Abstract
Notch receptors are expressed in neurons and glia in the adult nervous system, but why this expression persists is not well-understood. Here we examine the role of the Notch pathway in the postnatal mouse main olfactory system, and show evidence consistent with a model where Notch2 is required for maintaining sustentacular cell function. In the absence of Notch2, the laminar nature of these glial-like cells is disrupted. Hes1, Hey1, and Six1, which are downstream effectors of the Notch pathway, are down-regulated, and cytochrome P450 and Glutathione S-transferase (GST) expression by sustentacular cells is reduced. Functional levels of GST activity are also reduced. These disruptions are associated with increased olfactory sensory neuron degeneration. Surprisingly, expression of Notch3 is also down-regulated. This suggests the existence of a feedback loop where expression of Notch3 is initially independent of Notch2, but requires Notch2 for maintained expression. While the Notch pathway has previously been shown to be important for promoting gliogenesis during development, this is the first demonstration that the persistent expression of Notch receptors is required for maintaining glial function in adult.
Keywords: Notch, glia, olfactory, sustentacular, neurodegeneration, Hes1, Hey1, Six1, Notch2, GST
Introduction
The Notch pathway is involved in a wide array of cell fate decisions during development (Louvi and Artavanis-Tsakonas, 2006). However, Notch receptors are also expressed in the adult in astrocytes, Müller glia, olfactory ensheathing glia, and Bergmann glia (Carson et al., 2006; Furukawa et al., 2000; Givogri et al., 2006; Higuchi et al., 1995; Irvin et al., 2001; Tanaka et al., 1999). Although some of these glia are likely to represent progenitor populations (Gaiano and Fishell, 2002), why Notch receptors continue to be maintained within apparently committed, mature glia is not known.
One hypothesis would be that Notch receptor expression is required to prevent a change in cell fate. Experiments in fly (Fortini et al., 1993) and in frog (Coffman et al., 1993) show that transient expression of Notch only temporarily alters cell fate. In postmitotic retinal neurons, misexpression of Notch1 can cause a subset of these neurons to become Müller glia (Jadhav et al., 2006). Thus, expression of Notch receptors even in differentiated cell-types can alter cell fate, suggesting that maintained expression of Notch receptors may be required to permanently establish fate, at least in some cell-types. On the other hand, activation of the Notch pathway in neural crest stem cells initiates an irreversible switch from neurogenesis to gliogenesis (Morrison et al., 2000). Similarly, in the CNS, brief induction of Notch1 or Notch3 signaling can irreversibly induce astrocyte differentiation (Tanigaki et al., 2001). These experiments suggest that transient activation of the Notch pathway is sufficient to promote gliogenesis.
An alternative hypothesis to explain the persistent expression of Notch receptors in adult would be that the Notch pathway acts to maintain function in adult glia. Notch1 and Notch2 are expressed in adult neurons (Sestan et al., 1999). Although studies on adult neuronal Notch function are rare, these studies indicate that the Notch pathway is employed in the adult in a manner distinct from its role during development. In adult hippocampal neurons, for example, reduced Notch1 expression impairs long-term potentiation (Wang et al., 2004). Notch receptors in the cortex are thought to influence neurite outgrowth (Sestan et al., 1999). Thus, the Notch pathway acts in the adult to influence and maintain neuronal function. But why Notch receptors are expressed in adult glia has yet to be examined.
Here we have examined the function of Notch2 in the postnatal mouse main olfactory epithelium. The nasal epithelium is a pseudostratified epithelium containing four major cell-types separated into distinct layers (Farbman, 1992). Sustentacular cells are located apically, with olfactory sensory neurons (OSNs) basal to these cells. Deep to the OSNs lie the progenitor cells of the epithelium, which can be divided into globose and horizontal cell-types. Although olfaction is mediated by receptors expressed by OSNs, the OSNs themselves are supported by sustentacular cells, which perform a number of glial-like functions (Nomura et al., 2004; Vogalis et al., 2005; Weiler and Farbman, 1998). For example, sustentacular cells express high levels of cytochrome P450 isoforms (Gu et al., 1998) as well as Glutathione S-transferases (GSTs; (Whitby-Logan et al., 2004)). Expression of these enzymes is consistent with sustentacular cells acting in a neuroprotective manner (Ling et al., 2004).
We show that Notch2 and Notch3 expression persists in postnatal and adult animals within sustentacular cells. We use a conditional mutant of Notch2 to show that the canonical Notch pathway is important for maintaining sustentacular cell function. In the absence of Notch2, the laminar organization of the epithelium is disrupted. Expression of the transcription factors Hes1, Hey1, and Six1, which are downstream effectors in the Notch pathway, are down-regulated in sustentacular cells. Morever, expression of cytochrome P450 isoforms and GST enzymes in the epithelia are reduced. Functional levels of GST activity are also reduced. These changes in the sustentacular layer are accompanied by neurodegeneration of OSNs, consistent with the interpretation that the glial-like function of sustentacular cells has been affected. This work therefore provides evidence that, in addition to its role during development in promoting gliogenesis, the Notch pathway is required for maintaining the function of differentiated glia in the adult. Finally, we show that Notch3 expression in Notch2 mutants is down-regulated, suggesting that maintenance of Notch3 expression also requires Notch2.
Materials and methods
Mice
All animal protocols were approved by Cornell's IACUC. Notch2 mutant mice were generated as previously described (McCright et al., 2006) and are maintained in a mixed 129Sv/C57BL/6 background. Notch2flox/flox (N2flox/flox) animals were crossed to Foxg1-Cre mice (Hebert and McConnell, 2000), also maintained in a mixed 129Sv/C57BL/6 background, to generate F1 N2flox/+;Foxg1Cre/+ animals. These mice were crossed to N2flox/flox animals to generate the mutant N2flox/flox;Foxg1Cre/+ animals and controls (N2flox/+;Foxg1Cre/+ and N2flox/flox). Within-litter, sex matched comparisons were performed for all studies. The day a vaginal plug was observed was termed day 0.5.
In situ hybridization
P0, 2.5 week-old, and adult (8-19 weeks) mice were euthanized and decapitated. Heads were embedded in OCT (TissueTek; Sakura-FineTek) and fresh-frozen in liquid nitrogen cooled isopentane. 10-20μm thick cryosections were collected and processed for single and double-label in situ hybridization as previously described (Williams et al., 2007). Probes used were cloned by PCR or purchased from clonesets. Probes corresponded to the following regions for each gene: Notch1 (nt 7819-9029), Notch2 (nt 594-1653), Notch3 (nt 519-1369 and 7038-7943), Hes1 (nt 234-1379), Hes5 (nt 15-1271), Scg10 (BMAP clone 30G10), Mash1 (nt 664-1810), OMP (IMAGE clone IRAKp961I03127Q), Hey1 (nt 781-1288), Six1 (NIA 7.4k clone H4070G06). Sections were hybridized at 62-70°C.
TUNEL
10μm sections were fixed and rinsed with PBS before incubating in pre-cooled ethanol:glacial acetic acid (2:1) for 5 min. at −20°C. After rinsing with PBS, endogenous peroxidase activity was quenched by incubating in 3% hydrogen peroxide for 10 min. at room temperature. After further PBS washes, slides were incubated with equilibration buffer (Chemicon) for 10 minutes and then incubated with terminal transferase (New England Biolabs (NEB)) in incubation buffer (1x CoCl2 (NEB), 1x restriction buffer 4 (NEB), 0.5 nM biotin-dUTP (Roche)) for 3.5 hrs at 37°C. Reactions were stopped with stop buffer (Chemicon), rinsed with PBS, incubated with streptavidin-HRP (Zymed) and reacted using the AEC staining protocol (Zymed).
Mash1 and TUNEL Morphometry
Sections from matched littermates were serially photographed. Number of positive signals/mm were obtained by drawing a line between positive signals, counting the number of positive spots within this range, and obtaining an average. The spots that were connected was arbitrarily chosen, but selected so as not to have extreme distances between spots. To compensate for the arbitrary nature of the selection, between 5-26 mm (Mash1) and 3-18 mm (TUNEL) of linear distance were counted and averaged per animal. Cells were binned into basal, neuronal, and apical layers based upon their relative location within the epithelium. Cells were only defined as apical if they were clearly adjacent to the lumen. Cells were only defined as basal if they were within 2-3 cell diameters of the lamina propria.
RNA isolation
Whole epithelia were dissected out and flash-frozen in liquid nitrogen. Total RNA was purified using Trizol reagent as per manufacturer's instructions (Invitrogen).
RT-PCR
5μg of total RNA was primed with oligo-dT and reverse transcribed using Superscript III (Invitrogen). The product was precipitated and 1/100th of the reaction was used per PCR reaction. A control RNA sample containing no reverse transcriptase was performed for all three RNA samples. PCR primers for Notch2 were identical to those described previously (Carson et al., 2006). We found that the Notch3 sequences described in this paper overlapped with a Notch pseudogene. We designed new primers for Notch3 with the following sequences: AAGGTGGAAAGTGCATAGACAAG and ATCTTGTAGGCAGTCCCGAGTAT to produce a product of 506 bp.
Northern Blot
10μg of total RNA from P0, 2.5 week, and 3 month old adult epithelia was electrophoresed through an agarose gel containing 2% formaldehyde in Hepes buffer. The RNA was transferred to a nylon-backed membrane and the filter hybridized in Church buffer at 65°C (Church and Gilbert, 1984). After washing, membranes were exposed to Biomax MS film (Kodak).
Immunohistochemistry
Epithelia from adult matched Notch2 mutants and wild-type controls were fixed in Bouin's fixative (LabChem) overnight at room temperature and washed in 70% ethanol. Samples were embedded in wax and 5μm sections were deparaffinized and processed as described previously (Carson et al., 2006) except samples were microwaved in citric acid. NOTCH3 antibody (Santa Cruz Biotechnology #sc-5593) at a dilution of 1:50 was applied overnight at 4°C, and bound antibody was detected using a FITC goat anti-rabbit secondary (Vector Labs) and an Alexa488 anti-FITC tertiary antibody (Molecular Probes). Sections were imaged on a Leica DMRE upright microscope fitted with bandpass filters.
Histology
Samples were embedded in wax and processed for histology by the Cornell Diagnostic Laboratory as described (Luna, 1992; Luna et al., 1968; Preece, 1972).
Scanning electron microscopy
Samples were fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate (pH 7.4) at 4°C for at least 24 hours. Samples were then treated in 30% potassium hydroxide as described (Nomura et al., 2004), washed in 0.1M sodium cacodylate, fixed overnight in 1% osmium tetroxide (0.1M sodium cacodylate; Electron Microscopy Sciences), washed, and dehydrated through an ethanol gradient containing 70% ethanol with 2% uranyl acetate. Samples were dried in a critical point dryer (Bal-Tec), cracked, and sputter-coated in Au/Pd for 2 min. (Bal-Tec). Samples were viewed on a Hitachi S4500 SEM at an accelerating voltage of 10kV.
DAPI
Cryostat sections were fixed in buffered 4% paraformaldehyde, stained (15 min. at 1 μg/ml in 1x PBS; Roche), washed, and mounted in 90% glycerol containing 0.5% npropyl gallate and 20 mM Tris pH 8.0.
Quantitative RT-PCR
qPCR was performed using Universal ProbeLibrary (UPL; Roche) reagents, and appropriate primers were designed using the Roche Universal ProbeLibrary Assay Design website (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp). Each RNA sample was tested in triplicate and the data normalized using rodent GAPDH (Applied Biosystems) as an endogenous control.
| Gene | Left primer | Right primer | UPL# |
|---|---|---|---|
| Cyp1a2 | gactgactcccacaactctgc | gaacgccatctgtaccactg | 19 |
| Cyp2a5 | accaaggacaccaagtttcg | agagcccagcataggaaaca | 52 |
| Cyp2g1 | tgatgccacatttcagtcct | ccttggaccgaagtacacagt | 21 |
| GST mu1 | gcagctcatcatgctctgtta | ttttctcagggatggtcttca | 106 |
| GST mu2 | agttggccatggtttgctac | agcttcatcttctcagggagac | 106 |
GST assay
Dissected epithelia were snap frozen in liquid nitrogen before being homogenized in a solution of 10% glycerol, 1 mM EDTA, 0.1M DTT, 1 mM PMSF (Sigma), and 1 mM PTU (Sigma) using a TissueLyser (Qiagen). Homogenates were centrifuged for 20 min. at 10,000g and the supernatant quantified with a BCA protein assay (Pierce). GST activity toward 1-chloro-2,4-dinitrobenzene (CDNB; Sigma), a generic substrate, was determined spectrophotometrically as previously described (Habig and Jakoby, 1981; Whitby-Logan et al., 2004) using 10μ□per assay and GSH (Sigma) at 2.5 mM. Change in absorbance at 340 nm was recorded at 30 second intervals for at least 5 minutes. Each reaction was performed in duplicate or triplicate. Statistical comparison of mutant and control slopes was performed using the JMP statistical package (SAS Institute).
