Abstract
BACKGROUND
This is a double blind, placebo-controlled trial that evaluated the efficacy of disulfiram, naltrexone and their combination in patients with co-occurring cocaine and alcohol dependence.
METHODS
208 patients were randomized to disulfiram (250mg/day), naltrexone (100mg/day), the combination, or placebo for 11 weeks. Outcomes were in-trial abstinence from cocaine and/or alcohol.
RESULTS
Few safety concerns were reported, although medication adherence was low in a number of patients for both medications, alone or in combination. In the primary analyses (GEE modeling), abstinence from cocaine as measured by cocaine-negative urines and days of self-reported abstinence from cocaine or alcohol did not differ between placebo and any of the medication groups. However, patients taking disulfiram (alone or in combination) were most likely to achieve combined abstinence from cocaine and alcohol. Secondary analyses revealed that patients taking the disulfiram-naltrexone combination were most likely to achieve 3 consecutive weeks of abstinence from cocaine and alcohol.
CONCLUSION
There was an association between disulfiram treatment and abstinence from cocaine and alcohol. More patients taking the disulfiram-naltrexone combination achieved 3 consecutive weeks of abstinence in treatment than placebo-treated patients.
Keywords: combining medications, cocaine, alcohol, disulfiram, naltrexone, clinical trial, medication nonadherence
1. Introduction
Patients who have co-occurring cocaine and alcohol dependence comprise a large proportion of the cocaine-addicted population (Gossop, Manning, & Ridge, 2006b), and suffer more adverse addiction-related consequences, have greater psychosocial problems, are inherently inconsistent at showing up for treatment visits, and have higher rates of recidivism than patients dependent only on cocaine or on alcohol (Brady, Sonne, Randall, Adinoff et al., 1995; Carroll, Rounsaville, & Bryant, 1993; Flannery, Morgenstern, McKay, Wechsberg et al., 2004; Heil, Badger, & Higgins, 2001; Mengis, Maude-Griffin, Delucchi, & Hall, 2002). Also, concurrent use of cocaine and alcohol produces cocaethylene (Gossop, Manning, & Ridge, 2006a; Harris, Everhart, Mendelson, & Jones, 2003), an active transesterified metabolite associated with more lethality than cocaine alone (Pennings, Leccese, & Wolff, 2002) and toxicity (Harris et al., 2003; Hearn, Rose, Wagner, Ciarleglio et al., 1991; Pennings, Leccese, & Wolff, 2002).
Thus, this treatment-refractory population may require a pharmacological approach, added to traditional counseling, which effectively targets the potentially different neurobiology of the combination of cocaine and alcohol dependencies. Historically, there have been many attempts to test medications for the treatment of either cocaine or alcohol dependence alone. Presently, the Food and Drug Administration (FDA) has approved medications for treating alcohol dependence, but none have been approved for treating cocaine dependence (Vocci, Acri, & Elkashef, 2005). Notably, there have been few attempts to evaluate medications in clinical trials for treating the co-occurrence of cocaine and alcohol dependence.
One reasonable treatment approach of co-occurring cocaine and alcohol dependence, which has been tried but has thus far failed, is giving an FDA-approved alcohol-reducing medication (added to counseling) that will decrease alcohol drinking, and, in turn, indirectly promote cocaine abstinence. It is well known that the use of one addictive substance often leads to the use of another addictive one. However, two double-blind, placebo-controlled studies of 50mg/day of naltrexone, an FDA-approved medication for treating alcohol dependence, did not reduce alcohol or cocaine use in cocaine-alcohol dependent outpatients, compared to placebo treatment (Hersh, Van Kirk, & Kranzler, 1998; Schmitz, Stotts, Sayre, DeLaune et al., 2004). Nonetheless, some preliminary data have suggested that a higher dose of naltrexone (150mg/day) may be useful in reducing cocaine and alcohol use in cocaine-alcohol dependent patients (Oslin, Pettinati, Volpicelli, Wolf et al., 1999), but more so in men than in women (Pettinati, Kampman, Lynch, Suh et al., 2007).
Another rationale, and a novel approach, to treating the co-occurrence of cocaine and alcohol dependence is to combine two medications, one of which will decrease cocaine use and the other of which will decrease alcohol use. In recent years, there have been a series of double blind, placebo-controlled clinical trials of combination medications for treating primarily alcohol or drug dependence in patients without concomitant psychiatric disorders (see, e.g., Ait-Daoud, Johnson, Prihoda, & Hargita, 2001; Anton, O’Malley, Ciraulo, Cisler et al., 2006;Kampman, Dackis, Lynch, Pettinati et al., 2006;Kiefer & Wiedemann, 2004), including adding disulfiram to naltrexone (Petrakis, Poling, Levinson, Nich et al., 2005), or to acamprosate – another FDA-approved medication for treating alcohol dependence (Besson, Aeby, Kasas, Lehert et al., 1998). The treatment strategy tested in the present study was combining two medications (added to twice weekly sessions of cognitive behavioral therapy) to treat patients presenting with dual cocaine and alcohol dependence. One medication was selected to reduce cocaine use (disulfiram – see below the rationale for doing so), and the second medication was selected to reduce alcohol use -- plus its FDA approval in 1994 for treating alcohol dependence (naltrexone).
Selecting a medication to reduce cocaine use was a more difficult task than choosing one to reduce alcohol use because there are no FDA-approved medications for treating cocaine dependence. However, recent controlled trials of several medications, namely, disulfiram, topiramate, modafinil, and others, suggested that some medications added to counseling may provide an advantage over placebo treatment for cocaine dependence (See respectively: Carroll, Fenton, Ball, Nich et al., 2004; Dackis, Kampman, Lynch, Pettinati et al., 2005; Kampman, Pettinati, Lynch, Dackis et al., 2004). To date, however, disulfiram has had more published articles than any other medication in support for its use in the treatment of cocaine dependence (see Carroll et al., 2004; Carroll, Nich, Ball, McCance et al., 2000; Carroll, Nich, Ball, Mccance et al., 1998; Gossop & Carroll, 2006; Grassi, Cioceb, Dei Giudicib, Antonillia et al., 2007), particularly in men (Nich, McCance-Katzb, Petrakis, Cubellsa et al., 2004; Petrakis, Carroll, Nich, Gordon et al., 2000).
