Abstract
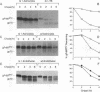
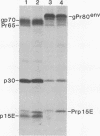
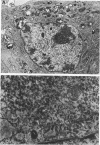
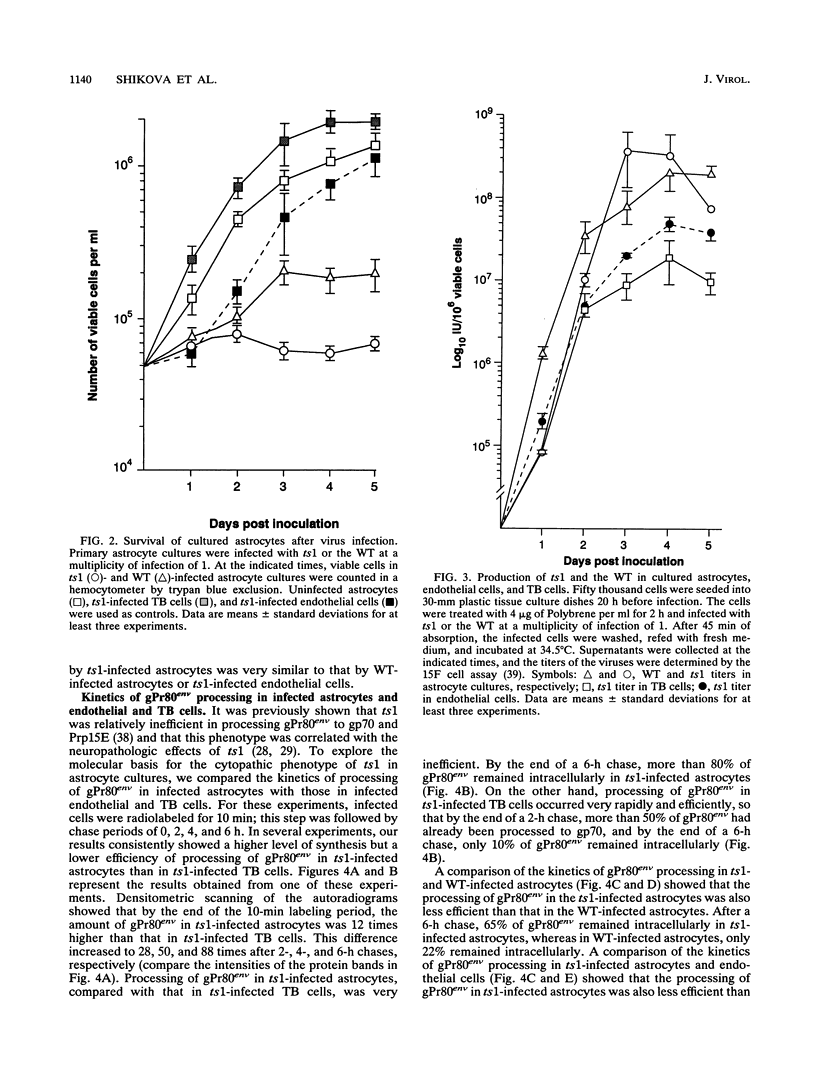
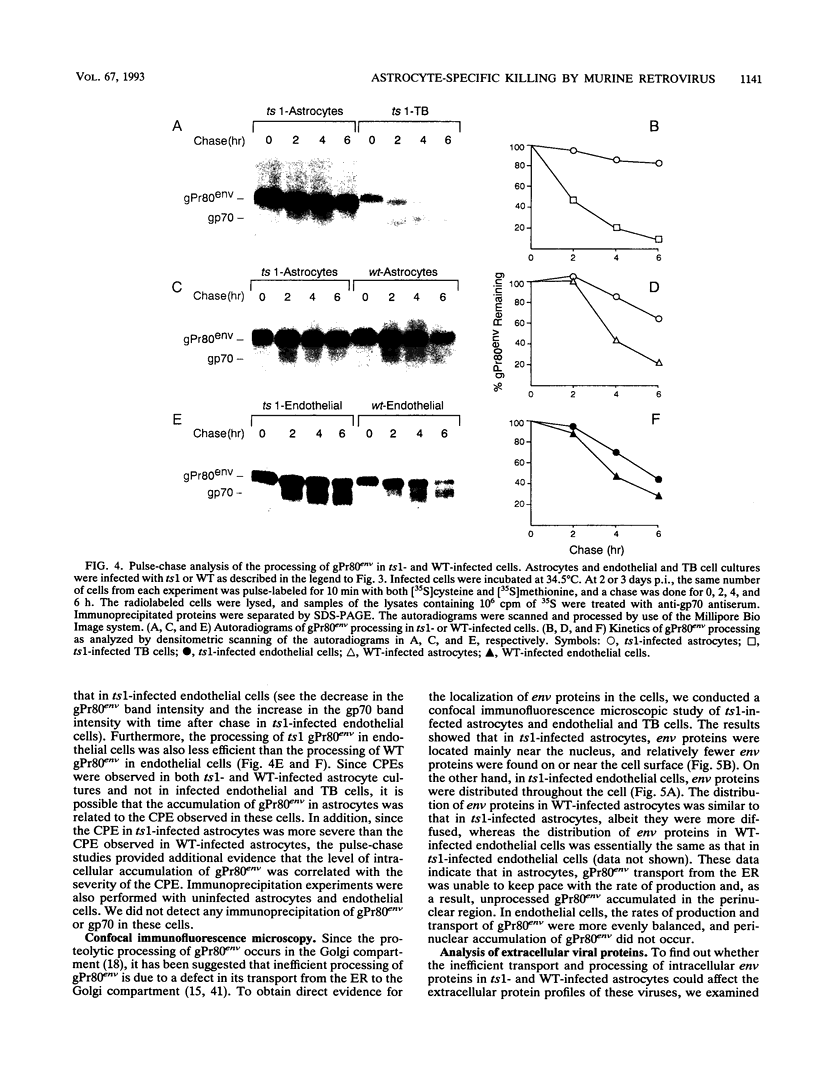
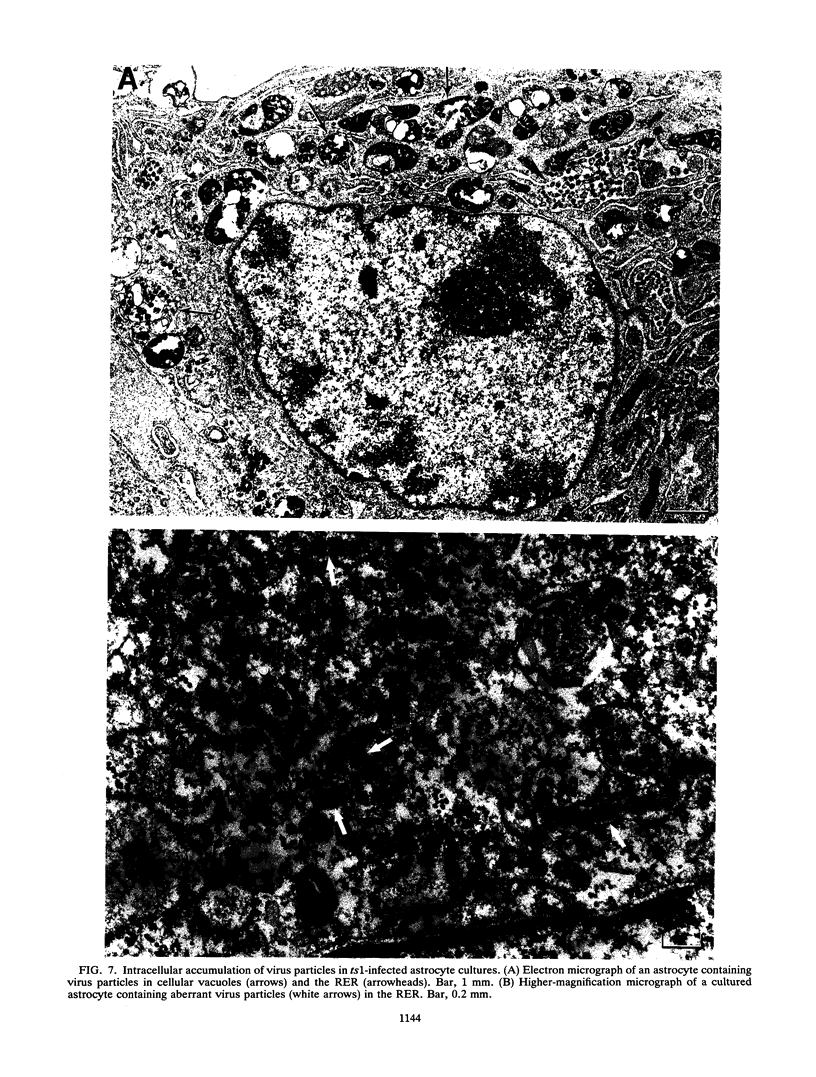
ts1 is a highly neuropathogenic and lymphocytopathic mutant of Moloney murine leukemia virus TB (MoMuLV-TB). We previously reported that the primary neuropathogenic determinant of ts1 maps to a single amino acid substitution, Val-25-->Ile, in precursor envelope protein gPr80env. This Val-25-->Ile substitution apparently renders gPr80env inefficient for transport from the endoplasmic reticulum to the Golgi apparatus. These findings suggest that the cytopathic effect of ts1 in neural cells might be due to the accumulation of gPr80env in the endoplasmic reticulum. Since endothelial and glial cells are targets of ts1 infection in the central nervous system, we established primary endothelial and astrocyte cultures to investigate the mechanism of cell killing caused by ts1. A continuous cell line, TB, was used as a control. Our results showed that both ts1 and MoMuLV-TB replicated and induced a cytopathic effect in astrocyte cultures, albeit to different degrees; ts1 appeared to be more lethal than MoMuLV-TB. On the other hand, ts1 and MoMuLV-TB infections of endothelial or TB cells were not cytopathic. The cytopathic effect in infected astrocytes correlated with the inefficiency of gPr80env transport and the intracellular accumulation of gPr80env as well as aberrant virus particles.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banapour B., Marthas M. L., Munn R. J., Luciw P. A. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). Virology. 1991 Jul;183(1):12–19. doi: 10.1016/0042-6822(91)90113-p. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R., Kleinschmidt A., Ludvigsen A., Mellert W., Neumann M., Herrmann R., Khim M. C., Burny A., Müller-Lantzsch N., Stavrou D. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992 Mar;6(3):273–285. [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi F., Fuerstenberg S., Gidlund M., Asjö B., Fenyö E. M. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987 Apr;61(4):1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofinis G., Papadaki L., Sattentau Q., Ferns R. B., Tedder R. HIV replicates in cultured human brain cells. AIDS. 1987 Dec;1(4):229–234. [PubMed] [Google Scholar]