Results
Expression of Notch receptors in the postnatal main olfactory epithelium
We examined the expression of Notch1-4 using in situ hybridization. Although Notch receptors have been previously examined for expression in the olfactory system, the various studies have not been entirely consistent with one another. Notch1 is found in the basal epithelium by Orita et al. (Orita et al., 2006), Mitsiadis et al. (Mitsiadis et al., 2001), Doi et al. (Doi et al., 2004), and Lindsell et al. (Lindsell et al., 1996), but not by Carson et al. (Carson et al., 2006). Doi et al. do not observe any Notch2 expression at all within the epithelium, whereas Carson et al., Mitsiadis et al., and Lindsell et al. show Notch2 expression within the sustentacular layer. Finally, Notch3 is not detected in the sustentacular layer by Carson et al., but is by Mitsiadis et al. However, Doi et al. detected Notch3 in the basal OE at postnatal stages.
Given the variation in described expression patterns for the Notch receptors, we reexamined the expression patterns for the four receptors during postnatal stages using in situ hybridization. We find Notch1 is expressed from P0 to adult in the basal epithelium, consistent with Orita et al. but not Carson et al. Clusters of Notch1 expressing cells are observed at all stages (Fig. 1A-C), although this expression is significantly reduced by adulthood (defined as 8 weeks and older). Interestingly, expression of Notch1 appears to be restricted to the dorsal recess (data not shown). In contrast, we found that Notch2 is apically expressed within the sustentacular layer at all stages (Fig. 1D-F), consistent with Carson et al. but not Doi et al. We found that Notch3 expression is present in the sustentacular layer from P0 through adulthood (Figs 1G-I). Both Notch2 and Notch3 are also expressed in the lamina propria (data not shown; (Carson et al., 2006)). Notch4 expression is only found within the lamina and not within the epithelium itself (data not shown).
Figure 1. Expression of Notch receptors during postnatal stages.
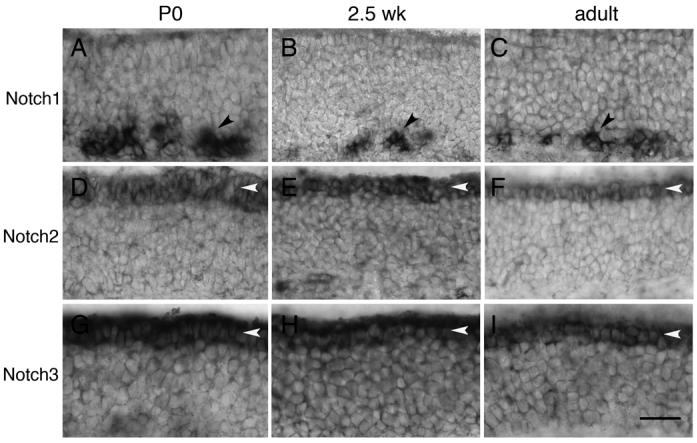
A-C) Notch1 is expressed in the basal epithelium (black arrowheads) by a subset of cells located primarily in the dorsal recess. This expression is present at P0, 2.5 weeks, and in adult (8-19 weeks). D-F) Notch2 is expressed by sustentacular cells (white arrowheads) at P0, 2.5 weeks, and adult. Expression is also detected in the lamina propria (data not shown). G-I) Notch3 is expressed by sustentacular cells (white arrowheads) at P0, 2.5 weeks, and adult. Expression is also detected in the lamina propria (data not shown). Scale bar=40μm.
These data suggested that Notch2 and Notch3 are co-expressed within sustentacular cells. To more closely examine this expression, we performed a series of double-label in situ hybridization experiments on adult epithelia. To first confirm that expression does occur within sustentacular cells, we used a known marker of sustentacular cells, Carbonyl reductase 2 (Cbr2) (Yu et al., 2005). Expression of Notch2 clearly colocalizes with Cbr2 (Fig. 2A-C). To show that expression does not occur within the neuronal layer, we used Olfactory marker protein (OMP) ((Margolis, 1982), Fig. 2D-F). Finally, we asked whether or not Notch2 and Notch3 are co-expressed among sustentacular cells, and found significant overlapping expression (Fig. 2G-I). These results show that Notch2 and Notch3 are expressed within sustentacular cells. Neuronal expression was not detected using these approaches.
Figure 2. Notch receptor expression in sustentacular cells.
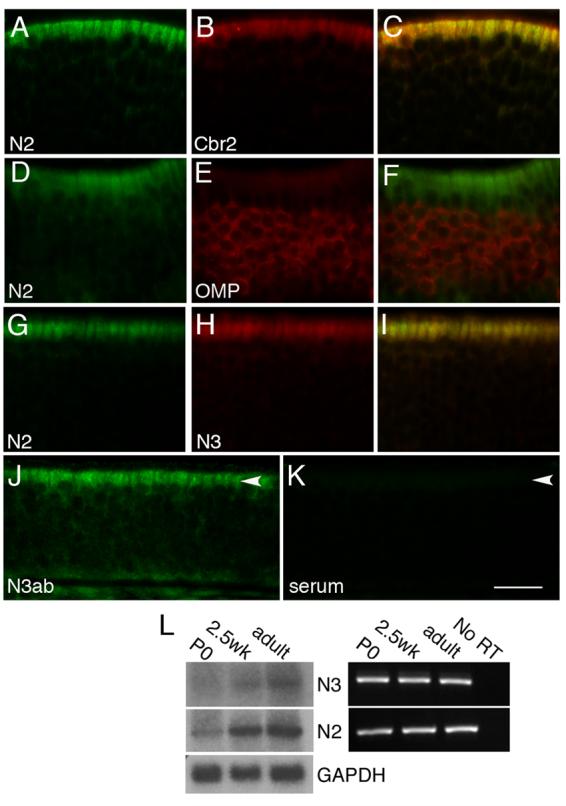
A-C) Double-label in situ hybridization in adult epithelia of Notch2 (A) and Cbr2 (B), an enzyme expressed by sustentacular cells, show that both are localized to the sustentacular layer (C). D-F) Notch2 (D) and OMP (E), a marker for mature OSNs, are not co-expressed in the neuronal layer (F). G-I) Notch2 (G) and Notch3 (H) are both expressed in the sustentacular layer (I). J) Immunohistochemical detection of NOTCH3 protein in adult animals in sustentacular cells (arrowhead). K) No signal is detected in sustentacular cells (arrowhead) in the absence of anti-NOTCH3 antibody. Scale bar=25μm for A-I, 17μm for J-K. L) RNA isolated from whole epithelia was used for Northern blot and RTPCR. Notch2 and Notch3 message can be detected at P0, 2.5 weeks, and adult stages. GAPDH was used as a loading control for the Northern blot.
We were surprised at the observed expression of Notch3, as prior studies using immunohistochemistry had not detected Notch3 in the sustentacular layer (Carson et al., 2006; Doi et al., 2004), and RT-PCR experiments suggested the transcript was not present in adult (Carson et al., 2006). We therefore performed additional tests to validate our observed Notch3 expression pattern. We cloned two non-overlapping regions of Notch3 and used each for in situ hybridization (see Materials and methods). Notch3 was detected with both probes in the sustentacular layer (data not shown). We also performed immunohistochemistry and detected expression of NOTCH3 protein in adult animals in sustentacular cells (Fig. 2J). No expression was detected in the sustentacular layer in the absence of primary antibody (Fig. 2K). Finally, Northern blot analysis and RT-PCR with whole epithelial RNA isolated from all three postnatal stages clearly detected Notch3 RNA (Fig. 2L). Collectively, these experiments show that Notch3 is expressed postnatally in the epithelium by sustentacular cells.
Conditional elimination of Notch2 with Foxg1-cre
The continued expression of Notch2 and Notch3 within the sustentacular layer during postnatal stages led us to further investigate the role of Notch receptor expression in the adult. Sustentacular cells make up 15-25% of all olfactory epithelial cells (Farbman, 1992). Based on similarities in function with glia, these cells are thought to act as support cells for OSNs, and have been termed “glial-like” (Weiler and Farbman, 1998). What is the purpose of the maintained expression of Notch2 and Notch3 in postnatal sustentacular cells? We used a conditional null mutant of Notch2 (McCright et al., 2006) and a null mutant of Notch3 (Krebs et al., 2003) to examine the effects of removing these Notch receptors on sustentacular cells. We used the Foxg1-Cre line (Hebert and McConnell, 2000) to selectively inactivate Notch2 in the epithelium and telencephalon. Although it has previously been shown that Foxg1Cre/+ animals have haploinsufficient phenotypes within the CNS (Shen et al., 2006), no qualitative differences were observed among the two control populations (N2flox/+;Foxg1Cre/+ and N2flox/flox; data not shown).
To show that Foxg1-Cre is expressed within the epithelium, we crossed these mice with a Rosa26-LacZ reporter line (Soriano, 1999). Expression of β-galactosidase is detected throughout the epithelium (Fig. 3A). To confirm the efficacy of the deletion, we performed in situ hybridization using a probe corresponding to the exon deleted in the conditional Notch2 mutant (Fig. 3B,C). No signal was observed in mutant animals (Fig. 3C) as compared against controls (Fig. 3B). These experiments show that Notch2 has been effectively deleted from the sustentacular layer during development.
Figure 3. Notch2 is effectively deleted in mutant mice and mutant animals are smaller than control siblings.
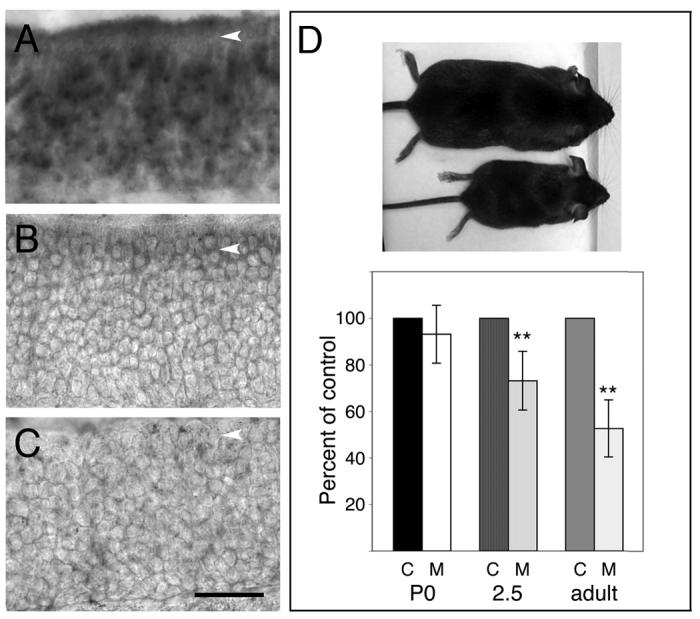
A) Foxg1-cre animals (Hebert and McConnell, 2000) were crossed to the Rosa26-LacZ reporter strain (Soriano, 1999). Sections from P0 progeny were assayed for β-galactosidase activity and counterstained with eosin. β-galactosidase expression was detected in the sustentacular layer (arrowhead) and throughout the apical-basal extent of the epithelium. B) Exon 3 (260 bp; deleted in N2flox/flox; Foxg1-cre animals (McCright et al., 2006)) was used as probe to detect expression of Notch2 in control and mutant animals. Message could be detected within sustentacular cells of adult control animals (arrowhead). C) No Notch2 message could be detected with the exon 3 probe in N2flox/flox; Foxg1-cre animals (arrowhead). D) Notch2 mutant animals were smaller than their control siblings (top mouse-control adult; bottom mouse-mutant adult). Animals were weighed at P0, 2.5 weeks, and adult (8-19 weeks) stages to determine when this weight difference became apparent. At P0, the average weight of controls (c-control, 1.6±0.1g) did not differ significantly from mutants (m-mutant, 1.8±0.2g; n=4 pairs; p=0.3, Student's t-test). However, by 2.5 weeks, mutants were on average 27% less in weight than control siblings (n=8 pairs; control: 8.1±1.4g; mutant: 5.8±1.0g; p=0.003). Although adult animals were weighed at different ages (8-19 weeks), control siblings were always compared with mutant siblings. On average, mutants weighed 47% less than controls (n=13 pairs; control: 24.7g±4.3; mutant: 12.8g±3.0; p=2.1×10−8, Student's t-test). Scale bar=40μm.