Disulfiram originally was approved in 1951 for promoting abstinence from alcohol, but it has been used only modestly since its approval to treat alcohol dependence (Suh, Pettinati, Kampman, & O’Brien, 2006). While disulfiram has an appeal in the context of the present study because of its known action in deterring alcohol use, it was selected for the present study primarily as a treatment for reducing cocaine use. Disulfiram’s mechanism of action for reducing cocaine use is believed to be different from its mechanism for promoting abstinence from alcohol. Essentially, disulfiram’s effect on decreasing alcohol use is that it inhibits the enzyme, aldehyde dehydrogenase, which is necessary for fully metabolizing alcohol, leaving an increased concentration of acetaldehyde, which causes unpleasant sensations (Suh et al., 2006). Potentially, disulfiram’s mechanism of action for reducing cocaine use is that it inhibits dopamine beta-hydroxylase, an enzyme that normally converts dopamine to noradrenaline (Kosten, George, & Kosten, 2002; McCance-Katz, Kosten, & Jatlow, 1998a), and hence, increases the concentration of dopamine more than what is observed when cocaine is taken alone. Furthermore, disulfiram, when coupled with cocaine use, impedes cocaine metabolism by inhibiting plasma and microsomal carboxylesterases and plasma cholinesterase (McCance-Katz, Kosten, & Jatlow, 1998b). In some studies, the combination of disulfiram and cocaine have resulted in a higher than expected plasma-cocaine concentration, and a longer than expected cocaine-elimination half-life (Hameedi, Rosen, McCance-Katz, McMahon et al., 1995; McCance-Katz, Kosten, & Jatlow, 1998a). These effects of the disulfiram-cocaine interaction might result in an exacerbation of negative effects of cocaine, such as anxiety, paranoia, and cardiovascular response (McCance-Katz, Kosten, & Jatlow, 1998a, 1998b).
In a controlled clinical trial for treating cocaine dependence with disulfiram, Carroll and colleagues (Carroll et al., 2004) found that disulfiram significantly reduced cocaine use in patients with cocaine dependence. Of interest, however, disulfiram did not seem to provide the same advantage over placebo in reducing cocaine use in cocaine-dependent patients who regularly drank a substantial amount of alcohol. The results of this Carroll study, while encouraging for treating cocaine dependence, did not address how to treat the highly prevalent cocaine-dependent group with alcohol dependence.
With respect to the treatment in the present study for the patient’s alcohol dependence, the daily, oral version of naltrexone was selected to be given in combination with disulfiram. Naltrexone is an opioid receptor antagonist that has been found in a number of trials to reduce heavy drinking in alcoholics. (See review by Pettinati, O’Brien, Rabinowitz, Wortman et al., 2006). A preponderance of evidence from pre-clinical and clinical studies suggest that the therapeutic effect of naltrexone in alcoholism stems from its ability to attenuate alcohol-induced euphoria (O’Malley, Jaffe, Chang, Schottenfeld et al., 1992; Volpicelli, O’Brien, Alterman, & Hayashida, 1990) by inhibiting the release of beta-endorphin (Volpicelli, Watson, King, Sherman et al., 1995). Notably, naltrexone may also have direct therapeutic effects on cocaine use, independent of its effects on alcohol consumption, although to date, clinical trials have not proven its effectiveness in reducing cocaine use at the 50mg/day dose (Hersh, Van Kirk, & Kranzler, 1998; Schmitz et al., 2004). Furthermore, studies have failed to demonstrate that naltrexone has an effect on cocaine euphoria in humans (Sofuoglu, Singha, Kosten, McCance-Katz et al., 2003; Walsh, Sullivan, Preston, Garner et al., 1996). Nonetheless, pre-clinical studies have demonstrated that cocaine releases beta-endorphin (Beakeland, Lundwall, Kissin, & Shanahan, 1971), and that naltrexone and other opioid antagonists reduce rates of cocaine self-administration in animals (Paille, Guelfi, Perkins, Royer et al., 1995; Pelc, Verbanck, Le Bon, Gavrilovic et al., 1997; Sass, Soyka, Mann, & Zieglgansberger, 1996). Chronic exposure to both alcohol and cocaine result in dynorphin upregulation (Dackis & O’Brien, 2003), which may contribute to dopamine hypoactivity in both conditions (De Witte, Littleton, Parot, & Koob, 2005; Fuller & Gordis, 2004; Suh et al., 2006). This neuroadaptation may be reversed by naltrexone’s antagonism of kappa-opioid receptors, by which dynorphin inhibits dopamine activity. In summary, the present study evaluated in a controlled clinical trial the effect of treating cocaine-alcohol dependence with the combination of disulfiram and naltrexone added to twice weekly sessions of cognitive behavioral therapy.
2. Methods
2.1 Patients
The patients were 208 men and women between the ages of 18 and 65 who had a DSM-IV diagnosis of both cocaine and alcohol dependence. Patients with dependence on substances other than cocaine and alcohol, except nicotine addiction, were excluded. Patients needed to have used in the month before treatment a minimum of $100 worth of cocaine and drank an average of 12 standard alcoholic drinks a week. Psychiatric exclusion criteria included active psychosis, mania, dementia, or the need for treatment with psychiatric medications. Medical exclusion criteria included pregnancy, breastfeeding, active hepatitis, and significant hepatocellular injury. If clinically indicated, patients had to successfully complete outpatient alcohol detoxification, i.e., achieve 3 days of abstinence from alcohol, before starting study medication. The study was approved by the University of Pennsylvania’s Institutional Review Board, and all patients who were included in the study had signed informed consents prior to screening.
2.2 Procedures
Patients were treatment-seeking cocaine users recruited through community advertisements and professional referrals. Those patients who appeared to meet criteria in a telephone interview were invited to the Treatment Research Center (TRC) for a screening evaluation. Eligible patients entered a 13-week trial that included a 2-week pre-treatment/screening phase and an 11-week randomized medication phase.
During the 4 years of recruitment for this trial, 434 cocaine-alcohol dependent patients were invited to screen for this study. Of these, 342 (79%) signed consent and agreed to begin screening. There were 97 consented patients (28%) who never returned after signing consent for the first screening session. Of those remaining (245 consented patients), 37 (15%) were excluded during the screening period based on the specific eligibility criteria: 10 were excluded for medical reasons (e.g. taking disallowed medications, significantly elevated blood pressure or higher than 3.5 times normal liver function tests), 18 were excluded for not meeting cocaine and/or alcohol dependence diagnostic criteria, and 9 were excluded for disallowed psychiatric diagnoses (e.g. bipolar disorder or psychosis), or for concomitant opiate abuse or dependence. The remaining 208 patients entered the randomized medication phase of the trial.
2.2.1 Design
Randomized patients were assigned to 250mg/day of disulfiram (DISULF; n=53), 100mg/day of naltrexone (NTX; n = 52), the combination of disulfiram and naltrexone (DISULF+NTX; n=49), or to double placebo (PLAC; n=54). Patients received their first dose of randomized medications in the 3rd week of the study, following 2 weeks of screening. Patients were assigned for the first 2 days of the 11-week treatment phase to receive either 50 mg/day of naltrexone or placebo in the morning; and, then on the 3rd day of the treatment week, 100 mg/day of naltrexone or placebo in the morning. Patients were also assigned to receive 250 mg of disulfiram, starting in the first treatment week on the 5th day. In the 4th week of treatment, patients who tolerated the medications would be prescribed the maximum doses of 250 mg/day of disulfiram (or matching placebo) and 100 mg/day of naltrexone (or matching placebo). Patients were continued at these daily dosages of each medication or placebo through to end of 11 treatment weeks, or Week 13 since signing consent. In the last 2 weeks of the trial, both medications were tapered, and pharmacotherapy was completed by the end of Week 11 (Week 13 since signing consent). All patients received the same number of pills (active medication and/or placebo pills), regardless of group or week of treatment. Study medication was dispensed in clearly labeled blister cards at a once-a-week medical evaluation visit.