- Crise B., Rose J. K. Human immunodeficiency virus type 1 glycoprotein precursor retains a CD4-p56lck complex in the endoplasmic reticulum. J Virol. 1992 Apr;66(4):2296–2301. doi: 10.1128/jvi.66.4.2296-2301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Bresser J., Stevenson M., Sakai K., Evinger-Hodges M. J., Volsky D. J. Susceptibility of human glial cells to infection with human immunodeficiency virus (HIV). FEBS Lett. 1987 Mar 9;213(1):138–143. doi: 10.1016/0014-5793(87)81479-5. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Sakai K., Bresser J., Stevenson M., Evinger-Hodges M. J., Volsky D. J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987 Dec;61(12):3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow S. W., Poss M. L., Hoover E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J Acquir Immune Defic Syndr. 1990;3(7):658–668. [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Kessler S. W., Orenstein J. M., Justement J. S., Jaffe E. S., Fauci A. S. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988 Nov 11;242(4880):919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps C. A., Lin Y. C., Wong P. K. Oligomerization and transport of the envelope protein of Moloney murine leukemia virus-TB and of ts1, a neurovirulent temperature-sensitive mutant of MoMuLV-TB. Virology. 1991 Oct;184(2):687–694. doi: 10.1016/0042-6822(91)90438-h. [DOI] [PubMed] [Google Scholar]
- Kawamura I., Koga Y., Oh-Hori N., Onodera K., Kimura G., Nomoto K. Depletion of the surface CD4 molecule by the envelope protein of human immunodeficiency virus expressed in a human CD4+ monocytoid cell line. J Virol. 1989 Sep;63(9):3748–3754. doi: 10.1128/jvi.63.9.3748-3754.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Sasaki M., Nakamura K., Kimura G., Nomoto K. Intracellular distribution of the envelope glycoprotein of human immunodeficiency virus and its role in the production of cytopathic effect in CD4+ and CD4- human cell lines. J Virol. 1990 Oct;64(10):4661–4671. doi: 10.1128/jvi.64.10.