Unexpectedly, we found that mutant mice were significantly smaller than their heterozygous littermates (Fig. 3D). Although there was no apparent weight difference at P0, at later stages mutant animals weighed 27% (2.5 weeks) and 47% (adult) less than controls. In preliminary studies, we had found that Foxg1 is expressed within Rathke's pouch at embryonic day 10 (E10). Similarly, Notch2 is also expressed in the pouch at E10 (data not shown). As activated Notch2 has been shown to affect pituitary differentiation (Raetzman et al., 2006), one interpretation is that Notch2 deletion in the pituitary leads to the reduced size of the mutant animals. Consistent with this, Notch2 mutant pituitaries are significantly smaller in size than that of wild-type littermates (data not shown). Although mutant animals were born in approximately Mendelian ratios (21%), nearly two-thirds did not survive to adulthood. While the mortality rate was high, we did not find any evidence of non-specific effects upon the olfactory system, as described below.
Absence of Notch2 disrupts epithelial organization
We initially used histological stains to assess the overall structure of the epithelium in mutants. In sagittal sections of adult Notch2 mutants, clear alterations in hematoxylin/eosin and Bielschowsky staining were observed (Fig. 4). Hematoxylin/eosin staining revealed that the relatively uniform spacing of sustentacular nuclei seen in control animals was disrupted in regions in the mutant (compare Fig. 4A,B). The morphology of the nuclei was also altered, and they appeared smaller and more irregularly shaped. Pyknotic nuclei could be seen as well in the epithelium (Fig. 4B). Bielschowsky staining was used to reveal axonal and dendritic processes of OSNs. Significantly fewer dendritic processes were observed in the mutant, leading to gaps and a reduction in the number of dendritic tufts at the apical surface (Fig. 4C,D).
Figure 4. Disruption of epithelial structure in adult Notch2 mutants.
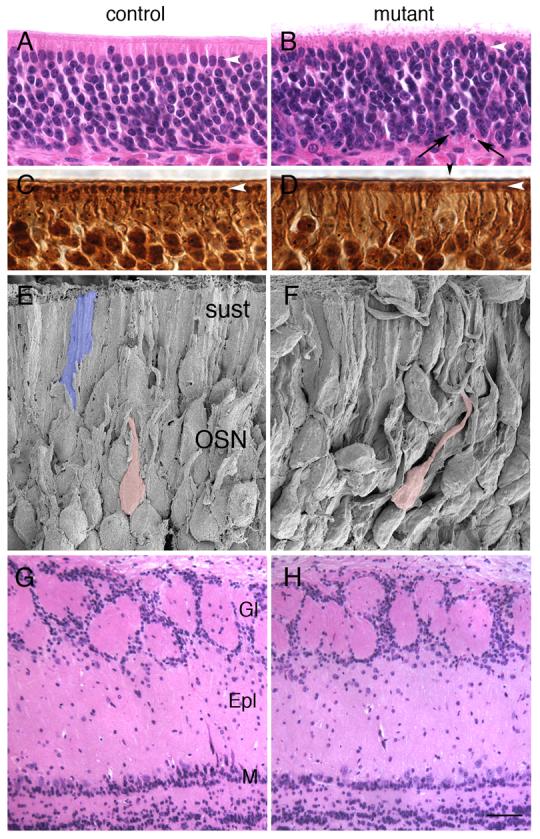
White arrowheads indicate sustentacular layer in A-D. A,B) Hematoxylin and eosin staining of control (A) and mutant (B) adult animals. Disruption of the organization of the sustentacular layer is apparent in mutants as compared with controls (white arrowhead). Pyknotic nuclei (black arrows) could be seen in mutant sections. C,D) Bielschowsky staining revealed gaps (black arrowhead) in the relatively uniform distribution of dendritic tufts (white arrowhead) in mutants (D) as compared against controls (C). E,F) Scanning electron micrograph from control (E) and mutant (F) adult animals show increased disorganization within the neuronal layer. Compare dendritic path of two neurons pseudocolored pink. Typical sustentacular cell pseudocolored blue (E). G,H) Hematoxylin and eosin staining of control (G) and mutant (H) adult olfactory bulbs. No differences in laminar structure were observed. Gl: glomerular layer, Epl: external plexiform layer, M: mitral layer. Scale bar=20μm for A-B, 10μm for C-D, 5μm for E-F, 62.5μm for G-H.
The histological analysis suggested that the laminar nature of the epithelium had been disrupted. Using scanning electron microscopy, we were able to confirm this disorganization. In wild-type animals, OSNs are organized into relatively ordered arrays (Fig. 4E). However, in three different mutants, regions of the epithelium can be found where OSNs appear disorganized (Fig. 4F), consistent with what was observed with the hematoxylin/eosin and Bielschowsky analysis.
We next examined olfactory bulbs of mutant mice for defects. Because of the observed difference in size between mutant and control animals and the potential effect of Notch2 on the pituitary, we based our study on data from mice bearing a knockout mutation in IGF1 (Pichel et al., 2003). Hypophysectomized animals show highly reduced levels of IGF1 (Murphy et al., 1987), and IGF1 mutants weigh less than controls at embryonic day 18.5 (E18.5). In addition, the mitral layer is disorganized and disrupted, and 70% of mitral neurons are missing in these IGF1 mutants. In contrast, no alterations in the laminar structure of the mitral layer were observed in Notch2 mutant bulbs (Fig. 4G,H).
The histological and SEM analysis showed that the epithelium appeared disorganized. However, we noted that this disorganization was not generally observed throughout the epithelium. While some areas were significantly disrupted, other areas appeared relatively normal. The variability in phenotype led us to examine the overall structure in a coronal plane of the epithelium. Strikingly, in several mutants, significant differences in the thickness of the epithelium could be observed as compared with controls (Fig. 5). DAPI staining showed that some regions of the epithelium were dramatically thinner than others (Fig. 5B,D; compare with Fig. 5A,C). These differences are highlighted in cartoon form in Fig. 5E,F. This variability in epithelial thickness was not present in all mutants. However, all of the phenotypes described below were present in varying degrees within all Notch2 mutant animals.
Figure 5. Variability in epithelial degeneration in Notch2 mutants.
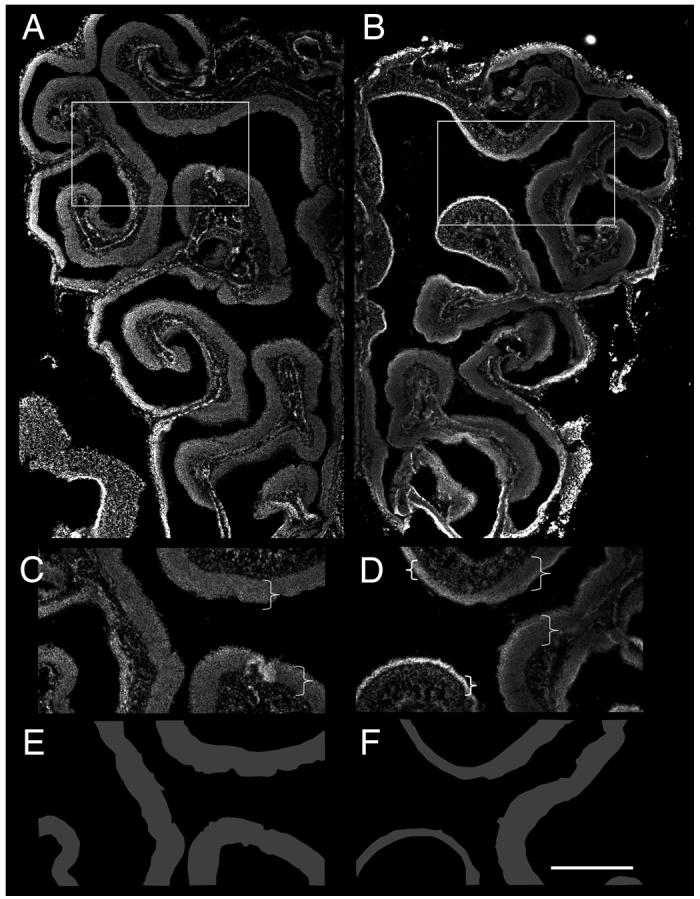
A-B) Low power images of DAPI stained coronal sections from control (A) and mutant (B) epithelium. Boxed areas are represented in (C) and (D). Brackets highlight regions of epithelia (C,D). E,F) Tracing of epithelia of in (C) and (D) to illustrate variability in mutant epithelium as compared with control. Scale bar=500μm for A-B, 300μm for C-D.
Neurodegeneration in Notch2 mutants within the epithelium
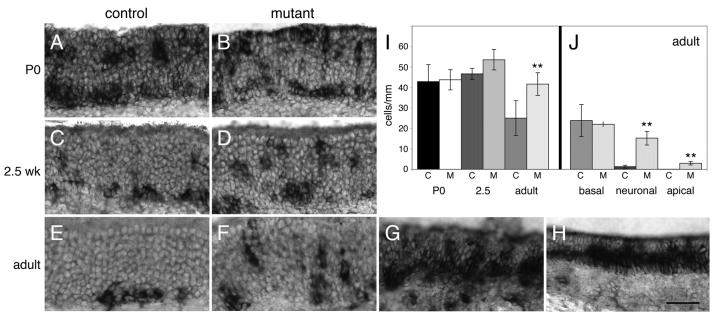
The DAPI stained images (Fig. 5) clearly showed significant degeneration had occurred in some mutants. However, it was unclear whether other areas of the epithelium that were apparently unchanged in thickness and other mutants that did not show dramatic alterations in epithelial thickness would also show the presence of degeneration. Moreover, it was unclear whether both neurons and sustentacular cells were being affected. We therefore used TUNEL analysis to examine and quantify the levels of apoptosis in adult Notch2 mutants and to define the laminar location of this expression (see Materials and methods; 3-8mm of linear distance counted per adult). We defined apical TUNEL-positive cells as those adjacent to the lumen, and basal TUNEL-positive cells as being within 2-3 cell diameters of the basal lamina. Most of the observed TUNEL signal appeared to be within the neuronal layer (Fig. 6B; compare with 6A), although occasional cells were detected in the sustentacular and basal layer. Quantification of TUNEL signal (n=3 pairs; see Materials and methods) showed no significant difference in the number of TUNEL-positive cells per millimeter in the basal layer of adults (Fig. 6D). Significant differences did exist between mutant and control in the apical layer, although the overall number of positive cells was small (0.3 cells/mm vs 0/mm). The great majority of the increase in TUNEL-expressing cells were located within the neuronal layer.
Figure 6. Increased apoptosis in Notch2 mutants.