The disulfiram target dose of 250mg/day is the one that is recommended in its label. Naltrexone’s recommended daily dose is 50mg/day, but for this study we chose a target dose of 100mg/day because this higher daily dose will last longer in patients –better ensuring the medication’s action in patients if they tend to be medication nonadherent; also, our prior studies suggested that doses higher than 50mg/day may result in better outcomes in patients with the combination of cocaine and alcohol dependence (Oslin et al., 1999; HM Pettinati et al., 2007). In addition to medication or placebo pills, participants received twice-weekly individual cognitive-behavioral therapy (CBT) utilizing the CBT therapy manual and supporting materials that were developed for the National Institute on Alcohol Abuse and Alcoholism Project MATCH (Kadden, Litt, Cooney, & Busher, 1992). The basic format was accepted, although specific procedures were adapted for treatment of both cocaine and alcohol dependence by our group. CBT was provided by Master’slevel therapists with training in delivering CBT. Therapists received weekly supervision by a doctoral-level therapist with over 15 years experience in treating cocaine dependence. In addition, individual therapy sessions were audiotaped and a random sample of tapes from each therapist was listened to by the therapists’ supervisor to address any CBT drift.
2.2.2 Measures
Psychiatric diagnoses were obtained by master’s level clinicians using the Structured Clinical Interview for DSM–IV (SCID) (First, Spitzer, Gibbon, & Williams, 1995). Medical screening included a complete medical history and physical examination conducted by a certified nurse practitioner. Pre-treatment laboratory testing included a chemistry screen, bilirubin, complete blood count, urinalysis, urine pregnancy testing and a 12 lead EKG. Breathalyzer readings were collected at each visit throughout the study to ensure that research data were not collected from patients with a positive breathalyzer.
The primary in-trial outcome measure of daily alcohol use was the patient’s self-report collected via the Timeline Followback method (TLFB) (Sobell & Sobell, 1992). The TLFB is a 15–30 minute, semi-structured interview that uses the recall of critical life events and a personalized calendar to prompt recall of alcohol drinking frequency (days) and quantity (number of drinks per day) during the inquiry period (up to 90 days). The recalled information on alcohol use is recorded for each day on the personalized calendar. The TLFB method has been adapted by our laboratory, including the personalized calendar, to also collect information from the patient about daily use of cocaine or other abused drugs. Thus, a similar procedure was used in this study to recall daily use of cocaine, plus dollars worth of cocaine used per day. The TLFB was administered by a research technician, who had received training in administering the TLFB. The TLFB was given in screening to record use of alcohol and cocaine in the 3 months immediately preceding treatment entry. Use information was also collected for each day in treatment, updated at each research visit to create a continuous daily record of cocaine and alcohol use or abstinence in the 3 months prior to treatment and all during the treatment period. A 30-day alcohol and drug use self-report was also collected at a single follow-up visit that was scheduled for 6 months after the end of the trial to assess the patient’s status following treatment. Urine drug screen (UDS) tests were obtained 3 times weekly during the 2 screening weeks and the 11 weeks while in treatment.
Urine collection was not observed but urine specimen temperature was monitored. A urine specimen was also collected at a single follow-up visit that was scheduled for 6 months after the end of the trial to assess the patient’s status following treatment. Urine specimens with lower than 90 degrees, or greater than 100 degrees, Fahrenheit were not accepted. (Fewer than 1% urine specimens were outside this range). Specimens were analyzed for benzoylecgonine (BE) by fluorescent polarization assay. Specimens were analyzed qualitatively >/= 300 ng/ml of BE was considered drug-positive, indicating recent cocaine use.
Cocaine abstinence was determined for each week by 3 weekly cocaine negative urine samples. If any samples during a given study week were positive for BE or missing, the week was coded as non-abstinent. For alcohol, abstinence from alcohol for a given study week was determined by self-report derived from the TLFB method. Any alcohol use during a given study week resulted in that week being coded as a non-abstinent week.
In addition to examining cocaine and alcohol outcomes separately, we also chose to evaluate outcomes that reflected the combination of both alcohol and cocaine use. One of these outcomes involved creating an abstinence index that combined the separately constructed cocaine and alcohol use/non-use, and then repeating outcome analyses with this index as the primary outcome measure. A secondary analysis with this combined abstinence index compared the number of patients who were able to achieve 3 consecutive weeks of continuous abstinence from both alcohol and cocaine while in treatment. The latter measure has been used in a number of studies because it is thought to represent a primary goal of short-term treatment, which is to get patients to achieve a stable period of continuous abstinence. Abstinence from cocaine for approximately 3–4 consecutive weeks was been shown to be predictive of long-term cocaine abstinence (Carroll, Rounsaville, Gordon, Nich et al., 1994; Kosten, Morgan, Falcione, & Schottenfeld, 1992). We, therefore, evaluated the proportion of patients in each of the four treatment groups (DISULF, NTX, DISULF + NTX, PLAC) that were able to attain 3 consecutive weeks of abstinence from both cocaine and alcohol while in treatment.
Other measures included the Hamilton Rating Scales of Depression (Hamilton, 1960) and of Anxiety (Hamilton, 1959). They were administered at pre-treatment and at the end of treatment to measure the number of mood and anxiety symptoms. Adverse events were measured at each medical evaluation visit using the Systematic Assessment for Treatment Emergent Effects (Rabkin, Markowitz, Ocepek-Welikson, & Wager, 1992).
2.3 Statistical Analysis
The main analyses in this paper were by intention-to-treat. The two primary outcome measures were: 1) abstinence from cocaine; and 2) abstinence from alcohol. In addition, a combination measure of abstinence from both cocaine use (based on UDS) and alcohol use (based on TLFB) was also created for both primary and secondary analyses. Secondary outcomes included abstinence from heavy drinking (defined as drinking 5 or more standard drinks in one day for men and 4 or more for women). The patients were initially compared on a variety of demographic and pre-treatment characteristics, using logistic regression for categorical characteristics, and linear regression (ANOVA) for continuous characteristics, to assess how well the randomization had balanced patient characteristics across the four treatment groups.
Primary and secondary outcomes were analyzed using generalized estimating equations (GEE) models. The models included terms for use status during the pre-randomization phase, medication group (disulfiram present/absent; naltrexone present/absent), together with linear and quadratic time effects, and some group by time interactions. In fitting these models to the data, terms were included if they were significant at the 5% level, and lower order effects contained in a significant interaction effect were also included. Empirical (sandwich) standard errors were used to assess significance. Thus, the primary outcome analyses did not include additional covariates, but characteristics that showed significant imbalance across the groups were considered for inclusion as covariates in supplementary analyses.
