. Biochem Biophys Res Commun. 1998;246:142–147. doi: 10.1006/bbrc.1998.8590. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Brain Res Mol Brain Res. 1997;45:13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Sumner BE, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in male rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63:241–247. doi: 10.1016/s0169-328x(98)00281-2. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci. 2004;24:10628–10635. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Nomura M, Akama KT, Alves SE, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-beta in midbrain serotonergic systems. Neuroscience. 2005;130:445–456. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- O'Brien ML, Park K, In Y, Park-Sarge OK. Characterization of estrogen receptor-beta (ERbeta) messenger ribonucleic acid and protein expression in rat granulosa cells. Endocrinology. 1999;140:4530–4541. doi: 10.1210/endo.140.10.7032. [DOI] [PubMed] [Google Scholar]
- Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2007;19:189–197. doi: 10.1111/j.1365-2826.2006.01525.x. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Inoue S, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Cross-inhibition of both estrogen receptor alpha and beta pathways by each dominant negative mutant. FEBS Lett. 1998a;423:129–132. doi: 10.1016/s0014-5793(98)00079-9. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998b;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun. 1998c;243:122–126. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Osterlund M, Dahllund J, Appelqvist T, Lindstrom E, Ryan CN, Witt MR. Estrogen receptor beta agonists reduce exaggerated swim test immobility in a genetic animal model of depression; Society for Neuroscience 36th Annual Meeting; Atlanta, GA.. 2006. Abstract 476.11. [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem. 1997;272:25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Pak T, Chung W, Handa R. Estrogen receptor-beta mediates DHT-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007 doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin-releasing hormone promoter activity. Endocrinology. 2006;147:1924–1931. doi: 10.1210/en.2005-1297. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Dorce VA. Influences of estrogen and/or progesterone on some dopamine related behavior in rats. Gen Pharmacol. 1990;21:83–87. doi: 10.1016/0306-3623(90)90600-q. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Brain Res Mol Brain Res. 1999;67:165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998;139:1082–1092. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Platania P, Laureanti F, Bellomo M, Giuffrida R, Giuffrida-Stella AM, Catania MV, Sortino MA. Differential expression of estrogen receptors alpha and beta in the spinal cord during postnatal development: localization in glial cells. Neuroendocrinology. 2003;77:334–340. doi: 10.1159/000070899. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Handa RJ. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci Lett. 2000;288:115–118. doi: 10.1016/s0304-3940(00)01221-0. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Res Mol Brain Res. 2000;80:260–268. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142:2039–2049. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul JM, van den Bosch FR, de Kloet ER. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J Endocrinol. 1987;115:459–567. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berl) 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Santagati S, Melcangi RC, Celotti F, Martini L, Maggi A. Estrogen receptor is expressed in different types of glial cells in culture. J Neurochem. 1994;63:2058–2064. doi: 10.1046/j.1471-4159.1994.63062058.x. [DOI] [PubMed] [Google Scholar]
- Schaufele F, Chang CY, Liu W, Baxter JD, Nordeen SK, Wan Y, Day RN, McDonnell DP. Temporally distinct and ligand-specific recruitment of nuclear receptor-interacting peptides and cofactors to subnuclear domains containing the estrogen receptor. Mol Endocrinol. 2000;14:2024–2039. doi: 10.1210/mend.14.12.0572. [DOI] [PubMed] [Google Scholar]
- Sencar-Cupovic I, Milkovic S. The development of sex differences in the adrenal morphology and responsiveness in stress of rats from birth to the end of life. Mech Ageing Dev. 1976;5:1–9. doi: 10.1016/0047-6374(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Shapiro RA, Xu C, Dorsa DM. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor alpha and beta. Endocrinology. 2000;141:4056–4064. doi: 10.1210/endo.141.11.7796. [DOI] [PubMed] [Google Scholar]
- Sharifi N, Reuss AE, Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology. 2002;143:2503–2507. doi: 10.1210/endo.143.7.8897. [DOI] [PubMed] [Google Scholar]
- Shima N, Yamaguchi Y, Yuri K. Distribution of estrogen receptor beta mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat Sci Int. 2003;78:85–97. doi: 10.1046/j.0022-7722.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroendocrinol. 2005;17:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE. Detection of estrogen receptor alpha and beta messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-beta in rat vasopressin and oxytocin neurons. J Neurosci. 2003;23:4261–4269. doi: 10.1523/JNEUROSCI.23-10-04261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somponpun SJ, Johnson AK, Beltz T, Sladek CD. Osmotic regulation of estrogen receptor-beta expression in magnocellular vasopressin neurons requires lamina terminalis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R465–R473. doi: 10.1152/ajpregu.00478.2003. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- Stenoien DL, Mancini MG, Patel K, Allegretto EA, Smith CL, Mancini MA. Subnuclear trafficking of estrogen receptor-alpha and steroid receptor coactivator-1. Mol Endocrinol. 2000;14:518–534. doi: 10.1210/mend.14.4.0436. [DOI] [PubMed] [Google Scholar]
- Sun J, Baudry J, Katzenellenbogen JA, Katzenellenbogen BS. Molecular basis for the subtype discrimination of the estrogen receptor-beta-selective ligand, diarylpropionitrile. Mol Endocrinol. 2003;17:247–258. doi: 10.1210/me.2002-0341. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Lund TD, Price RH, Handa RJ. Sex differences in the hypothalamo-pituitary-adrenal axis: novel roles for androgen and estrogen receptors. Recent Res Dev in Endocrinol. 2001:69–86. [Google Scholar]
- Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305:37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Varghese FP, Brown ES. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim Care Companion J Clin Psychiatry. 2001;3:151–155. doi: 10.4088/pcc.v03n0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman GH. Non-steroidal subtype selective estrogens. Curr Med Chem. 2005;12:1077–1136. doi: 10.2174/0929867053764662. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 2004;181:223–231. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13:442–452. doi: 10.1046/j.1365-2826.2001.00653.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144:3067–3075. doi: 10.1210/en.2003-0064. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2006 doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Inoue S, Ogawa S, Ishii Y, Hiroi H, Ikeda K, Orimo A, Muramatsu M. Agonistic effect of tamoxifen is dependent on cell type, ERE-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors alpha and beta. Biochem Biophys Res Commun. 1997;236:140–145. doi: 10.1006/bbrc.1997.6915. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–1685. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta, 17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerghet M, Mullins C, Williamson M, Bessert D, Skoff R. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and beta in oligodendrocytes. J Neurochem. 2004;89:674–684. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]