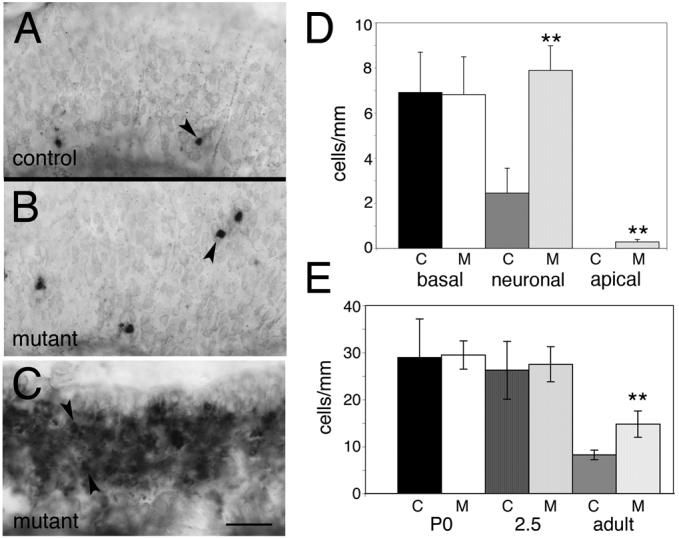
A) TUNEL staining was used to assay levels of apoptotic cell death in control animals. Apoptotic cells were sparsely distributed in adult animals, and were generally detected in the basal and neuronal layers (arrowhead). B) TUNEL staining in Notch2 mutants appeared more widespread, with many areas outside the basal layer showing increased expression relative to control (arrowhead). C) TUNEL staining in some regions of Notch2 mutant epithelia was dramatically elevated (e.g. arrowheads). D,E) Quantitation of TUNEL expression. “C” indicates control and “M” indicates mutant. D) The distribution of TUNEL positive cells in control and mutant adults were binned by location into basal, neuronal, and apical layers. No significant differences were observed in the basal layer (control: 6.9±1.8 cells/mm; mutant: 6.8±1.7 cells/mm; p=0.7; Student's t-test). However, in the neuronal layer (control: 2.4±1.1 cells/mm; mutant: 7.9±1.1 cells/mm; p=0.005) and in the apical layer (control: 0 cells/mm; mutant: 0.3±0.1 cells/mm; p=0.01) significant differences were observed. The bulk of the TUNEL-positive cells in the mutant were present in the neuronal layer. E) Quantification of total TUNEL cells per millimeter throughout the apical-basal extent of the epithelium was performed in P0 (n=3 pairs: 6-18mm/animal), 2.5 week-old (n=3 pairs: 7-15mm/animal), and adult (n=3 pairs: 3-8mm/animal) control and mutant animals. No significant differences were detected at P0 (control: 29.0 ±8.2 cells/mm; mutant: 29.5±3.0 cells/mm; p=0.9), or at 2.5 weeks (control: 26.3±6.2 cells/mm; mutant: 27.5±3.7 cells/mm; p=0.8). However, in adult animals, there were significant differences between control and mutant (control: 8.2±1.0 cells/mm; mutant: 14.8±2.8 cells/mm; p=0.02; asterisks indicate significant differences relative to control). Quantitation of data in (D) and (E) excluded all areas similar to those shown in (C), as it was impossible to determine the number of positive cells per millimeter in such regions. Scale bar=25μm.
Interestingly, we found that some regions of the mutant epithelium showed dramatically higher TUNEL expression (Fig. 6C). We note that the thickness of the epithelium was not a strict predictor of the degree of observed TUNEL expression. In areas of the epithelium that were extremely thin (e.g. Fig. 5D), little or no TUNEL expression was detected, presumably because relatively few cells remained. Areas that did have extremely high TUNEL expression were not included in our quantitation as it was impossible to identify individually stained cells. As a result, our analysis underestimates the level of cell death in mutants.
The overall increase in neurodegeneration seen in adult mutants led us to ask when this phenotype first occurred. We examined TUNEL expression in P0 and 2.5 week-old animals (n=3 pairs for each timepoint; 6-18mm/P0 animal, and 7-15mm/2.5 week animal). Quantitation showed no difference in the total number of TUNEL-positive cells per millimeter between mutant and control (Fig. 6E). No difference was observed in the apical-basal distribution of TUNEL-positive cells at either stage as well (data not shown). Collectively, these results show that neurodegeneration does not occur during early postnatal life, but does increase as the animal ages. To control for potential non-specific apoptosis that may occur as a result of altered Notch2 function in the pituitary, we examined mutant adult bulbs for evidence of increased TUNEL expression. No differences were observed between mutants and controls (data not shown), suggesting that widespread apoptosis throughout the nervous system is not present.
Deletion of Notch2 does not alter sustentacular cell fate in the adult
One possible reason for the persistent expression of Notch2 in postnatal sustentacular cells may be to prevent alterations in sustentacular cell fate. It has previously been shown that Hes1 is important for repressing neuronal fate in sustentacular cells (Cau et al., 2000). In the absence of Hes1, increased numbers of OSNs are produced. It was hypothesized that Notch receptor expression is required for mediating the effects of Hes1 on sustentacular cells during development (Cau et al., 2000). If Notch reprises this role in the adult olfactory system, the loss of Notch2 may lead to a change in cell fate among sustentacular cells. We therefore examined the epithelia of mutant animals at P0 (n=18), 2.5 weeks (n=8) and adult (8-19 weeks; n=13) for alterations in cell fate using a variety of neuronal markers.
We initially screened adult epithelia for Scg10, a marker of early neuronal development (Pellier-Monnin et al., 2001), and Olfactory marker protein (OMP), a marker of mature OSNs (Danciger et al., 1989). In adult animals, the location of Scg10 expression is similar in controls (Fig. 7A) and mutants (Fig. 7B). The expression of Scg10, however, is more variable in the mutant. In some areas, expression of Scg10 is significantly increased relative to control (Fig. 7B left panel vs. Fig. 7A), while in others, Scg10 expression is patchy, with areas of weak or absent expression (Fig. 7B right panel vs Fig. 7A). No expression of Scg10 was observed apically in the sustentacular layer.
Figure 7. Cell fate is unaffected in Notch2 mutant adults.
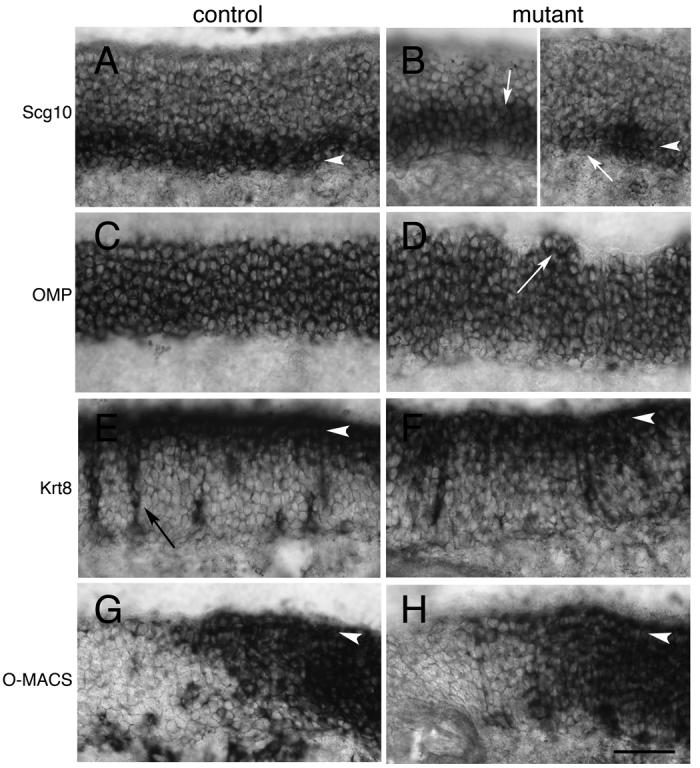
>A) Scg10 expression in adult control animals is basally located in developing immature OSNs (arrowhead). B) Expression of Scg10 can be detected in Notch2 mutants (arrowhead), but this expression is uneven. Some areas have significantly elevated expression (B-left panel; white arrow), while others have patchy or no Scg10 expression (B-right panel; white arrow). C) OMP expression in control animals is detected in mature OSNs within the neuronal layer. D) OMP expression in mutant animals is similar to that in control. However, the apical surface of OMP expression is less laminar in appearance. In several areas, OMP expression appears to extend into the apical layer (white arrow). E) Control expression of Cytokeratin8 (Krt8) is detected in the sustentacular layer (arrowhead) and in Bowman's glands (black arrow). F) Krt8 is detected in the sustentacular layer of mutant animals (arrowhead). G) O-MACS expression in control animals is detected in the dorsal-most zone of the epithelium (arrowhead shows sustentacular layer). H) O-MACS is also detected in a similar pattern in Notch2 mutants in the sustentacular layer (arrowhead). Scale bar=50μm.
Like Scg10, OMP is expressed in mutant epithelia (Fig. 7C,D). However, in adult mutants, the apical surface of OMP expression was often irregular (Fig. 7D). This expression correlates with the disorganized, laminar structure observed with the histological stains (Fig. 4B). Occasionally, OMP expression was seen to extend into the apical layer (Fig. 7D). We interpret this to indicate that OSNs are being displaced into the apical layer due to disruption of the sustentacular layer (Fig. 4B, F).
We also examined expression of several genes that are markers of sustentacular fate. Cytokeratin8 (Krt8) is a structural protein expressed by sustentacular cells (Suzuki and Takeda, 1991). Krt8 expression appeared relatively unaffected in mutants (Fig. 7 E,F). Similarly, we looked at expression of O-MACS, a member of the CoA-synthetase family that is expressed exclusively in the dorsal epithelium in a zonal manner (Oka et al., 2003). O-MACS is expressed by sustentacular, neuronal, and basal cells. Its expression was unaffected in the mutant, demonstrating that zonal identity for these dorsal-most sustentacular cells was not affected by the absence of Notch2 (Fig. 7G,H). Finally, we looked at expression of Carbonyl reductase2 (Cbr2), an enzyme produced by sustentacular cells that is thought to be important for clearance of odorants (Yu et al., 2005). Expression of Cbr2 in general appeared similar between mutant and control, although the level of expression seemed somewhat reduced in the mutant (data not shown). Collectively, these experiments show that no dramatic change in cell fate markers have occurred in either the sustentacular layer or in the neuronal layer of Notch2 mutants. However, disruption of the normal domains of OMP expression and Scg10 were visible. Although most regions of the epithelia showed variation in the laminar expression of OMP (Fig. 7D), in some areas of mutant epithelium, OMP expression was dramatically affected (Fig. 8A,B). The epithelium in these regions was highly variable in thickness, and OMP expression was strongly reduced and sometimes absent. We also examined mutant olfactory bulbs using a variety of probes, including Neurotensin, IGF1, and Glutamate receptor (subunit 1). No differences were seen between mutants and controls (data not shown).
Figure 8. Extreme examples of degenerating epithelia in Notch2 mutants.
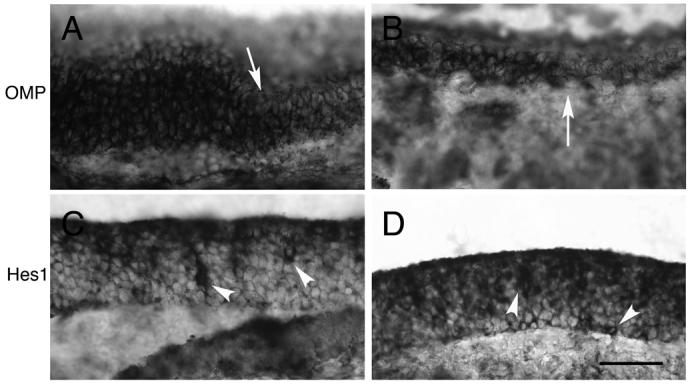
All images are taken from Notch2 adult mutants. A,B) OMP expression is dramatically affected in some areas of epithelia (areas to right of arrows). C,D) Hes1 expression is strongly affected in some areas of mutant epithelia, with an increase in the number of Hes1-positive cells in the basal and neuronal layers (arrowheads). Scale bar=50μm.