With respect to the repeated binary outcomes of abstinence from cocaine, alcohol, and both, missing weeks were imputed as positive/use, which is a standard practice in clinical trials for which cocaine abstinence is a primary outcome (Shoptaw, Kintaudi, Charuvastra, & Ling, 2002). The protocol called for three urines to be collected per patient each week, yielding a possible 39 urines per patient, 6 during the 2-week pre-randomization phase, and 33 during the 11-week treatment phase. GEE approaches work best when the data are in the form of a smaller number of repeated measurements for each of many patients (Sharples & Breslow, 1992), so we chose to reduce the number of time points per patient by coding each of the 11 weeks as a use or non-use week, rather than including each urine per week in the model. Using weeks as the time units of analysis, rather than visits, also reduces the risk of overestimating drug use due to BE carryover. The urines submitted during the pre-randomization phase were used to determine use/non-use status prior to randomization, and this status was included as a covariate in the GEE model. To assess the sensitivity of the results to the imputation of missing urines/weeks as positive/use, we re-ran the analyses with missing observations ignored, and also performed pattern-mixture analyses. Our pattern-mixture analyses compared subjects that provided complete data versus data from those that prematurely left the study or provided only partial data (Hedeker & Gibbons, 1997; Park & Lee, 1999). Pattern-mixture models augment the GEE models described above by including a variable describing the pattern of missing data in the study as a main effect and as an interaction effect with the group and time variables. This allows us to model differences in longitudinal behavior across missing data patterns, and to assess whether there are group by missing-pattern interaction effects. In our models, we used a binary variable as an indicator of missing pattern, distinguishing between subjects who provided complete data and those who missed at least one visit. As each of these groups included at least two time points, this allowed us to examine the influence of missing pattern on rate of change over time. As the results of the “missing = use” and “missing = ignored” analyses were virtually identical, we report on the “missing = use” and pattern-mixture analyses only.
We also assessed the effects of medication adherence and medication exposure on the comparisons of medication groups. We used the number of pills taken over number of pills prescribed for an individual patient while in treatment (measure of medication adherence), and the number of pills taken over the maximum number of pills available to any given patient who completed the full 11 weeks of treatment (medication exposure). Beyond the intent-to-treat analysis, this study repeated the same sets of analyses for the subset of patients who took at least 80% of the medication while in treatment, as well as for those “exposed” to 80% of medication taken by completers. While these analyses are not bias-protected by the initial randomization, they provide a useful measure of whether overall medication effects are affected by nonadherence while in treatment, as well as by poor exposure to a full course of pharmacotherapy.
3. Results
3.1 Demographic and Pre-Treatment Alcohol and Cocaine Use
The average age of the patients for the N=208 sample was approximately 41 years old. Most were African American men (88.9%) and most smoked crack cocaine (78.9%). The mean number of years of education completed for the sample was 12.3 years. On average, patients had said they had in the month prior to treatment used cocaine 45.8% of the days, and drank alcohol on 56% days, with 48.8% heavy drinking days in the same pre-treatment month.
Overall, the four study groups, DISULF, NTX, DISULF-NTX, and PLAC were very similar in demographics and pre-treatment alcohol and cocaine use characteristics (see Table 1).
Table 1.
Patient characteristics and pretreatment measures of drug use, mood, and anxiety, expressed as percents or means (w/standard deviations).
| Variable | DISULF | NTX | DISULF+NTX | PLAC |
|---|---|---|---|---|
| N | 53 | 52 | 49 | 54 |
| Mean age | 42.1 (7.2) | 41.3 (6.8) | 40.3 (5.6) | 41.2 (7.5) |
| % Male | 67.9% | 75.0% | 67.3% | 70.4% |
| % African American | 88.7% | 86.5% | 87.8% | 92.6% |
| Mean years of education | 12.2 (2.6) | 12.2 (1.9) | 12.7 (1.7) | 12.1 (1.6) |
| Mean proportion of days employed in past 30 | 0.34 (0.3) | 0.33 (0.3) | 0.32 (0.3) | 0.27 (0.3) |
| % smoked route of cocaine use | 81.5% | 73.6% | 78.8% | 81.4% |
| Mean years cocaine use | 12.8 (6.3) | 13.8 (6.2) | 11.7 (6.8) | 14.4 (7.9) |
| Mean years alcohol problem use | 22.0 (7.9) | 22.6 (8.2) | 20.9 (7.7) | 20.7 (9.2) |
| % days cocaine use in past 30 | 45.5% (.25) | 48.8% (.24) | 45.9% (.28) | 43.0% (.25) |
| $ spent for cocaine in past 30 | $1153.5 (1680.6) | $1048.3 (1114.8) | $1081.6 (1401.8) | $1149.1 (1173.9) |
| % days alcohol use in past 30 | 55.3% (.28) | 56.8% (.25) | 54.1% (.28) | 57.0% (.25) |
| % days heavy alcohol use in past 30 | 49.4% (.25) | 47.0% (.24) | 49.1% (.29) | 49.7% (.25) |
| Mean drinks/drinking day in past 30 | 13.3 (10.9) | 12.7 (7.0) | 17.4 (19.0) | 14.3 (8.5) |
| Number of prior D&A treatments | 4.4 (8.3) | 3.2 (3.6) | 2.9 (2.8) | 3.8 (7.6) |
| Hamilton Anxiety Rating Scale | 7.8 (7.6) | 6.4 (6.4) | 9.6 (6.6) | 7.7 (6.7) |
| Hamilton Depression Rating Scale | 11.2 (10.8) | 8.9 (8.7) | 12.6 (8.9) | 9.4 (7.0) |
DISULF = disulfiram; NTX = naltrexone; PLAC = placebo; D&A = Drug and Alcohol
3.2 Treatment Attendance
Records of patient self-reported cocaine and alcohol use via the TLFB were complete for 85.1% of the sample. The patients on average attended 9.7 or 44% of a possible 22 CBT sessions, and attended at least one CBT session a week for a mean number of 6.2 or 56% of 11 weeks. There were no significant differences in either the mean number of CBT sessions or mean number of weeks where a CBT session was attended across the four groups. The percent of patients per treatment group who discontinued treatment before the end of the trial were: 22.6% DISULF, 32.7% NTX, 40.8% DISULF + NTX, and 40.7% PLAC. Chi-square tests showed no significant difference in discontinuation rates across the four groups (Pearson chi-square = 5.2, df = 3, p = 0.16).
3.3. Medication adherence while in treatment and medication exposure to full 11-week trial dosage
Medication adherence and exposure measures were based on pill counts, using returned blister cards and discussion with the patient at weekly intervals. Medication adherence was defined as the percentage of pills ingested based on the number prescribed while the patient was in the trial. Medication exposure is defined as the percentage of pills ingested based on the number of pills that comprised the “planned maximum dose”, i.e., the amount of pill taking expected in patients who completed 11 weeks of treatment and were 100% adherent over the 11-week trial.
There were 45.8% of patients who took 80% of their pills while in treatment, and this rate was comparable for all four treatment groups (chi square = 4.79, df = 3, p = 0.19), and there were also no differences in naltrexone versus disulfiram adherence rates in patients while in treatment.