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Meltzer M. S., Phipps T., Gendelman H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988 Aug;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautrat G., Suzan M., Salaun D., Corbeau P., Allasia C., Morel G., Filippi P. Human immunodeficiency virus type 1 infection of U937 cells promotes cell differentiation and a new pathway of viral assembly. Virology. 1990 Dec;179(2):749–758. doi: 10.1016/0042-6822(90)90142-e. [DOI] [PubMed] [Google Scholar]
- Poss M. L., Dow S. W., Hoover E. A. Cell-specific envelope glycosylation distinguishes FIV glycoproteins produced in cytopathically and noncytopathically infected cells. Virology. 1992 May;188(1):25–32. doi: 10.1016/0042-6822(92)90731-4. [DOI] [PubMed] [Google Scholar]
- Sawada M., Kondo N., Suzumura A., Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989 Jul 10;491(2):394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Szurek P. F., Floyd E., Yuen P. H., Wong P. K. Site-directed mutagenesis of the codon for Ile-25 in gPr80env alters the neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990 Nov;64(11):5241–5249. doi: 10.1128/jvi.64.11.5241-5249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Ball J. K., Wong P. K. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990 Feb;64(2):467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volsky B., Sakai K., Reddy M. M., Volsky D. J. A system for the high efficiency replication of HIV-1 in neural cells and its application to anti-viral evaluation. Virology. 1992 Jan;186(1):303–308. doi: 10.1016/0042-6822(92)90086-5. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., McCartney-Francis N., Morganti-Kossmann M. C., Kossmann T., Ellingsworth L., Mai U. E., Mergenhagen S. E., Orenstein J. M. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991 Apr 1;173(4):981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Floyd E., Szurek P. F. High susceptibility of FVB/N mice to the paralytic disease induced by ts1, a mutant of Moloney murine leukemia virus TB. Virology. 1991 Jan;180(1):365–371. doi: 10.1016/0042-6822(91)90041-9. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Knupp C., Yuen P. H., Soong M. M., Zachary J. F., Tompkins W. A. ts1, a Paralytogenic mutant of Moloney murine leukemia virus TB, has an enhanced ability to replicate in the central nervous system and primary nerve cell culture. J Virol. 1985 Sep;55(3):760–767. doi: 10.1128/jvi.55.3.760-767.