Abstract
Portal vein thrombosis (PVT) may not be an absolute contraindication for hepatic radiofrequency ablation (RFA). Although the data are sparse, PVT is commonly considered a contraindication to RFA. PVT has actually been described as a complication following RFA. RFA was used to treat a 3.9 × 2.9 cm primary hepatocellular carcinoma (HCC) in a patient with concomitant PVT without complication. RFA can be safely performed in this setting but further studies could clarify this issue.
Keywords: Hepatocellular carcinoma (HCC), Portal vein thrombosis (PVT), Radiofrequency ablation (RFA)
1. Introduction
The etiology of portal vein thrombosis (PVT) can be divided into the following groups: hypercoagulability, low portal flow, infections, inflammatory diseases and mechanical interventions [1]. PVT in the setting of primary or secondary hepatic tumors may be caused by low portal flow and hypercoagulability followed by mechanical interventions such as chemoembolization, transjugular intrahepatic portosystemic shunt (TIPS), hepatobiliary surgery and endoscopic sclerotherapy. The consequences of PVT are related to the extension of the thrombus [2]. Upstream from the thrombus, there is usually a small effect on the intestine, as long as the mesenteric venous arches remain patent. Downstream, the consequences for the liver are hardly recognized, with absent or transient clinical signs of liver disease (unless occurring with cirrhosis). PVT in patients with hepatocellular carcinoma (HCC) is a poor prognostic factor, even if the patient is receiving systemic [3] or intra-arterial [4] chemotherapy.
PVT has conventionally been considered a relative or absolute contraindication to transarterial chemoembolization (TACE), because of the potential risk of hepatic insufficiency resulting from ischemia following the procedure [5,6]. A few controlled studies have demonstrated that TACE may be a safe modality if patients have good hepatic function and adequate collateral circulation around the main portal vein [5,7]. Percutaneous radiofrequency ablation (RFA) is becoming a well-established, minimally invasive procedure for the treatment of primary and secondary hepatic tumors, which can be performed under conscious sedation in the outpatient setting with few side effects. The theoretical additional risk of RFA in the setting of PVT is unknown.
2. Case report
A 61-year-old White male with a 30-year history of hepatitis B presented 3 months before RFA with PVT, and gradual abdominal distention associated with nausea. He was treated with diuretics, beta-blockers and warfarin. Dynamic MRI revealed a 3.9 × 2.9 cm mass in the right posterior portion of the liver, segments 5 and 6 (Fig. 2A), with an intralumenal right portal vein thrombus. However, the left portal venous system was patent and there was collateralization surrounding the right portal vein. A multiphase CT scan (Fig. 1A) showed marked arterial enhancement on the arterial phase and rapid washout on the portal venous phase, most consistent with a hepatocellular carcinoma (HCC), and the presence of a right portal vein thrombus with surrounding collateralization and a patent left portal vein.
Fig. 2.
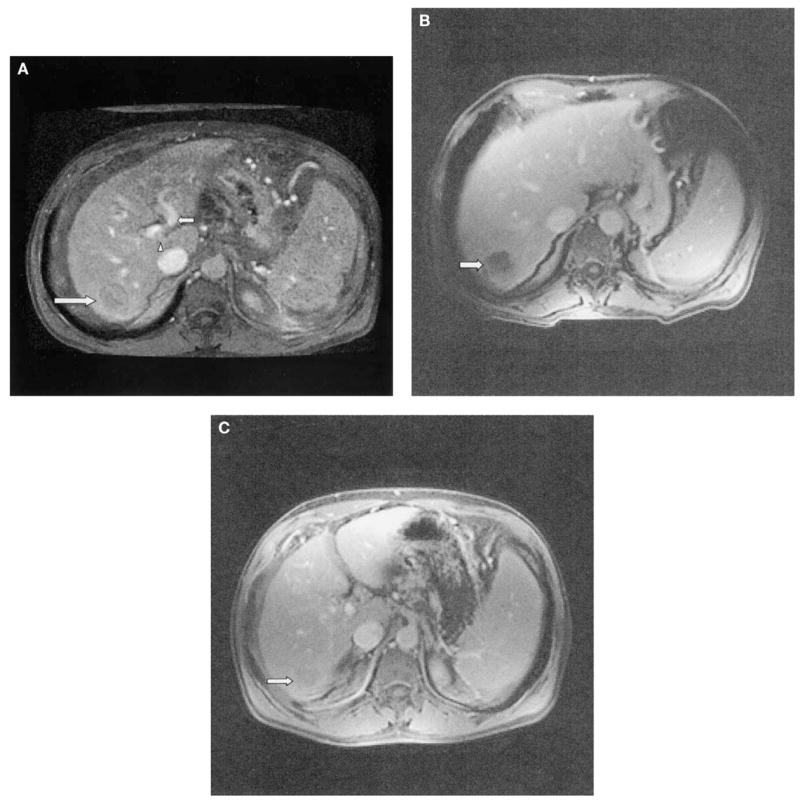
(A) Pre RFA treatment dynamic MRI T1-weighted image demonstrating hepatic mass (large arrow) and patent left portal vein (small arrow). The right portal vein thrombus is seen partially at this level (arrowhead). (B) Two-month follow-up dynamic MRI T1-weighted image shows no enhancement of the thermal lesion (arrow). (C) Six-month follow-up dynamic MRI T1-weighted image. The thermal lesion has shrunken considerably in size and there is no evidence of enhancement to suggest local recurrence (arrow).