Only 35.1% of the patients received 80% or more of the maximum possible number of disulfiram pills, compared to 58.7% of naltrexone, suggesting that patients received more exposure to naltrexone than disulfiram, although the difference in medication exposure rates between the two medications was not significant (chi square = 3.2; df = 3, p = 0.36). Nonetheless, the medication adherence and exposure rates indicated only a modest exposure to these medications in this trial, preventing us from conducting a meaningful post-hoc exploratory analyses of outcome results with only those patients who had either taken 80% of the medications while in the trial or had been exposed to at least 80% of the medications overall.
3.4 Primary and Secondary Outcome Analyses
Some key in-trial and end-of-trial variables representing cocaine and alcohol use, mood and anxiety are listed for informational purposes for all four groups in Table 2. Outcome analyses focused primarily on the urine drug screen results and self-reported cocaine and alcohol use, and these analyses are described below. As Table 2 shows, rates of self-reported abstinence for cocaine, any drinking, and heavy drinking, were high. For example, more than half of the placebo group reported no heavy drinking across the entire treatment period. Since the % days responses contained many zeroes, log transformations failed to adequately reduce skewness. As a result, we focused on monthly abstinence as our primary measure, rather than days of use.
Table 2.
In-trial continuous outcomes for cocaine and alcohol use, expressed as median percentages or medians (w/95% confidence intervals).
| DISULF | NTX | DISULF+NTX | PLAC | |
|---|---|---|---|---|
| N | 53 | 52 | 49 | 54 |
| In-Trial Outcomes | ||||
| % days cocaine use | 5.2% [2.6, 11.1] | 7.8% [2.9, 11.7] | 4.3% [1.3, 6.5] | 5.2% [2.6, 11.7] |
| % BE-negative urines (missing ignored) | 45.5% [27.3, 63.6] | 28.6% [9.1, 54.5] | 54.5% [40, 81.8] | 23.6% [0, 66.7] |
| % BE-negative urines (missing BE-positive) | 18.2% [9.1, 27.3] | 9.1% [0, 18.2] | 9.1% [0, 36.4] | 4.6% [0, 18.2] |
| $ spent for cocaine | $195 [50, 300] | $107.5 [50, 260] | $47.5 [20, 200] | $88.5 [45, 170] |
| % days alcohol use | 2.6% [1.3, 7.8] | 3.4% [1.3, 9.1] | 2.6% [1.3, 7.1] | 2.6% [0, 6.5] |
| % days heavy alcohol use | 0.6% [0, 2.6] | 0.6% [0, 3.9] | 1.3% [0, 3.9] | 0% [0, 1.3] |
| End-of-Tx Scale Scores | ||||
| Hamilton Anxiety Rating Scale | 2.0 [1.0, 4.0] | 3.0 [0.0, 6.0] | 5.0 [2.0, 7.0] | 3.5 [2.0, 8.0] |
| Hamilton Depression Rating Scale | 3.0 [1.0, 5.0] | 5.0 [3.0, 7.0] | 5.0 [2.0, 10.0] | 3.5 [2.0, 7.0] |
DISULF = disulfiram; NTX = naltrexone; PLAC = placebo; BE = benzoylecgonine
3.4.1 Cocaine Abstinence (Urine Drug Screens-UDS)
A full GEE model of the log-odds of missing and/or BE-positive urines showed no medication effects significant at the 5% level. All groups showed an increase in the odds of a missing and/or BE-positive urine across time, with the odds ratio increasing by a factor of 1.25 per week [p = 0.001; 95% CI =(1.09, 1.40)]. Patients who had provided BE-positive urines in the screening period had higher rates of missing and/or BE-positive urines during the treatment phase: OR = 5.21 [p < 0.001, 95% CI = (3.06, 8.88)].
3.4.2 Cocaine Abstinence (TLFB Self-report)
Of the 2,288 urine specimens expected to be collected during the treatment period, 1807 (78.9%) were actually collected. Of those collected, 1368 (75.7%) were consistent with self-report. Of the total collected urines, there were 358 (19.8%) urines that were positive for cocaine but patients denied cocaine use, and 81 (4.5%) urine specimens were found negative for cocaine, despite admission of cocaine use. Of the 481 missing urine specimens, 314 (65.2%) were self-reported by patients as positive for cocaine. These figures are comparable to previous studies using self-report data in cocaine dependent samples (Carroll et al., 2004; Hersh, Mulgrew, Van Kirk, & Kranzler, 1999; Zanis, McLellan, & Randall, 1994). This indicated that in this sample, self-reported cocaine use is relatively reliable.
A similar GEE model for self-reported cocaine use, with missing weeks regarded as use, showed a trend towards fewer patients using cocaine in the disulfiram groups [OR for use = 1.45, p = 0.06, 95% CI = (0.98, 2.16)]. However, the pattern of use across the four groups was about the same as in the UDS analyses. Patients who self-reported cocaine use in the screening period were significantly more likely to self-report cocaine use in the treatment phase (p < 0.001), but there were no significant time or medication effects.
3.4.3 Alcohol Abstinence (TLFB Self-report)
A similar GEE model for presence of any drinking showed linear and quadratic time trend interactions with the medication groups (p = 0.02 for the quadratic effect, and p = 0.04 for the linear effect). These interactions were due to the disulfiram only and naltrexone only groups showing faster initial rates of increase in drinking than the other two groups, followed by a leveling off later in the treatment phase. However, there were no significant medication group differences at any time point. A similar GEE model for presence of heavy drinking showed an overall increase in rates of alcohol use over time [OR = 1.13, p = 0.02, 95% CI = (1.02, 1.26)], but there were no significant effects for medication group.
3.4.4 Evaluation of Combined Abstinence from both Cocaine and Alcohol
A similar GEE model to what has just been described for evaluating separately cocaine and alcohol use during the trial was also performed for combined abstinence using a combination of UDS cocaine results and self-reported alcohol use. This analysis showed significantly more abstinence in patients taking disulfiram, OR = 1.64 [p = 0.04, 95% CI = (1.02, 2.65)], although abstinent days were not significantly different from the placebo group per se via post-hoc analyses. There were no significant time or naltrexone effects with the combined index of abstinence.
3.4.5 Pattern Mixture Models
We used a binary indicator of treatment completion as our pattern variable for these analyses, and included it as a main effect and interaction effect in the GEE models for the cocaine UDS, self-reported cocaine use, self-reported alcohol use, and combined cocaine/alcohol use analyses. None of the interactions between the treatment factors and the pattern variable were significant (all p-values > 0.3), suggesting that the effects of the medications did not differ across completers and non-completers. When the interaction terms were removed, and the pattern variable retained as a main effect only, we observed that disulfiram was associated with significantly lower rates of use as indexed by the cocaine UDS (p=0.02), cocaine self-report (p=0.04), and combined alcohol-cocaine use outcome variable (p=0.02). While interpretation of these results is complicated by the fact that we are considering a post-randomization variable, they provide support for the disulfiram effect observed in the “missing = use” urine analyses reported above.