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K. Moloney murine leukemia virus temperature-sensitive mutants: a model for retrovirus-induced neurologic disorders. Curr Top Microbiol Immunol. 1990;160:29–60. doi: 10.1007/978-3-642-75267-4_3. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Prasad G., Hansen J., Yuen P. H. ts1, a mutant of Moloney murine leukemia virus-TB, causes both immunodeficiency and neurologic disorders in BALB/c mice. Virology. 1989 Jun;170(2):450–459. doi: 10.1016/0042-6822(89)90436-4. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Russ L. J., McCarter J. A. Rapid, selective procedure for isolation of spontaneous temperature-sensitive mutants of Moloney leukemia virus. Virology. 1973 Feb;51(2):424–431. doi: 10.1016/0042-6822(73)90441-8. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Soong M. M., MacLeod R., Gallick G. E., Yuen P. H. A group of temperature-sensitive mutants of Moloney leukemia virus which is defective in cleavage of env precursor polypeptide in infected cells also induces hind-limb paralysis in newborn CFW/D mice. Virology. 1983 Mar;125(2):513–518. doi: 10.1016/0042-6822(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Soong M. M., Yuen P. H. Replication of murine leukemia virus in heterologous cells: interaction between ecotropic and xenotropic viruses. Virology. 1981 Mar;109(2):366–378. doi: 10.1016/0042-6822(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Yu Y., Kamps C. A., Yuen P. H., Wong P. K. Construction and characterization of expression systems for the env gene of ts1, a mutant of Moloney murine leukemia virus-TB. Virus Res. 1991 Mar;19(1):83–92. doi: 10.1016/0168-1702(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Nau C., Soong M. M., Wong P. K. Molecular cloning of two paralytogenic, temperature-sensitive mutants, ts1 and ts7, and the parental wild-type Moloney murine leukemia virus. J Virol. 1985 Apr;54(1):178–185. doi: 10.1128/jvi.54.1.178-185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary J. F., Knupp C. J., Wong P. K. Noninflammatory spongiform polioencephalomyelopathy caused by a neurotropic temperature-sensitive mutant of Moloney murine leukemia virus TB. Am J Pathol. 1986 Sep;124(3):457–468. [PMC free article] [PubMed] [Google Scholar]