Fig. 1.
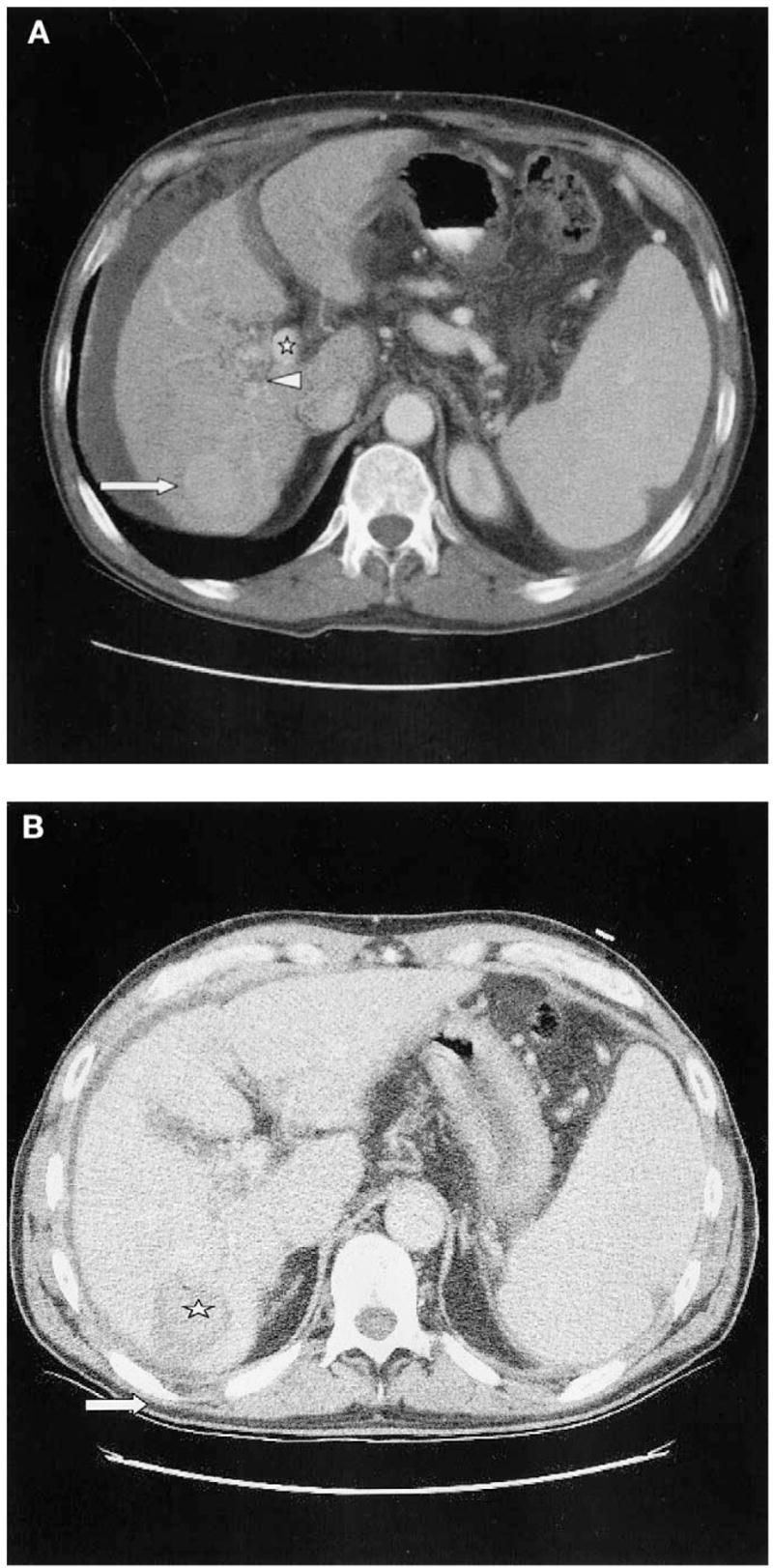
(A) Enhanced CT scan in the arterial phase before treatment shows enhancing hepatic tumor (large arrow), as well as right portal vein thrombus (arrowhead) with surrounding collateralization and cavernous transformation. The left portal vein remains patent (star). (B) Nonenhanced CT immediately following RFA treatment. Hyperdense regions (star) within treated area (arrow) are seen, most likely representing coagulative tissue.