3.4.6 Periods of Abstinence
A non-parametric approach to evaluating combined abstinence from both cocaine and alcohol during the trial compared the four treatment groups on the number of patients who achieved 3 consecutive weeks of continuous abstinence from both cocaine and alcohol while in treatment. This measure has been shown to be predictive of long-term cocaine abstinence in previous trials. In this trial, the ability to achieve 3 consecutive weeks of abstinence while in treatment was found to be a significant predictor of higher rates of abstinence from alcohol and cocaine during the trial and at the 6-month follow up visit (64% of patients attended the 6-month follow-up visit). For example, patients with 3 consecutive weeks of abstinence, compared to patients without 3 consecutive weeks of abstinence, submitted significantly more BE-negative urine specimens during the trial (24/33 or 73% vs 9/33 or 27% BE-negative specimens, respectively; t= −12.0, df=206, p < 0.001), reported a significantly lower percentage of drinking days during the trial (3.0% vs 10.3% days drinking, respectively; t = 4.7, df = 126, p < 0.001), and at the 6-month follow-up visit, were more likely to submit a BE-negative urine specimen (60% vs 30%, respectively; chi square = 13.4, df = 1, p = < 0.001), and self-report being abstinent from alcohol in the 30 days prior to their follow-up visit (17/43 (39.5%) vs 39/165 (23.6%), respectively; chi square = 4.4, df = 1, p = 0.04). Based on findings from previous trials, as well as data from this trial, the ability to achieve 3 weeks of continuous abstinence is a strong predictor of treatment response and long-term abstinence from both cocaine and alcohol.
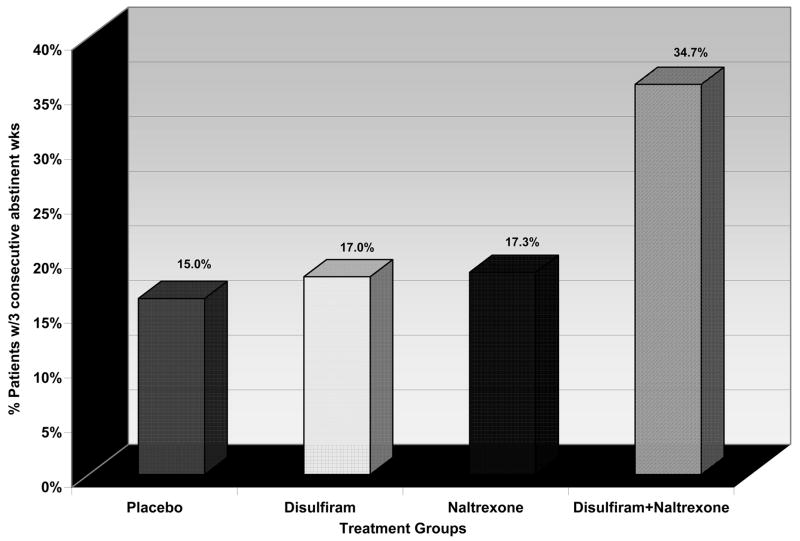
Figure 1 shows the proportion of patients in each of the four treatment groups who achieved 3 consecutive weeks of abstinence from both cocaine and alcohol during the trial. Significantly more patients treated with the combination of disulfiram and naltrexone achieved 3 consecutive weeks of abstinence from both cocaine and alcohol compared to the other 3 medication groups (DISULF+NTX combination = 34.7%, NTX = 17.3%, DISULF = 17.0%, PLAC = 15.0%; chi square = 7.8, df =3, p = .05).
Figure 1.
The percent of cocaine-alcohol dependent patients (via DSM-IV criteria) that achieved at least 3 consecutive weeks of abstinence from both cocaine and alcohol in an 11-week controlled clinical trial of four randomized groups, assigned to placebo, or 250mg/day of disulfiram, or 100mg/day of naltrexone, or the combination of these two medications at the dosages specified.
3.5 Adverse Events (AEs)
There were no participant deaths or serious medical conditions during the clinical trial. Adverse events or AEs ranged from mild to severe. The most frequently reported AEs were headache (64.4%), drowsiness (39.4%), anxiety/irritability (53.4%) and nausea (40.4%). Table 3 provides the prevalence of the most frequent AEs reported in this study (i.e., reported by 10% or more patients). Nausea and increased sexual desire were the only two events that differed across the groups. Differential reporting on these two events is predictable. With respect to nausea, the placebo group reported a lower frequency of nausea compared to the other three medication groups. With respect to increased sexual desire, the event is usually most salient in patients who become abstinent during treatment. Of note, the patient group with the highest frequency of reports of increased sexual desire was the group who were taking the combination of disulfiram and naltrexone. This also was the group who were most likely achieve 3 consecutive weeks of combined abstinence from both cocaine and alcohol while in treatment.
Table 3.
Patient percentages reporting frequent adverse events during treatment (frequent adverse events are those reported in 10% or more of patients).
| Adverse event | DISULF N=53 | NTX N=52 | DISULF+NTX N=49 | PLAC N=54 |
|---|---|---|---|---|
| Headache | 68% | 63% | 73% | 54% |
| Drowsiness | 42% | 40% | 49% | 28% |
| Anxiety/irritability | 55% | 46% | 61% | 52% |
| Nausea** | 42% | 38% | 57% | 26% |
| Upper respiratory tract infection | 45% | 38% | 43% | 50% |
| Decreased sexual desire | 32% | 29% | 33% | 28% |
| Increased sexual desire* | 11% | 19% | 33% | 28% |
| Vomiting | 17% | 23% | 29% | 19% |
| Skin rash | 15% | 8% | 10% | 9% |
| Difficulty achieving orgasm | 17% | 13% | 14% | 9% |
| Toothache | 15% | 21% | 10% | 11% |
| Diarrhea | 13% | 8% | 8% | 12% |
DISULF = disulfiram; NTX = naltrexone; PLAC = placebo
p = 0.05;
p = 0.01
4. Discussion
Co-occurring cocaine and alcohol dependence is highly prevalent in the United States and is also very difficult to treat successfully. Adding pharmacotherapies that target one or both of these dependencies to an accepted counseling/psychosocial treatment for cocaine and alcohol dependence, i.e., CBT, was evaluated in this double blind, placebo-controlled study combining disulfiram and naltrexone as a treatment regimen for attaining abstinence in cocaine and/or alcohol use. Disulfiram and naltrexone have both been approved by the FDA for alcohol dependence, with disulfiram also being shown to reduce cocaine use in cocaine dependent patients (Carroll et al., 2004). Thus, this trial evaluated the use of 100mg/day naltrexone, or 250mg/day disulfiram, or their combination, or placebo, with twice weekly CBT for 11 weeks in patients with co-occurring cocaine and alcohol dependence.
Using GEE analyses, in-trial reductions in cocaine and, independently, alcohol use occurred but were not significantly different on continuous outcome measures across the four study groups treated with either naltrexone or disulfiram or both or neither (placebo). Patients who had provided BE-positive urines in the screening period had higher rates of missing and/or BE-positive urines during treatment. In addition, patients who reported higher rates of heavy drinking in the pre-treatment period had higher rates of alcohol drinking during treatment. There was a finding that the patients from the two disulfiram groups tended to have greater combined abstinence from cocaine and alcohol during treatment.