Increase in progenitor cell number in Notch2 mutants
We next examined expression of Mash1, a marker of olfactory neuronal progenitors (Guillemot et al., 1993). During development, Mash1 labels cells that are migrating from the apical layer to the basal epithelium where they will give rise to progenitor populations (Cau et al., 2002). At P0, there are large numbers of Mash1-expressing cells in the neuronal and basal layer in control animals (Fig. 9A). A small number of positive cells can also be detected in the apical layer, as previously described (Cau et al., 2002). By 2.5 weeks, there are fewer Mash1-positive cells in the neuronal layer and an increasing number of basally located cells (Fig. 9C). In the adult, the great majority of Mash1-positive cells are basally located (Fig. 9E). As previously described (Manglapus et al., 2004), Mash1 expression in the dorsal recess tended to be lighter than those in the more ventral and lateral epithelium in adults. In P0 and 2.5 week-old mutant animals, the overall pattern of Mash1-expressing cells was similar to that of controls (Fig. 9B,D). However, in the adult mutant, increased numbers of Mash1 cells were observed in the neuronal and apical layer (Fig. 9F). As with OMP expression, in some regions of mutant epithelia, a dramatic increase in Mash1 expressing cells was detected (Fig. 9G,H).
Figure 9. Mash1 expression is elevated in adult mutants.
A,B) Mash1 expression in P0 control (A) and mutant (B) animals are similar. C,D) Mash1 expression in 2.5 week-old control (C) and mutant (D) are also similar. E,F) Multiple regions can be found in adult mutant animals that appear to have increased numbers of Mash1-expressing cells (F) as compared against control (E). G,H) In some extreme instances, very high numbers of Mash1-expressing cells can be found in regions of mutant epithelia (compare with (E)). I) Quantitation of the total number of Mash1-expressing cells per millimeter (c-control; m-mutant) showed no significant differences at P0 (8-15mm counted/animal; control: 42.9±8.3 cells/mm; mutant: 43.7±5.0 cells/mm; p=0.9, Student's t-test). No differences were found at 2.5 weeks (5-26mm counted/animal; control: 46.6±2.7 cells/mm; mutant: 53.5±10.3 cells/mm; p=0.3). However, significant differences were found in adult (7-14mm counted/animal; control: 25±8.5 cells/mm; mutant: 41.7±5.5 cells/mm; p=0.05; asterisks indicate significant differences relative to control). J) We quantified the distribution of Mash1-expressing cells in adults in the basal, neuronal, and apical layers of the epithelium. No significant differences were found in the basal layer (control: 23.8±7.9 cells/mm; mutant: 22.0±1.2 cells/mm; p=0.7). However, in the neuronal (control: 1.3±0.7 cells/mm; mutant: 15.2±3.3 cells/mm; p=0.004) and apical layers (control: 0.1±0.1 cells/mm; mutant: 3.0±0.8 cells/mm; p=0.008), significant differences were identified between mutant and control. Quantification does not include areas of epithelia with extremely high levels of Mash1 expression (e.g. G,H). Scale bar=50μm.
We quantified the distribution of Mash1 in P0 (n=3 pairs; 8-15mm/animal), 2.5 week (n=3 pairs; 5-26mm/animal), and adult (n=3 pairs; 7-14mm/animal) mutants and controls (Fig. 9I). No difference was observed in the total number of Mash1-expressing cells per millimeter at P0. Although no significant difference was observed in 2.5 week-old animals, there were areas of epithelia in the mutant which possessed increased numbers of Mash1-positive cells (data not shown). However, these regions constituted a relatively small proportion of the epithelium, and the overall quantitation showed no significant differences.
In adult mutants, there were significantly greater numbers of Mash1-positive cells per mm as compared with control (Fig. 9I). We quantified the apical-basal distribution of these cells in a manner analogous to that for the TUNEL analysis. Approximately equal numbers of cells are found in the basal layer between control and mutant (Fig. 9J). However, in both the neuronal and apical layers, significantly higher numbers of Mash1-positive cells were observed in the mutant. We note that we did not include any areas in this quantitation where there were excessively large numbers of Mash1-positive cells (e.g. Fig. 9G,H), as it was impossible to quantify the number of cells within these regions. Thus, as with the TUNEL analysis, this quantification represents an underestimate of the number of Mash1-positive cells and their distribution in Notch2 mutants. While the small but significant increase in apically-detected Mash1 expression is consistent with a cell fate change among sustentacular cells, only a small number of such cells are detected (Fig. 9J; 3.0 cells/mm vs 0.1 cells/mm). Moreover, the distribution of Mash1 cells in highly affected regions is reminiscent of what was observed in olfactory epithelia that have been chemically ablated (Manglapus et al., 2004). In these epithelia, both neurons and sustentacular cells have been ablated, and Mash1 cells can be observed in the apical layer of the regenerating epithelia. Over time, this expression disappears, and Mash1 is again primarily found in the basal epithelium.
Deletion of Notch2 alters expression of downstream Notch pathway effectors
Although Notch2 expression is widespread in the sustentacular layer, we were unable to identify widespread changes in cell fate among sustentacular cells in Notch2 mutants. We next considered that Notch2 may instead be required for maintained sustentacular function. It had previously been suggested, based upon the continued expression of Hes1 in the adult, that Hes1 may be required for maintaining the function of sustentacular cells (Manglapus et al., 2004). We therefore examined whether or not Hes1 and Hey1, which are both in vitro downstream targets of Notch2 (Maier and Gessler, 2000; Shimizu et al., 2002), are affected in mutants.
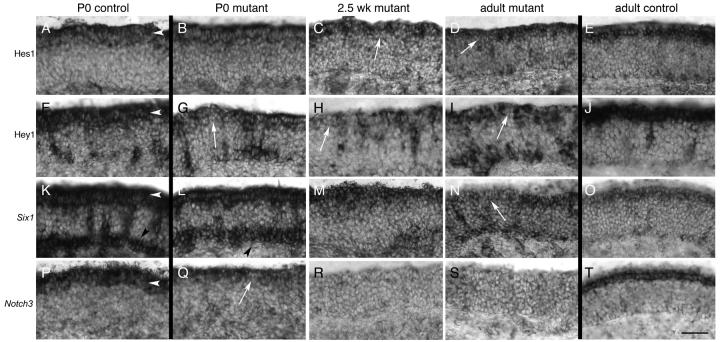
Hes1 is expressed in the sustentacular layer during postnatal stages in Notch2 mutants and in controls (Fig. 10A-E). At P0, Hes1 expression could be detected in the apical epithelium of Notch2 mutants. Although this expression appeared somewhat lighter than control, no significant differences were found (Fig. 10A,B). At 2.5 weeks, however, gaps in Hes1 expression could be detected apically (Fig. 10C), which increased in frequency in the adult (Fig. 10D). This can be contrasted with the relatively higher control expression of Hes1 in adults (Fig. 10E) and at 2.5 weeks (data not shown). Thus, although Hes1 was detected at all stages in Notch2 mutants, progressively fewer cells expressed Hes1 in the apical layer as the animal aged. While the reduced and intermittent apical Hes1expression was the predominant phenotype, in some areas of adult mutant epithelia, Hes1expression was dramatically increased (Fig. 8C,D). The epithelium was also reduced in thickness within these areas. The distribution of Hes1 was also markedly higher throughout the apical-basal extent. This distribution is reminiscent of what was observed in epithelia that have been ablated and are undergoing regeneration (Manglapus et al., 2004).
Figure 10. Down-regulation of Hes1, Hey1, Six1, and Notch3 in the absence of Notch2.
In all panels, arrowheads point to the position of the sustentacular layer. For comparison, P0 and adult controls for each probe are shown. A,E) Expression of Hes1 at P0 (A) and adult (E) control animals. B-D) No significant differences in Hes1 expression could be detected at P0 in mutants (B). Subtle differences were observed at 2.5 weeks, as there was an apparent decrease in the number of Hes1-expressing cells in the apical epithelium (C; arrow). However, a clear reduction in Hes1 expression could be seen in adult mutants (D: white arrow shows large gap in Hes1 expression). Hes1 positive cells can also be detected scattered in the neuronal and basal layers. F,J) Hey1 is evenly expressed in sustentacular cells at P0 (F) and in adult (J) controls. Hey1 is also expressed in an unknown population of cells distributed deep to the sustentacular layer. G-I) Hey1 expression is strongly affected at P0 (G), 2.5 weeks (H), and adult (I) in Notch2 mutants. Significant disruption of apical Hey1 expression is observed at all stages, with gaps in the normal, uniform apical expression (arrows). Increased expression is also seen in basal cells in mutants relative to controls (I). K,O) Expression of Six1 in P0 (K) and adult (O) control animals. Six1 is expressed in P0 animals strongly in the sustentacular and basal layers, and in cells distributed in the neuronal layer. Only a small number of basally-located cells express Six1 in control adults (O). L-N) Six1 expression in P0 (L) and 2.5 week (M) mutant animals appears minimally or subtly affected. However, in adult (N) mutant animals, gaps in Six1 expression could be seen apically (arrow). P,T) Expression of Notch3 in P0 (P) and adult (T) control animals. Q-S) Expression of Notch3 in P0 mutant (Q) animals is clearly detectable in the sustentacular layer, but this expression is more variable than control, and is also reduced in expression (arrow). Weak or no expression of Notch3 is observed in 2.5 week-old (R) or adult (S) Notch2 mutants. Scale bar=50μm.
An examination of Hey1 expression in P0, 2.5 week and in adult Notch2 mutants showed that, like Hes1, gaps and reduced levels of Hey1 are observed in the sustentacular layer in the adult (compare Fig. 10I with 10J). Thus, expression of both Hes1 and Hey1 are missing in a large subset of sustentacular cells in Notch2 mutants. Also like Hes1, this is often accompanied by increased numbers of Hey1-positive cells basally. However, Hes1 is only beginning to be affected in 2.5 week-old mutants and is more strongly affected in adults, while Hey1 is dramatically reduced at all stages (compare Fig. 10G with 10F). This suggests that although both Hes1 and Hey1 are downstream targets of the Notch receptors, there are differences in activation of these two transcription factors by the various receptors, as previously suggested (Shimizu et al., 2002).
Six1 has been shown in zebrafish to be affected by mutants in the Notch pathway (Bricaud and Collazo, 2006). We found that Six1 is expressed in the sustentacular layer in control animals at all ages (Fig. 10K,O). It is also strongly expressed basally in P0 controls, but this basal expression becomes weaker by 2.5 weeks, and is much reduced in expression by adulthood. In mutants, the pattern of Six1 distribution in P0 and 2.5 week-old appears minimally disrupted (Fig. 10L,M). No significant difference in expression between mutant and control could be detected, although expression appeared to be somewhat weaker in 2.5 week-old mutants. In contrast, in adult mutants, the uniform expression of Six1 is interrupted apically in mutants by domains that express little or no Six1 (Fig. 10N). The alterations in Hes1, Hey1, and Six1 show that transcriptional regulation is variably disrupted in the absence of Notch2 in an age-dependent manner. Hey1 is disrupted apically at all postnatal stages, Hes1 expression only appears altered beginning at 2.5 weeks, while Six1 is most strongly affected in adults.
Notch3 expression is absent in Notch2 adults
Both Notch2 and Notch3 are expressed in sustentacular cells (Fig. 1, 2). Both are known to activate Hes1 and Hey1 in vitro (Maier and Gessler, 2000; Shimizu et al., 2002) and are thought to serve similar functions in vivo (Louvi and Artavanis-Tsakonas, 2006). We were therefore surprised at the fact that there was reduced Hes1 and Hey1 in Notch2 mutants, as we expected that Notch3 might compensate for the lack of Notch2. Interestingly, we found that Notch3 expression was strongly affected. In P0 mutants, Notch3 was still detectable in the sustentacular layer, although expression was reduced relative to control (compare Fig. 10Q with 10P). Occasional gaps in Notch3 expression could also be seen at P0. We found Notch3 could also be detected in E14 mutants (data not shown). But in both 2.5 week-old animals (Fig. 10R) and in adult mutants (Fig. 10S), Notch3 expression was essentially absent. Unlike the expression of Hes1, Hey1, and Six1, which are still present in the adult at a low level, Notch3 expression was undetectable by in situ hybridization. This surprising result suggests that Notch3 expression initially does not require Notch2. However, as development proceeds, maintenance of Notch3 directly or indirectly requires Notch2.