Relevant history included colon resection 10 years ago secondary to carcinoma in situ, multiple abdominal surgeries for hernias and an appendectomy. Laboratory findings revealed the following: platelets — 71,000 (normal value: (162–380) × 103/mm3); PT — 15.9 (normal values: 11.8–14.7 s); PTT — 46.8 (normal values: 23.4–34.5 s); total bilirubin — 1.3 (normal values: 0.2–1.2 mg/dl); direct bilirubin — 0.5 (normal values: 0–0.3 mg/dl); alkaline phosphatase — 229 (normal values: 36–124 IU/L); CEA — 0.6 (normal values: 0–2.5 μg/l; albumin — 3 (normal values: 3.5–4.8 g/dl)); acute care chemistry 7 panel, normal; normal transaminases. The hepatic mass was consistent with an HCC, given its growth, characteristic arterial phase enhancement, rapid portal venous phase washout, history of hepatitis B and a 20G core biopsy showing marked atypical cells suggesting HCC. An Investigational Review Board (IRB) consult was not obtained because the RFA system is Food and Drug Administration (FDA) 510k cleared for soft tissue ablation. Similar systems have clearance for unresectable liver lesions.
Following written informed consent, standard local anesthesia and conscious sedation were achieved with subcutaneous 1% lidocaine and intravenous midazolam and fentanyl. A pre-RFA paracentesis was performed with 700 cc of serosanguinous ascitic fluid removed with a 5Fr needle sheath. Four 180-cm2 grounding pads were placed on patients thighs and RFA of the hepatic tumor was performed using a three-needle cluster, 17.5-gauge, 20 cm long, cooled tip, 2.5-cm probe (Radionics, Burlington, MA). CT findings following the procedure demonstrated the treated lesion as a low-attenuation area measuring 3.0 × 3.5 cm, with no contrast enhancement, overlapping the previous enhancing lesion (Fig. 1B). Prophylactic ciprofloxacin and metronidazole were given for 5 days, given the presence of the ascites. Laboratory results following the procedure were stable with no evidence for bleeding, complications or altered laboratory values. The following morning the patient was feeling well and was discharged.
Follow-up dynamic MRI and CT of the liver were performed at 2 months and at 6 months following the RFA. Two-month MRI showed a nonenhancing thermal lesion measuring 4.0 × 3.7 cm (Fig. 2B). Six-month MRI showed shrinkage and no evidence for pathologic enhancement of the thermal lesion, then measuring 3.3 × 3.0 cm (Fig. 2C).
3. Discussion
RFA is a thermal treatment that produces localized lesion destruction by heating to temperatures that exceed 50 °C (usually approaching temperatures around 80–110 °C), for 12 to 30 min resulting in denaturation and death of the targeted cells. RFA is advantageous because it can be performed under conscious sedation on an outpatient basis. Lately, there has been a growing multidisciplinary consensus regarding the promising therapeutic role of RFA for primary and secondary hepatic tumors. Early optimistic studies demonstrate favorable morbidity, mortality, recurrence and survival rates using this technique [8,9].