Using a non-parametric approach, which has been associated with clinical significance, we found that significantly more patients treated with the combination of disulfiram and naltrexone achieved 3 consecutive weeks of continuous abstinence from both cocaine and alcohol while in treatment, compared to the other 3 treatment groups. The achievement of 3 consecutive weeks of abstinence from both cocaine and alcohol was also shown in this study to be a significant predictor of more abstinence during the trial and at a 6-month follow-up visit.
The apparent discrepancy in results between the GEE analyses and a non-parametric approach that targets 3 consecutive weeks of abstinence may indicate a difficulty in the sensitivity of traditional outcome measures to target levels of treatment response in the patient groups who traditionally include many patients who are treatment nonadherent. Essentially, the patients in this study demonstrated less than optimal medication adherence while in treatment (< 50% took 80% of their medications), and also the majority of patients went to fewer than 50% of their CBT sessions. Treatment nonadherence was comparable across the four study groups.
Taking fewer pills than prescribed for the full treatment period has also been consistently reported in alcohol dependent populations for both disulfiram (Fuller, Rieckmann, McCarty, Smith et al., 2005) and naltrexone (Pettinati et al., 2006; Volpicelli, Rhines, Rhines, Volpicelli et al., 1997). While typically disulfiram has had much higher nonadherence problems than naltrexone (De Witte et al., 2005; Fuller & Gordis, 2004; Suh et al., 2006), in this study, the medication adherence rates for patients taking 80% of their medications while in treatment were found to be similar for the two medications, although there was a nonsignificant difference that indicated patients overall ingested more naltrexone than disulfiram (medication exposure rates). Still, medication exposure to either medication was modest at best, suggesting that the majority of the patients took less than 80% of the targeted 11-week course of the expected medication regimen. Because patients with both cocaine and alcohol dependence tend to have high treatment nonadherence rates, it is possible that the high nonadherence and poor medication exposure rates were attributable to the patient population and not the medications selected.
Also, there were no serious adverse effects. General adverse effects were in the mild to moderate range and typical for these medications. No patient was discontinued or said they would discontinue treatment because of a physical complaint that was reported as an adverse event.
Limitations to this study are that patients in this study agreed to participate in a research clinical trial and, therefore, may be different from clinical patients who are not asked to meet research eligibility criteria. In addition, the high levels of patient nonadherence to treatment regimens may have weakened the analysis to provide statistically confident results.
In summary, the lack of a consistent effect in the GEE outcome analyses using continuous outcome measures suggested that these treatments alone or in combination are unlikely to be robust enough to generalize an advantage to most patients who enter treatment with both cocaine and alcohol dependence. On the other hand, nonparametric analyses that were used in this trial suggested that some of the patients in this trial benefited from the combination of 250mg/day of disulfiram plus 100mg/day of naltrexone for 11 weeks in that increases in combined abstinence from cocaine and alcohol could be observed in over 30% of the patients who received both active medications. Patient adherence to medication regimens were generally poor, and nonadherence likely contributed to the inconsistency in results. Clinically, we would need to identify the patients whom we think will adhere to taking medications before initiating either or both of these medications. This study provides some modest evidence that treating co-occurring cocaine and alcohol dependence with a combination of disulfiram and naltrexone may be beneficial for some patients.
Acknowledgments
The work was supported by grants from the National Institute on Drug Abuse (P60 DA05186 to Dr. O’Brien; P50 DA12756 to Dr. Pettinati). Dupont Pharmaceuticals generously donated naltrexone and matching placebo.
We thank Thea Gallis, Thomas Whittingham, William Dundon, and Donna Giles for their technical assistance.
Footnotes
A portion of this paper was presented in a symposium conducted at the annual meeting of the College on Problems of Drug Dependence, Orlando, Florida.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Daoud N, Johnson BA, Prihoda TJ, Hargita ID. Combining ondansetron and naltrexone reduces craving among biologically predisposed alcoholics: preliminary clinical evidence. Psychopharmacology. 2001;154(1):23–27. doi: 10.1007/s002130000607. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Journal of the American Medical Association. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Beakeland F, Lundwall L, Kissin B, Shanahan T. The correlates of outcome in disulfiram treatment of alcoholism. Journal of Nervous and Mental Disease. 1971;53:1–9. doi: 10.1097/00005053-197107000-00001. [DOI] [PubMed] [Google Scholar]
- Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: a controlled study. Alcoholism: Clinical & Experimental Research. 1998;22(3):573–579. doi: 10.1111/j.1530-0277.1998.tb04295.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne S, Randall CL, Adinoff B, Malcolm R. Features of cocaine dependence with concurrent alcohol abuse. Drug & Alcohol Dependence. 1995;39(1):69–71. doi: 10.1016/0376-8716(95)01128-l. [DOI] [PubMed] [Google Scholar]
- Carroll K, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Archives of General Psychiatry. 2004;61(3):264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Nich C, Ball S, McCance E, Frankforter T, Rounsaville B. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction. 2000;95(9):1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- Carroll K, Nich C, Ball S, Mccance E, Rounsaville B. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll K, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. Journal of Studies on Alcohol. 1993;54(2):199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- Carroll K, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Archives of General Psychiatry. 1994;51(3):177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Dackis C, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. Glutamatergic agents for cocaine dependence. Annals of the New York Academy of Sciences. 2003;1003:328–345. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19(6):517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM IV Axis I Disorders -Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute, New York: Biometrics Research Department; 1995. [Google Scholar]
- Flannery BA, Morgenstern J, McKay J, Wechsberg WM, Litten RZ. Co-occurring alcohol and cocaine dependence: recent findings from clinical and field studies. Alcoholism: Clinical & Experimental Research. 2004;28(6):976–981. doi: 10.1097/01.alc.0000128232.30331.65. [DOI] [PubMed] [Google Scholar]
- Fuller R, Gordis E. Does disulfiram have a role in alcoholism treatment today. Addiction. 2004;99:21–24. doi: 10.1111/j.1360-0443.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- Fuller R, Rieckmann T, McCarty D, Smith KW, Levine H. Adoption of naltrexone to treat alcohol dependence. Journal of Substance Abuse Treatment. 2005;28(3):273–280. doi: 10.1016/j.jsat.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Gossop M, Carroll K. Disulfiram, cocaine, and alcohol: Two outcomes for the price of one? Alcohol & Alcoholism. 2006;41(2):119–120. doi: 10.1093/alcalc/agl003. [DOI] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G. Concurrent use and order of use of cocaine and alcohol: behavioural differences between users of crack cocaine and cocaine powder. Addiction. 2006a;101(9):1292–1298. doi: 10.1111/j.1360-0443.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G. Concurrent use of alcohol and cocaine: differences in patterns of use and problems among users of crack cocaine and cocaine powder. Alcohol & Alcoholism. 2006b;41(2):121–125. doi: 10.1093/alcalc/agh260. [DOI] [PubMed] [Google Scholar]