To determine whether or not the converse is true, and if Notch3 also influences Notch2 expression, we examined Notch3 mutants (Krebs et al., 2003) for phenotypes in the adult olfactory epithelium. We found that expression of Notch2 was unaffected in Notch3 mutants (data not shown), suggesting that Notch3 is not required for either the initiation or maintenance of Notch2 expression. We also found no histological or molecular alterations with any of the markers used to analyze Notch2 mutants (data not shown). Unfortunately, efforts to generate the triple mutant (N2flox/flox;Foxg1Cre/+;N3−/−) have been unsuccessful so far. Together, these results suggest that Notch2 can compensate for Notch3, but that Notch3 can only partially compensate for Notch2.
Impairment of sustentacular cell function
One of the hypothesized roles of sustentacular cells is that they function in a neuroprotective role towards OSNs (Whitby-Logan et al., 2004). This is based in large part on the fact that sustentacular cells express high levels of enzymes known to participate in metabolic modification of toxicants. These include cytochrome P450 isoforms (Ling et al., 2004) and Glutathione S-transferases (Weech et al., 2003; Whitby-Logan et al., 2004). Indeed, the epithelium can display higher levels of P450 activity than the liver (Hadley and Dahl, 1983). Moreover, some cytochrome P450 forms are either expressed most highly in the epithelium or are uniquely expressed in the epithelium (Ling et al., 2004). Where expression of these enzyme isoforms has been examined, the majority are expressed primarily or exclusively in sustentacular cells and/or in Bowman's glands. There are three major P450 isoforms – Cyp1a2, Cyp2a5, and Cyp2g1 - which collectively constitute >35% of the total P450 in the epithelium (Gu et al., 1998). At least a dozen other isoforms are also expressed (Ling et al., 2004). Similarly, of the various GST isoforms, mu1 and mu2 are most highly expressed (Ben-Arie et al., 1993).
We hypothesized that impairment of sustentacular cell function may ultimately lead to neuronal degeneration. Toxicology experiments have shown that exposure to some nasal inhalants can lead to cell death in select regions of the epithelium, which is attributable to airflow-driven deposition in these areas (Harkema et al., 2006). Notably, these areas include the dorsal medial meatus and proximal regions of the lateral and middle medial meatus, areas which are strongly affected in several of our mutants (Fig. 5). Thus, the variability of our phenotype may be dependent, at least in part, upon the ability of sustentacular cells to respond to environmental inhalants and the frequency to which these cells are exposed to airflow bearing these inhalants.
We therefore analyzed by quantitative RT-PCR the level of RNA expression of several major P450 and GST isoforms in the epithelium (Fig. 11A). This analysis showed decreases of 40-50% in four of five genes assayed. These results indicate that expression of these metabolically important modification enzymes are down-regulated, consistent with a reduction in the ability of sustentacular cells to detoxify and/or modify inhalants and other toxins. Interestingly, Cyp2a5 appeared to be modestly up-regulated in two samples but strongly down-regulated in a third. The first two samples were from animals that were 4 weeks of age while the third sample is from one that is 9 weeks old. As we have shown previously, there appears to be a progressive increase in neurodegeneration from 2.5 weeks to adulthood (8 weeks or older). As such, RNA levels of Cyp2a5 may be minimally affected at 1 month, but are eventually down-regulated as the animal ages. We also performed a functional analysis to determine if the level of GST activity in mutant epithelia correlates with the decrease in GST mu1 and mu2 RNA. Graphing of GST activity (n=3) clearly revealed lower levels in mutants as compared with controls (Fig. 11B). The ratio of the two slopes show that the mutant pairs possessed 80-87% of control levels of GST activity (Fig. 11C), confirming GST levels are impaired in Notch2 mutants.
Figure 11. Quantitation of P450 and GST expression and determination of GST activity.
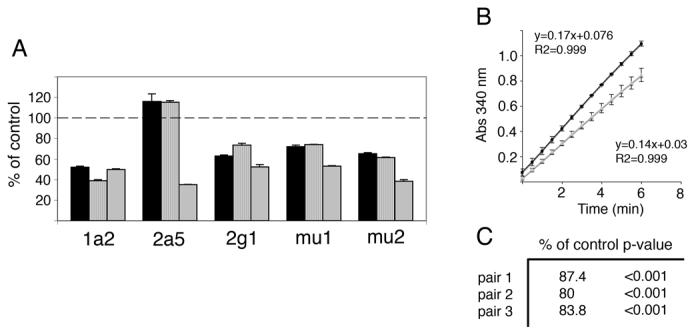
A) Quantitative RT-PCR of P450 and GST isoform expression levels in three mutant epithelia as compared with control. Reduction of P450 isoforms Cyp1a2 and Cyp2g1 and GST isoforms mu1 and mu2 RNA are seen in all three mutant epithelia relative to control. Expression of Cyp2a5 is somewhat increased in two mutants, but strongly decreased in the third mutant. This may be a result of the ages of the mutant samples. The first two epithelia are obtained from 1 month old animals, while the third is from an animal 9 weeks old. B) Example graph of GST activity from one paired mutant and control assay. Black line (circles) represents control epithelial extract and gray line (triangles) represents mutant epithelial extract. Graph indicates increasing accumulation of GS-DNB conjugate over time, as determined by absorbance at 340nm. Note reduced slope of mutant relative to control. C) Percent of control GST activity from three separate mutant-control epithelial pairs.
Discussion
Here we show evidence consistent with a model where Notch2 acts to maintain sustentacular function in the adult main olfactory epithelium. In the absence of Notch2, a progressive reduction of Hes1, Hey1, and Six1 expression occurs as postnatal development proceeds. Further, we show that sustentacular cell function is impaired, as assessed by RNA expression of P450 and GST isoforms and functional assays of GST activity. These alterations in sustentacular cell morphology, gene expression, and function are associated with neurodegeneration among OSNs. Finally, we show that Notch2 is required to maintain, but not initiate, Notch3 expression. Notch3, however, is not required for Notch2 initiation or maintenance of expression.
Notch2 and Notch3 are co-expressed in sustentacular cells
We have demonstrated that Notch2 and Notch3 are co-expressed within the sustentacular layer of the epithelium. There is conflicting evidence regarding the expression of Notch3 in the olfactory system. Our results are inconsistent with other studies that show no expression of Notch3 in sustentacular cells (Carson et al., 2006; Doi et al., 2004). We use in situ hybridization with two non-overlapping probes, double-label in situ hybridization, immunohistochemistry, Northern blot analysis, and RT-PCR to show Notch3 RNA and protein are present in the epithelium and are expressed by sustentacular cells. We cannot explain why our results vary from these other studies. It is possible that the antibody used for immunohistochemistry by Carson et al. and Doi et al. may not be the same as that used in these studies (see Materials and methods).
Sustentacular cells possess many similarities with glial cells. Like glial cells, sustentacular cells express high levels of cytochrome P450 isoforms (Chen et al., 1992) and Glutathione S-transferases (Krishna et al., 1994), and participate in detoxification. Like Müller glia, sustentacular cells span the epithelium and encapsulate OSN cell bodies, forming columns of cells within the epithelia (Nomura et al., 2004). Similar to glia, sustentacular cells electrically isolate OSNs, and ensure that their dendrites do not make direct contact with one another (Getchell et al., 1984). Collectively, these functions indicate that sustentacular cells support and maintain OSN function. However, they also express markers more commonly found on keratinocytes, including cytokeratins (Suzuki and Takeda, 1991). These cells have therefore been termed “glial-like” instead of glia (Weiler and Farbman, 1998). Despite the inferred function of sustentacular cells in supporting OSNs, this has not been formally demonstrated. Part of this is due to a lack of any genetic model or chemical ablation paradigm that selectively removes sustentacular cells while leaving OSNs intact.
Notch2 mutants do not possess widespread alterations in sustentacular cell fate
We initially hypothesized that the persistence of Notch2 expression in adult may be required to prevent alterations in cell fate by sustentacular cells. Although transient expression of the Notch pathway is sufficient to initiate an irreversible switch from gliogenesis to neurogenesis in some systems (Morrison et al., 2000; Tanigaki et al., 2001), in others, transient expression only temporarily alters cell fate (Dorsky et al., 1997; Fortini et al., 1993). As Notch receptor function had not been previously examined in the olfactory system, it was unclear which paradigm would apply to sustentacular cell development. We found alterations in Hes1, Hey1, and Six1 expression in Notch2 mutants. All three transcription factors have been shown to repress neuronal cell fate during development. Loss of Hes1 in the epithelium leads to increased numbers of OSNs (Cau et al., 2000). Misexpression of Hey1 promotes astrocyte formation by repressing Mash1 and Math3 (Sakamoto et al., 2003). Finally, Six1 acts in zebrafish to repress neuronal differentiation (Bricaud and Collazo, 2006). Thus, all three genes affected in Notch2 mutants have been shown to inhibit neuronal cell fate during development.
But despite the known effects of Hes1, Hey1, and Six1 on cell fate during development, our results suggest that once the fate of sustentacular cells has been determined, this process is not reversible, consistent with other studies (Morrison et al., 2000; Tanigaki et al., 2001). If significant alteration in cell fate had occurred among sustentacular cells, we would expect to find a large increase in neuronal marker expression in the apical epithelium. However, markers of neuronal fate, such as Scg10 and OMP, were not widely expressed in mutants in the sustentacular layer. Moreover, markers of sustentacular fate, including Krt8 and Cbr2, are still expressed in apparently normal expression patterns. This argues against a wholesale change in cell fate by sustentacular cells. However, we cannot exclude the possibility that other markers not tested here may produce results more consistent with a change in cell fate.
One other possible interpretation for the continued postnatal expression of Notch2 in the sustentacular layer may be to maintain progenitor populations within the apical epithelium. This would be analogous to its proposed role in the cortex to inhibit differentiation among progenitor cells (Ever and Gaiano, 2005). However, there appears to be little evidence that sustentacular cells harbor a population of progenitor cells in the adult. First, proliferation occurs primarily in the basal layer in adults (Smart, 1971; Weiler and Farbman, 1998). Second, transplantation experiments have not demonstrated that sustentacular cells are able to function as stem cells (Chen et al., 2004). Finally, in cases where chemical ablation has been used to destroy sustentacular and neuronal cells within the epithelium, sustentacular cells are replaced by dividing basal cells (Manglapus et al., 2004). Thus, results from mitotic profile analyses, transplantation studies, and ablation experiments show adult sustentacular cells are likely to represent a population of mature, differentiated cells.
Notch2 is required for maintaining sustentacular function
Our experiments are most consistent with the interpretation that Notch2 is required to maintain sustentacular cell function. We demonstrate reduced RNA levels for major P450 and GST isoforms known to be expressed by sustentacular cells, as well as show an overall reduction in GST activity in mutants. Consistent with a loss of the normal, supporting function of sustentacular cells, these alterations are associated with neurodegeneration among OSNs. One possible interpretation for the variability in our phenotype may be the relative exposure within the epithelium to airflow patterns during inhalation. Toxicology experiments have shown that inhaled compounds, including that from soiled cage bedding (Mery et al., 1994), can have differential effects on various regions of the epithelium due to airflow-driven deposition. Although we found that nearly all areas of the epithelium possessed higher TUNEL and Mash1 expression than controls, some areas were often more highly affected, consistent with these prior studies.
How does Notch2 act to maintain sustentacular cell function once cell fate has been established? The ability of the Notch pathway to influence a wide variety of different developmental decisions is thought to be context dependent (Gaiano and Fishell, 2002). Although Hes1 and Hey1 are canonical downstream effectors of the Notch receptors, a handful of other genes have been identified that are also directly activated by Notch. Brain lipid binding protein (BLBP), for example, has been shown to be a target of the Notch pathway during radial glia formation (Anthony et al., 2005). Although Hes5 is also activated during the genesis of radial glia, BLBP expression occurs after Hes5. The sequential nature of Hes5 and BLBP expression suggests that the panoply of genes that are activated by the Notch pathway can change over time. Thus, early in radial glial development, Hes genes would be expressed in response to Notch signaling, presumably to repress Mash1 and inhibit neural differentiation. After this process, glial transcription targets, such as BLBP, are then expressed, leading to activation of a glial differentiation pathway.