A few case reports have described PVT as a complication of percutaneous RFA of hepatic tumors [10,11]. PVT is considered a poor prognostic factor with HCC, and for years was considered a contraindication for TACE, based on the presumption that these patients are prone to hepatic insufficiency resulting from the ischemic insult. A few well-controlled studies in recent years have shown that TACE may be a safe procedure in the PVT setting if the patients have good hepatic function and collateral circulation around the main portal vein [5,7].
There is sparse data directly addressing the issue of RFA in the setting of PVT, and there have been several studies excluding the use of RFA in the HCC and PVT setting [12], with the latter considered a contraindication secondary to theoretical risks including hepatic infarct or insufficiency. Goldberg et al. [13] demonstrated that intraoperative occlusion of the portal vein increases the destruction volume during a single RFA, thereby potentially enhancing the efficacy of this therapy without negative outcome. The Pringle maneuver [14] (transient occlusion of the portal vein at the level of the porta hepatis) is a well-tolerated, common and well-established surgical technique. It is possible that PVT will assist with perfusion-mediated phenomena in a similar way.
Many patients with liver tumors and PVT have few options and RFA may not be absolutely contraindicated. There is little published data to support the common belief of whether RFA is contraindicated in this population. It is possible that old literature related to chemoembolization has influenced the RFA practice in this setting, which could result in withholding a potentially beneficial therapy.
References
- 1.Janssen HL. Changing perspective in portal vein thrombosis. Scand J Gastroenterol Suppl. 2000;232:69–73. [PubMed] [Google Scholar]
- 2.Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol. 2000;32:865–71. doi: 10.1016/s0168-8278(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 3.Okada S, Okazaki N, Nose H, Yoshimori M, Aoki K. Prognostic factors in patients with hepatocellular carcinoma receiving systemic chemotherapy. Hepatology. 1992;16:112–7. doi: 10.1002/hep.1840160119. [DOI] [PubMed] [Google Scholar]
- 4.Akashi Y, Korceda C, Enomoto S, Uchiyama S, Mizuno T, Shiozaki Y, Sameshima Y, Inoue K. Prognosis of unresectable hepatocellular carcinoma: an evaluation based on multivariate analysis of 90 cases. Hepatology. 1991;14:262–8. [PubMed] [Google Scholar]
- 5.Pentecost MJ, Daniels JR, Teitelbaum GP, Stanley P. Hepatic chemoembolization: safety with portal vein thrombosis. J Vasc Interv Radiol. 1993;4:347–51. doi: 10.1016/s1051-0443(93)71873-4. [DOI] [PubMed] [Google Scholar]
- 6.Allison DJ, Jordan H, Hennessy O. Therapeutic embolisation of the hepatic artery: a review of 75 procedures. Lancet. 1985;1(16):595–9. doi: 10.1016/s0140-6736(85)92142-7. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–94. [PubMed] [Google Scholar]
- 8.Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, Servadio G, Rizzatto G, Mueller PR, Del Maschio A, Gazelle GS. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202:195–203. doi: 10.1148/radiology.202.1.8988211. [DOI] [PubMed] [Google Scholar]
- 9.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–68. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 10.Catalano O, Esposito M, Nunziata A, Siani A. Multiphase helical CT findings after percutaneous ablation procedures for hepatocellular carcinoma. Abdominal Imaging. 2000;25:607–14. doi: 10.1007/s002610000076. [DOI] [PubMed] [Google Scholar]
- 11.Francica G, Marone G, Solbiati L, D’Angelo V, Siani A. Hemobilia, intrahepatic hematoma and acute thrombosis with cavernomatous transformation of the portal vein after percutaneous thermoablation of a liver metastasis. Eur Radiol. 2000;10:926–9. doi: 10.1007/s003300051038. [DOI] [PubMed] [Google Scholar]
- 12.Montorsi M, Santambrogio R, Bianchi P, Opocher E, Zuin M, Bertolini E, Bruno S, Podda M. Radiofrequency interstitial thermal ablation of hepatocellular carcinoma in liver cirrhosis. Role of the laparoscopic approach Surg Endosc. 2001;15:141–5. doi: 10.1007/s004640000242. [DOI] [PubMed] [Google Scholar]
- 13.Golberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, Compton CC, Solbiati L, Gazelle GS. Percutaneous radio-frequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101– 11. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 14.Patterson FJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–65. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]