- Grassi M, Cioceb A, Dei Giudicib F, Antonillia L, Nencinia P. Short-term efficacy of Disulfiram or Naltrexone in reducing positive urinalysis for both cocaine and cocaethylene in cocaine abusers: A pilot study. Pharmacological Research. 2007;55(2):117–121. doi: 10.1016/j.phrs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biological Psychiatry. 1995;37(8):560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Psychiatry. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Everhart ET, Mendelson J, Jones RT. The pharmacology of cocaethylene in humans following cocaine and ethanol administration. Drug & Alcohol Dependence. 2003;72(2):169–182. doi: 10.1016/s0376-8716(03)00200-x. [DOI] [PubMed] [Google Scholar]
- Hearn WL, Rose S, Wagner J, Ciarleglio A, Mash DC. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacology, Biochemistry & Behavior. 1991;39(2):531–533. doi: 10.1016/0091-3057(91)90222-n. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of Random-Effects Pattern-Mixture Models for Missing Data in Longitudinal Studies. Psychological Methods. 1997;2(1):64–78. [Google Scholar]
- Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. Journal of Studies on Alcohol. 2001;62(1):14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- Hersh D, Mulgrew CL, Van Kirk J, Kranzler HR. The validity of self-reported cocaine use in two groups of cocaine abusers. Journal of Consulting & Clinical Psychology. 1999;67(1):37–42. doi: 10.1037//0022-006x.67.1.37. [DOI] [PubMed] [Google Scholar]
- Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology. 1998;139(1–2):44–52. doi: 10.1007/s002130050688. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Cooney NL, Busher DA. Relationship between role-play measures of coping skills and alcoholism treatment outcome. Addictive Behaviors. 1992;17(5):425–437. doi: 10.1016/0306-4603(92)90003-e. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Dackis C, Lynch KG, Pettinati H, Tirado C, Gariti P, Sparkman T, Atzram M, O’Brien CP. A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug & Alcohol Dependence. 2006;85(2):129–137. doi: 10.1016/j.drugalcdep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug & Alcohol Dependence. 2004;75(3):233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Wiedemann K. Combined therapy: what does acamprosate and naltrexone combination tell us? Alcohol & Alcoholism. 2004;39(6):542–547. doi: 10.1093/alcalc/agh093. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opinion on Investigational Drugs. 2002;11(4):491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Morgan CM, Falcione J, Schottenfeld RS. Pharmacotherapy for cocaine-abusing methadone-maintained patients using amantadine or desipramine. Archives of General Psychiatry. 1992;49(11):894–898. doi: 10.1001/archpsyc.1992.01820110058009. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biological Psychiatry. 1998a;43(7):540–543. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug & Alcohol Dependence. 1998b;52(1):27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Mengis MM, Maude-Griffin PM, Delucchi K, Hall SM. Alcohol use affects the outcome of treatment for cocaine abuse. American Journal on Addictions. 2002;11(3):219–227. doi: 10.1080/10550490290087992. [DOI] [PubMed] [Google Scholar]
- Nich C, McCance-Katzb E, Petrakis I, Cubellsa J, Rounsaville B, Carroll K. Sex differences in cocaine-dependent individuals’ response to disulfiram treatment. Addictive Behaviors. 2004;29(6):1123–1128. doi: 10.1016/j.addbeh.2004.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Archives of General Psychiatry. 1992;49(11):881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Pettinati HM, Volpicelli JR, Wolf AL, Kampman KM, O’Brien CP. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. Journal of Substance Abuse Treatment. 1999;16(2):163–167. doi: 10.1016/s0740-5472(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30(2):239–247. [PubMed] [Google Scholar]
- Park T, Lee SY. Simple pattern-mixture models for longitudinal data with missing observations: analysis of urinary incontinence data. Statistics in Medicine. 1999;18(21):2933–2941. doi: 10.1002/(sici)1097-0258(19991115)18:21<2933::aid-sim233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. British Journal of Psychiatry. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- Pennings EJM, Leccese AP, Wolff FAd. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97(7):773–783. doi: 10.1046/j.1360-0443.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Carroll K, Nich C, Gordon L, McCance-Katz E, Frankforter T, Rounsaville B. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95(2):219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B, Group VANEVIMS. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry. 2005;57(10):1128–1137. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Kampman K, Lynch K, Suh J, Dackis C, Oslin D, O’Brien C. Gender Differences with High Dose Naltrexone in Cocaine and Alcohol Dependent Patients. Journal of Substance Abuse Treatment. 2007 doi: 10.1016/j.jsat.2007.05.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati H, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. Journal of Clinical Psychopharmacology. 2006;26(6):610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Markowitz JS, Ocepek-Welikson K, Wager SS. General versus systematic inquiry about emergent clinical events with SAFTEE: Implications for clinical research. Journal of Clinical Psychopharmacology. 1992;12:3–10. doi: 10.1097/00001573-199202000-00002. [DOI] [PubMed] [Google Scholar]
- Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Archives of General Psychiatry. 1996;53(8):673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J. Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. American Journal on Addictions. 2004;13(4):333–341. doi: 10.1080/10550490490480982. [DOI] [PubMed] [Google Scholar]
- Sharples K, Breslow N. Regression analysis of correlated binary data: some small sample results for the estimating equation approach. Journal of Statistical Computation and Simulation. 1992;42:1–20. [Google Scholar]
- Shoptaw S, Kintaudi PC, Charuvastra C, Ling W. A screening trial of amantadine as a medication for cocaine dependence. Drug & Alcohol Dependence. 2002;66(3):217–224. doi: 10.1016/s0376-8716(01)00205-8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Allen J, editor. Measuring Alcohol Consumption. Totowa, NJ: Humana Press Inc; 1992. pp. 41–65. [Google Scholar]
- Sofuoglu M, Singha A, Kosten TR, McCance-Katz FE, Petrakis I, Oliveto A. Effects of naltrexone and isradipine, alone or in combination, on cocaine responses in humans. Pharmacology Biochemistry and Behavior. 2003;75(4):801–808. doi: 10.1016/s0091-3057(03)00157-6. [DOI] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: a half of a century later. Journal of Clinical Psychopharmacology. 2006;26(3):290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. American Journal of Psychiatry. 2005;162(8):1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, O’Brien CP, Alterman AI, Hayashida M. Naltrexone and the treatment of alcohol dependence: initial observations. In: RL, editor. Opiods, Bulimia, and Alcohol Abuse & Alcoholism. New York, NY: Springer-Verlag; 1990. pp. 195–214. [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance.[see comment] Archives of General Psychiatry. 1997;54(8):737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. American Journal of Psychiatry. 1995;152(4):613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. The Journal of Pharmacology and Experimental Therapeutics. 1996;279(2):524–538. [PubMed] [Google Scholar]
- Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug & Alcohol Dependence. 1994;35(2):127–132. doi: 10.1016/0376-8716(94)90119-8. [DOI] [PubMed] [Google Scholar]