We propose that a similar model holds in the olfactory epithelium. During development, activation of Hes1 by Notch receptors is important to inhibit neuronal differentiation by sustentacular cell precursors. This is supported by the observation that in Hes1 mutants excess numbers of OSNs are produced (Cau et al., 2000). However, once this cell-fate decision has been established, The Notch pathway then acts to promote glial differentiation. This is likely to involve Hes1 and Hey1, which act with or upon other genes, such as Six1, ultimately leading to expression of cell-type specific markers such as Krt8 and other genes necessary for sustentacular function (e.g. GSTs). During postnatal stages, maintenance of sustentacular function requires continued expression of Notch2. In combination with Notch3, Hes1, Hey1, and Six1 continue to be expressed within the postnatal and adult animal.
An alternative interpretation to our results is that Notch2 may also be expressed in OSNs at levels below our limit of detection, and that loss of Notch2 within neurons leads to neurodegeneration. However, others have also shown Notch2 is apically expressed at various developmental and postnatal stages (Carson et al., 2006; Lindsell et al., 1996; Mitsiadis et al., 2001). Moreover, we examined expression of Notch2 as early as E10. At all epithelial stages where the laminar structure of the epithelium could be distinguished, Notch2 was expressed only by sustentacular cells (data now shown). Nevertheless, mutations in Notch receptors have previously been shown to be directly associated with neuronal apoptosis (Mason et al., 2006). Thus, we cannot formally rule out that the absence of low levels of Notch2 within OSNs forms the basis of our phenotype.
Another interpretation of our results would be that loss of Notch2 during developmental stages may somehow affect neuronal development, leading to increased apoptotic susceptibility at postnatal stages. However, we examined embryonic Notch2 E14 mutants (n=3) for various neuronal and sustentacular markers and found no overt phenotypes (data not shown). Thus, it is unlikely that our results can be explained by an early, embryonic effect that would influence postnatal survival of OSNs or sustentacular function.
Notch2 affects Notch3 expression in the epithelium
An unexpected result from these studies is that there exists communication among Notch receptors within the sustentacular layer. Initiation of Notch3 appears to be independent of Notch2, as there is clear expression of Notch3 in sustentacular cells at P0 (Fig. 10) and in E14 mutants (data not shown). But in 2.5 week-old postnatal Notch2 mutants and in adult, Notch3 expression essentially is undetectable. The converse was not true, as Notch2 expression was unaffected in Notch3 mutants. This suggests that Notch3 expression is directly or indirectly dependent upon Notch2, but Notch2 expression is independent of Notch3. How or why Notch3 is affected by Notch2 is unknown. While we have no mechanistic explanation for the loss of Notch3 in Notch2 adults, one possibility is that the reduced levels of Hey1 and Hes1 may lead to a feedback loop that results in the elimination of Notch3 expression in the mutant. Classic studies in Drosophila have shown that expression of Delta by presumptive neuroblasts is regulated indirectly by upregulation of Enhancer of split expression caused by Notch activation. This in turn leads to a positive feedback loop where Delta expression is down-regulated as Notch activation increases (Heitzler et al., 1996). In the cochlea of mammalian Hes1 mutants, Notch1 expression is reduced, suggesting Notch1 expression is dependent upon the Hes genes to some extent for expression (Zine and de Ribaupierre, 2002). If a similar model holds in the sustentacular layer, we would predict that the presence of Notch3 at P0 and at E14 is able to partially activate Hey1 and Hes1. But the overall reduction in Hey1 and Hes1 activation would ultimately lead to lower levels of Notch3. Over time, this feedback loop would reduce or eliminate Notch3. In the absence of both Notch2 and Notch3, Hes1 and Hey1 expression would be down-regulated, affecting Six1 and other as yet unidentified genes, ultimately impacting sustentacular function. Genetic proof that Notch2 and Notch3 interact would require the generation of triple mutants of Notch2, Foxg1-Cre, and Notch3, but these efforts have so far proven unsuccessful.
Prior studies have shown that the various Notch receptors may indirectly interact with one another by differential binding or activation of downstream Notch effectors. Notch3 has been shown in vitro to antagonize Notch1 activity (Beatus et al., 1999), while Notch2 has been shown to antagonize Notch1 and Notch3 activity (Shimizu et al., 2002). On the other hand, chimeric fusions of Notch1 and Notch2 suggest functional redundancy between these receptors (Kraman and McCright, 2005). It has similarly been argued in Notch3 mutants that the unaltered expression of Notch1 and Notch2 suggests functional redundancy among the Notch receptors at E13.5 (Kitamoto et al., 2005). However, Notch3/Notch1 double mutants show no synergistic effects, suggesting a lack of functional overlap prior to E9.5 (Krebs et al., 2003). These various conflicting results suggest that Notch receptors may function either in a similar or antagonistic manner depending upon the specific context in which the receptors are expressed. In none of these studies, however, has it been suggested that the receptors directly or indirectly regulate the expression of one another. Outside of the nervous system, transfection of the Notch2 intracellular domain into C2C12 myoblasts decreases Notch3 expression, while transfection of Notch2 siRNA increases Notch3 levels (Ono et al., 2007). Thus, manipulation of Notch2 has been shown to affect Notch3 in a non-neuronal system.
Non-specific neurodegeneration is not observed in Notch2 mutants
We have described alterations in Hes1, Hey1, Six1, and Notch3 expression in Notch2 mutants, as well as increased neurodegeneration of OSNs. Notch2 is also known to be important for pituitary development (Raetzman et al., 2006). Given the reduced size and weight of Notch2 mutants, our phenotypes may be caused indirectly by non-specific effects associated with pituitary defects. We found that Foxg1 is expressed in the pituitary (data not shown), and therefore it is likely that Notch2 pituitary function has been impaired in these mutants. We are able to functionally separate some of our phenotypes from any potential effects caused by pituitary impairment. At P0, we have observed alterations in Hey1 and Notch3 expression. However, there is no apparent difference in weight between mutant and controls at this stage. Thus, it is unlikely that pituitary impairment would be the primary reason for the change in Hey1 and Notch3 expression. Although Hes1 and Six1 are relatively unaffected at P0, they are affected in older mutants. We observed clear weight differences at 2.5 weeks and in adult between mutants and controls, and therefore are unable to directly show that Hes1 and Six1 are down-regulated in response to absence of Notch2 in the epithelium. However, given the extensive evidence that Hes1 is a downstream target of the Notch receptors, and that Six1 is affected by mutations in the Notch pathway, it seems reasonable to assume that these effects are also due to loss of Notch2.
We have also shown increased neurodegeneration in adult mutant epithelia. This phenotype cannot be directly separated from any potential indirect effects associated with pituitary impairment. Indeed, IGF1 has been shown to be important for neuronal survival (Russo et al., 2005). However, when we examined olfactory bulbs for increased apoptosis by TUNEL, we found no difference between mutant and control (data not shown). Thus, there appears to be no widespread, non-specific increase in apoptosis in Notch2 mutants. We have also compared our mutant phenotype with those of others affecting pituitary development. IGF1 mutants are reduced in weight relative to control at E18.5 (Pichel et al., 2003). In our Notch2 mutants, no weight differences were observed at P0. IGF1 mutants also show disruptions in bulbar architecture and variably reduced bulbs that are smaller in size due in part to reduced numbers of mitral neurons (Pichel et al., 2003). As disrupting bulbar structure can cause apoptosis of OSNs (Costanzo and Graziadei, 1983), we examined Notch2 mutant bulbs at P0, 2.5 weeks, and adults using histological stains and by in situ hybridization. We did not observe any alteration of the mitral layer at any stage, as described for IGF1 mutants, nor did the size of the bulb appear to be affected. No difference in gene expression were identified in mutant bulbs as compared with controls. As such, several distinctions can be made between our Notch2 mutants and those affecting IGF1. Given the presumed supporting role that sustentacular cells play in maintaining OSN function, it seems reasonable to assume that loss of Notch2 in sustentacular cells ultimately would affect OSN survival. Nevertheless, we cannot completely exclude the possibility that impairment of other hormonal aspects of pituitary function may lead to increased neurodegeneration in the epithelium.
Feedback between the sustentacular and basal layers
In the adult Notch2 mutant, the absence of both Notch2 and Notch3 is associated with down-regulation of Hes1 in the apical layer. Concomitant with this, we noticed an increase in Hes1 expression basally. As mentioned previously, this is consistent with chemical ablation studies showing the generation of Hes1-expressing cells basally following ablation (Manglapus et al., 2004). These cells will presumably migrate apically and continue differentiating into sustentacular cells. Similarly, Hey1 and Six1 expression are also upregulated basally in Notch2 mutant adults.
As both Hes1 and Hey1 are expressed apically during development, we have assumed that they are activated in response to expression of Notch2 and Notch3. However, in adult Notch2 mutants and in chemical ablation paradigms, it is unclear how these basally-derived replacement sustentacular cells are able to initiate expression of Hes1 and Hey1. One possibility is that Notch1, which we have shown is expressed in a subset of basal cells postnatally, mediates this expression. Upregulation of progenitor cell division by Notch1 in response to injury can lead to increased numbers of glial cells being produced (Givogri et al., 2006). Alternatively, we note that Hes1 has been shown during early epithelial development to be expressed in a Mash1-independent manner (Cau et al., 2000). Only after E10 does Hes1 appear to require Mash1 for expression. Perhaps this basal Hes1 expression follows a similar mechanism, and does not require Notch for its initial expression.
We observed an increase in Hes1, Hey1, and Six1 expression basally despite the fact that the apical layer was still present in Notch2 mutants. Although alterations in the structure of the sustentacular layer have occurred, O-MACS, Krt8, and Cbr2 are still expressed by cells in the apical layer. Thus, it is intriguing to speculate why basal cells are triggered to produce these presumed replacement sustentacular cells if at least some markers of sustentacular cells are still present. One possibility is that a feedback mechanism associated either with neurodegeneration of OSNs or with disruption in sustentacular function is detected by basal cells. GDF11 (Wu et al., 2003) and Npy (Hansel et al., 2001) provide feedback to basal cells to modulate neurogenesis. Perhaps a similar mechanism regulates production of sustentacular cells by basal cells.
The Notch pathway and neurodegeneration
Sustentacular cells have long been called support cells for OSNs because of their many glial-like properties. Our observed degeneration phenotype also has parallels with the role of astrocytes in neurodegeneration. In amyotrophic lateral sclerosis (ALS), Huntington's Disease, and Alzheimer's Disease (AD), alterations in astrocyte function strongly affects the survival and likelihood of injury to neurons (Maragakis and Rothstein, 2006). As a result, the astrocytes may still be present but the supported neurons will die. In keeping with these findings, sustentacular cell death appears to be minimal in Notch2 mutants despite the increase in OSN degeneration.
In addition, Notch1 has been associated with Alzheimer's disease (Berezovska et al., 1998). While a great deal of attention has focused on the possible role of Notch1 expression in neurons as contributing towards the progression of this disease (Sestan et al., 1999), it is tempting to speculate that one explanation for the loss of nasal sensitivity that often precedes diagnosis of Alzheimer's disease may be due to loss of Notch2 expression in sustentacular cells leading secondarily to OSN degeneration.
Conclusions
The Notch pathway plays multiple, pleiotropic roles during development, and its effect is dependent upon the specific temporal and cellular context. Notch receptor expression in progenitor cells is thought to maintain these cells in an undifferentiated state, while at later stages the Notch pathway is involved in delineating neuronal versus glial fates. Still later, Notch receptors may act to promote astrocyte fate while inhibiting oligodendrocyte fate. In the adult, Notch receptors may act to regulate neurite outgrowth. Thus, while the immediate downstream effectors of the Notch pathway may remain the same, the functional output of the genes that are activated are likely to vary based upon the specific cell-type and timing of differentiation.
Here we have provided evidence to support the model that Notch2 acts to maintains sustentacular function in the epithelium. Although the Notch receptors are expressed in the adult nervous system, most efforts have centered around understanding Notch receptor function in neurons (Presente et al., 2001; Presente et al., 2004; Wang et al., 2004). We propose that persistent Notch receptor expression in at least some glial populations is important for maintaining their functional role in supporting neuronal survival.
Acknowledgments
We acknowledge the generous support of the Center for Vertebrate Genomics at Cornell (SR) and the NIH to TG (NS036437) and DML (DC007489). We thank members of the Lin lab for helpful discussions and Pat Fischer for help with immunohistochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 2005;19:1028–33. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatus P, Lundkvist J, Oberg C, Lendahl U. The notch 3 intracellular domain represses notch 1-mediated activation through Hairy/Enhancer of split (HES) promoters. Development. 1999;126:3925–35. doi: 10.1242/dev.126.17.3925. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Khen M, Lancet D. Glutathione S-transferases in rat olfactory epithelium: purification, molecular properties and odorant biotransformation. Biochem J. 1993;292(Pt 2):379–84. doi: 10.1042/bj2920379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovska O, Xia MQ, Hyman BT. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:738–45. doi: 10.1097/00005072-199808000-00003. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26:10438–51. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson C, Murdoch B, Roskams AJ. Notch 2 and Notch 1/3 segregate to neuronal and glial lineages of the developing olfactory epithelium. Dev Dyn. 2006;235:1678–88. doi: 10.1002/dvdy.20733. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–80. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–32. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–74. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Chen Y, Getchell ML, Ding X, Getchell TV. Immunolocalization of two cytochrome P450 isozymes in rat nasal chemosensory tissue. Neuroreport. 1992;3:749–52. doi: 10.1097/00001756-199209000-00007. [DOI] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984;81:1991–5. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–71. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Graziadei PP. A quantitative analysis of changes in the olfactory epithelium following bulbectomy in hamster. J Comp Neurol. 1983;215:370–81. doi: 10.1002/cne.902150403. [DOI] [PubMed] [Google Scholar]
- Danciger E, Mettling C, Vidal M, Morris R, Margolis F. Olfactory marker protein gene: its structure and olfactory neuron-specific expression in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:8565–9. doi: 10.1073/pnas.86.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Ishida H, Nibu K. Notch expression in developing olfactory neuroepithelium. Neuroreport. 2004;15:945–7. doi: 10.1097/00001756-200404290-00003. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Ever L, Gaiano N. Radial ‘glial’ progenitors: neurogenesis and signaling. Curr Opin Neurobiol. 2005;15:29–33. doi: 10.1016/j.conb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfaction. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–7. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–94. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–90. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Margolis FL, Getchell ML. Perireceptor and receptor events in vertebrate olfaction. Prog Neurobiol. 1984;23:317–45. doi: 10.1016/0301-0082(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- Gu J, Zhang QY, Genter MB, Lipinskas TW, Negishi M, Nebert DW, Ding X. Purification and characterization of heterologously expressed mouse CYP2A5 and CYP2G1: role in metabolic activation of acetaminophen and 2,6-dichlorobenzonitrile in mouse olfactory mucosal microsomes. J Pharmacol Exp Ther. 1998;285:1287–95. [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Habig WH, Jakoby WB. Assays for differentiation of glutathione Stransferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hadley WM, Dahl AR. Cytochrome P-450-dependent monooxygenase activity in nasal membranes of six species. Drug Metab Dispos. 1983;11:275–6. [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–4. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34:252–69. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development. 1996;122:161–71. doi: 10.1242/dev.122.1.161. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Kiyama H, Hayakawa T, Hamada Y, Tsujimoto Y. Differential expression of Notch1 and Notch2 in developing and adult mouse brain. Brain Res Mol Brain Res. 1995;29:263–72. doi: 10.1016/0169-328x(94)00257-f. [DOI] [PubMed] [Google Scholar]
- Irvin DK, Zurcher SD, Nguyen T, Weinmaster G, Kornblum HI. Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J Comp Neurol. 2001;436:167–81. [PubMed] [Google Scholar]
- Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci U S A. 2006;103:18998–9003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T, Takahashi K, Takimoto H, Tomizuka K, Hayasaka M, Tabira T, Hanaoka K. Functional redundancy of the Notch gene family during mouse embryogenesis: analysis of Notch gene expression in Notch3-deficient mice. Biochem Biophys Res Commun. 2005;331:1154–62. doi: 10.1016/j.bbrc.2005.03.241. [DOI] [PubMed] [Google Scholar]
- Kraman M, McCright B. Functional conservation of Notch1 and Notch2 intracellular domains. Faseb J. 2005;19:1311–3. doi: 10.1096/fj.04-3407fje. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–43. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- Krishna NS, Getchell TV, Getchell ML. Differential expression of alpha, mu, and pi classes of glutathione S-transferases in chemosensory mucosae of rats during development. Cell Tissue Res. 1994;275:435–50. doi: 10.1007/BF00318813. [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8:14–27. doi: 10.1006/mcne.1996.0040. [DOI] [PubMed] [Google Scholar]
- Ling G, Gu J, Genter MB, Zhuo X, Ding X. Regulation of cytochrome P450 gene expression in the olfactory mucosa. Chem Biol Interact. 2004;147:247–58. doi: 10.1016/j.cbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Luna LG. Histopathologic methods and color atlas of special stains and tissue artifacts. American Histolabs; Gaitheresburg, MD: 1992. [Google Scholar]
- Luna LG, Armed Forces Institute of Pathology (U.S.) Armed Forces Institute of Pathology (U.S.) Manual of histologic staining methods of the Armed Forces Institute of Pathology. Blakiston Division; New York: 1968. [Google Scholar]
- Maier MM, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem Biophys Res Commun. 2000;275:652–60. doi: 10.1006/bbrc.2000.3354. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–33. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–89. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Margolis FL. Olfactory marker protein (OMP) Scand J Immunol Suppl. 1982;9:181–99. doi: 10.1111/j.1365-3083.1982.tb03764.x. [DOI] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. 2006;28:49–57. doi: 10.1159/000090752. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- Mery S, Gross EA, Joyner DR, Godo M, Morgan KT. Nasal diagrams: a tool for recording the distribution of nasal lesions in rats and mice. Toxicol Pathol. 1994;22:353–72. doi: 10.1177/019262339402200402. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Gayet O, Zhang N, Carroll P. Expression of Deltex1 during mouse embryogenesis: comparison with Notch1, 2 and 3 expression. Mech Dev. 2001;109:399–403. doi: 10.1016/s0925-4773(01)00534-2. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Bell GI, Friesen HG. Growth hormone stimulates sequential induction of c-myc and insulin-like growth factor I expression in vivo. Endocrinology. 1987;120:1806–12. doi: 10.1210/endo-120-5-1806. [DOI] [PubMed] [Google Scholar]
- Nomura T, Takahashi S, Ushiki T. Cytoarchitecture of the normal rat olfactory epithelium: light and scanning electron microscopic studies. Arch Histol Cytol. 2004;67:159–70. doi: 10.1679/aohc.67.159. [DOI] [PubMed] [Google Scholar]
- Oka Y, Kobayakawa K, Nishizumi H, Miyamichi K, Hirose S, Tsuboi A, Sakano H. O-MACS, a novel member of the medium-chain acyl-CoA synthetase family, specifically expressed in the olfactory epithelium in a zone-specific manner. Eur J Biochem. 2003;270:1995–2004. doi: 10.1046/j.1432-1033.2003.03571.x. [DOI] [PubMed] [Google Scholar]
- Ono Y, Sensui H, Okutsu S, Nagatomi R. Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J Cell Physiol. 2007;210:358–69. doi: 10.1002/jcp.20838. [DOI] [PubMed] [Google Scholar]
- Orita Y, Nagatsuka H, Tsujigiwa H, Yoshinobu J, Maeda Y, Kakiuchi M, Orita S, Takeuchi A, Takeda Y, Fukushima K, Nagai N, Nishizaki K. Expression of Notch1 and Hes5 in the developing olfactory epithelium. Acta Otolaryngol. 2006;126:498–502. doi: 10.1080/00016480500437419. [DOI] [PubMed] [Google Scholar]
- Pellier-Monnin V, Astic L, Bichet S, Riederer BM, Grenningloh G. Expression of SCG10 and stathmin proteins in the rat olfactory system during development and axonal regeneration. J Comp Neurol. 2001;433:239–54. doi: 10.1002/cne.1138. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Fernandez-Moreno C, Vicario-Abejon C, Testillano PS, Patterson PH, de Pablo F. Developmental cooperation of leukemia inhibitory factor and insulin-like growth factor I in mice is tissue-specific and essential for lung maturation involving the transcription factors Sp3 and TTF-1. Mech Dev. 2003;120:349–61. doi: 10.1016/s0925-4773(02)00449-5. [DOI] [PubMed] [Google Scholar]
- Preece A. A manual for histologic technicians. Little; Boston: 1972. [Google Scholar]
- Presente A, Andres A, Nye JS. Requirement of Notch in adulthood for neurological function and longevity. Neuroreport. 2001;12:3321–5. doi: 10.1097/00001756-200110290-00035. [DOI] [PubMed] [Google Scholar]
- Presente A, Boyles RS, Serway CN, de Belle JS, Andres AJ. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci U S A. 2004;101:1764–8. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–908. doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916–43. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Hirata H, Ohtsuka T, Bessho Y, Kageyama R. The basic helix-loop-helix genes Hesr1/Hey1 and Hesr2/Hey2 regulate maintenance of neural precursor cells in the brain. J Biol Chem. 2003;278:44808–15. doi: 10.1074/jbc.M300448200. [DOI] [PubMed] [Google Scholar]
- Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–6. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- Shen L, Nam HS, Song P, Moore H, Anderson SA. FoxG1 haploinsufficiency results in impaired neurogenesis in the postnatal hippocampus and contextual memory deficits. Hippocampus. 2006;16:875–90. doi: 10.1002/hipo.20218. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Saito T, Kumano K, Hamada Y, Hirai H. Functional diversity among Notch1, Notch2, and Notch3 receptors. Biochem Biophys Res Commun. 2002;291:775–9. doi: 10.1006/bbrc.2002.6528. [DOI] [PubMed] [Google Scholar]
- Smart IH. Location and orientation of mitotic figures in the developing mouse olfactory epithelium. J Anat. 1971;109:243–51. [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Takeda M. Keratins in the developing olfactory epithelia. Brain Res Dev Brain Res. 1991;59:171–8. doi: 10.1016/0165-3806(91)90097-3. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kadokawa Y, Hamada Y, Marunouchi T. Notch2 expression negatively correlates with glial differentiation in the postnatal mouse brain. J Neurobiol. 1999;41:524–39. [PubMed] [Google Scholar]
- Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Hegg CC, Lucero MT. Ionic conductances in sustentacular cells of the mouse olfactory epithelium. J Physiol. 2005;562:785–99. doi: 10.1113/jphysiol.2004.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–62. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weech M, Quash M, Walters E. Characterization of the mouse olfactory glutathione S-transferases during the acute phase response. J Neurosci Res. 2003;73:679–85. doi: 10.1002/jnr.10687. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation decrease in the olfactory epithelium during postnatal development. Ann N Y Acad Sci. 1998;855:230–4. doi: 10.1111/j.1749-6632.1998.tb10572.x. [DOI] [PubMed] [Google Scholar]
- Whitby-Logan GK, Weech M, Walters E. Zonal expression and activity of glutathione S-transferase enzymes in the mouse olfactory mucosa. Brain Res. 2004;995:151–7. doi: 10.1016/j.brainres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Williams EO, Xiao Y, Sickles HM, Shafer P, Yona G, Yang JY, Lin DM. Novel subdomains of the mouse olfactory bulb defined by molecular heterogeneity in the nascent external plexiform and glomerular layers. BMC Dev Biol. 2007;7:48. doi: 10.1186/1471-213X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Yu TT, McIntyre JC, Bose SC, Hardin D, Owen MC, McClintock TS. Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol. 2005;483:251–62. doi: 10.1002/cne.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine A, de Ribaupierre F. Notch/Notch ligands and Math1 expression patterns in the organ of Corti of wild-type and Hes1 and Hes5 mutant mice. Hear Res. 2002;170:22–31. doi: 10.1016/s0378-5955(02)00449-5. [DOI] [PubMed] [Google Scholar]